Abstract
Immune dysfunction is well documented during tumor progression and likely contributes to tumor immune evasion. CD8+ cytotoxic T lymphocytes (CTLs) are involved in antigen-specific tumor destruction and CD4+ T cells are essential for helping this CD8+ T cell-dependent tumor eradication. Tumors often target and inhibit T-cell function to escape from immune surveillance. This dysfunction includes loss of effector and memory T cells, bias towards type 2 cytokines and expansion of T regulatory (Treg) cells. Curcumin has previously been shown to have antitumor activity and some research has addressed the immunoprotective potential of this plant-derived polyphenol in tumor-bearing hosts. Here we examined the role of curcumin in the prevention of tumor-induced dysfunction of T cell-based immune responses. We observed severe loss of both effector and memory T-cell populations, downregulation of type 1 and upregulation of type 2 immune responses and decreased proliferation of effector T cells in the presence of tumors. Curcumin, in turn, prevented this loss of T cells, expanded central memory T cell (TCM)/effector memory T cell (TEM) populations, reversed the type 2 immune bias and attenuated the tumor-induced inhibition of T-cell proliferation in tumor-bearing hosts. Further investigation revealed that tumor burden upregulated Treg cell populations and stimulated the production of the immunosuppressive cytokines transforming growth factor (TGF)-β and IL-10 in these cells. Curcumin, however, inhibited the suppressive activity of Treg cells by downregulating the production of TGF-β and IL-10 in these cells. More importantly, curcumin treatment enhanced the ability of effector T cells to kill cancer cells. Overall, our observations suggest that the unique properties of curcumin may be exploited for successful attenuation of tumor-induced suppression of cell-mediated immune responses.
Keywords: CD4+/CD8+ T cell, Effector T cell, T-regulatory cell, FoxP3, Th1/Th2
Tumor progression often coincides with loss of proper cell-mediated immunity and reduced effector T-cell populations in the circulation. Patients and experimental animals with advanced cancer frequently display immune system dysfunctions,1 as indicated by reduced CD4+/CD8+ ratios2 and decreased T-cell proliferation.3 Depletion of the thymic CD4+CD8+ double-positive population in tumor-bearing animals, coupled with an increase in CD4−CD8− double-negative immature thymocytes, causes a decline in CD4+ and CD8+ effector populations in secondary immune organs and in circulation, weakens cellular defense mechanisms and induces thymic atrophy.4 Moreover, there is evidence for augmented apoptosis of T cells among cancer patients and mice with experimental tumors.5 Recent evidence from several laboratories, including our own, suggests that tumors may inhibit the development of an effective antitumor immune response either by directly inducing T-cell apoptosis or by sensitizing T lymphocytes to activation-induced cell death.6, 7, 8 There is evidence of increased apoptosis among CD8+ T cells in peripheral blood lymphocytes from cancer patients and animal models.4 These alterations correlate with the severity of the disease and with poor survival. Thus, tumor-induced immune suppression can add to the spread of the disease and create a barrier to immunotherapeutic interventions.
Tumors often contribute to a diminished proportion of effector T cells producing interferon (IFN)-γ and IL-2, thus decreasing the T helper 1 (Th1)-type immune response in the tumor-bearing host.9, 10, 11 IL-2 is a key cytokine in the regulation of the survival and expansion of peripheral T-cell populations, and IFN-γ is primarily responsible for activating and regulating the development and persistence of cytotoxic T lymphocytes (CTLs).12 CD8+ T cells secreting IFN-γ represent the effector Tc1 population, known to be essential for tumor eradication by cell-mediated immunity.13, 14 In cancer patients there is a shift from a type 1-mediated CD4+ T-cell response, which produces IFN-γ that is critical for the development of effective antitumor immunity, to a type 2 cytokine response (IL-4, IL-5 and IL-10), which typically mediates humoral immunity.15 Patients rendered disease free by primary tumor excision and/or immunotherapy revert to a predominance of IFN-γ-producing type 1 CD4+ T cells, suggesting that the tumor environment may be skewing the immune system towards a type 2 response.16
A growing tumor can evade immune surveillance by several mechanisms,17, 18 including manipulation of the microenvironment in a manner to induce negative regulation of immune responses within the host. These negative regulatory mechanisms include induction of tumor-specific T-cell tolerance mediated by T-regulatory (Treg) cells,19 a subset of CD4+ lymphocytes. Treg cells are a family of suppressor T cells which are further divided into two groups: thymic-derived ‘naturally occurring' Treg cells and induced/adaptive Treg cells generated extra thymically under certain conditions, including autoimmune diseases, transplantation tolerance and tumor immunity.20, 21, 22 Accumulating evidence suggests that Treg cells induced in tumor microenvironments are highly immunosuppressive.23 Relevant to antitumor immunotherapy, experimental models have shown that removal of Treg cells modifies the immune response to tumors.24 In mouse models, depletion of Treg cells with anti-CD25 antibody has been shown to enhance antitumor activity.25 It has also been suggested that the production of immunosuppressive cytokines by Treg cells is an important strategy employed by the growing tumor to evade immune surveillance.26, 27
Thus, therapeutic interventions designed to prevent tumor-induced downregulation of cell-mediated immune responses will be helpful to enhance antitumor immunity. In the current study, we characterized the mechanisms of tumor-induced immunosuppression and the role of curcumin (the major pigment of ground rhizome Curcuma longa, a known antioxidant with proven antitumor activity)28 in the prevention of immunosuppression caused by a progressing tumor. Reports of the immunomodulatory power of curcumin in tumor-bearing hosts are scarce. We recently demonstrated that curcumin reduced the overall systemic toxicity, while effectively lowering the tumor burden, and prevented tumor-induced T-cell depletion.28, 29, 30 This is the first time we are reporting the intricate mechanism of curcumin-induced immune restoration in tumor-bearing animals, and our results suggest that curcumin can prevent tumor-induced immunosuppression via restoration of effector T-cell populations, normalization of the cytokine profile and downregulation of Treg cell activity.
Results
Curcumin restores tumor-induced depletion of T cells in tumor-bearing hosts
To determine the effects of neoplastic conditions on the host's immune system, we used mice inoculated with Ehrlich's ascites carcinoma (EAC, a murine mammary carcinoma cell line). Lymphocytes from the peripheral blood, draining lymph nodes and tumor sites (tumor-infiltrating lymphocytes (TIL)) were isolated from tumor-bearing mice and compared with those of normal mice (Figure 1a). Analysis of circulating, lymph-node and tumor-site T-cell subpopulations of tumor-bearing mice revealed that both the helper T lymphocyte (CD4+) and the CTL (CD8+) populations were diminished in comparison to that of normal counterparts (Figure 1a). We have previously shown that curcumin induces apoptosis in human breast cancer and ascites carcinoma cells.26, 27, 28 Here we observed that administration of curcumin (50 mg/kg) to tumor-bearing animals prevented tumor-induced depletion of circulating and lymph-node T-cell populations and increased the number of CD4+ and CD8+ T cells present at the tumor site (Figure 1b).
Figure 1.
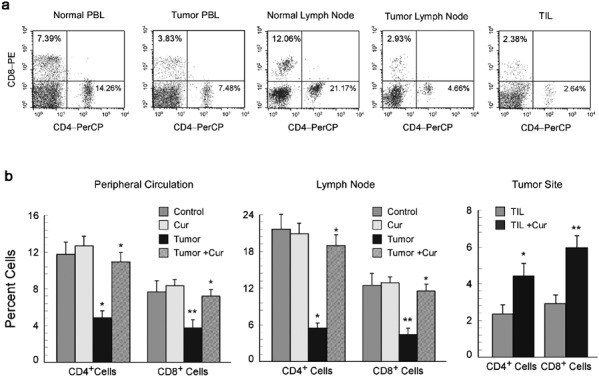
Curcumin restores tumor-induced depletion of T-cell populations. Lymphocytes from the peripheral circulation, draining lymph nodes and tumor sites of untreated or curcumin treated (50 mg/kg body weight) normal and EAC-bearing mice were analyzed by flow cytometry to quantitate percentages of CD4 and CD8 populations. (a) Flow cytometric dot plot presentation shows CD4+ and CD8+ T-cell populations in the peripheral circulation and lymph nodes of normal and tumor-bearing mice, as well as in the tumor sites. (b) Graphical representation of flow cytometric data showing percentages of CD4+ and CD8+ T cells in the peripheral circulation (left panel), lymph nodes (middle panel) and tumor sites (right panel) of curcumin-treated and untreated tumor-bearing mice. The values are mean±SEM of five independent experiments. *P<0.001 and **P<0.005 when compared with respective untreated/normal controls. EAC, Ehrlich's ascites carcinoma.
Curcumin restores central memory T cell (TCM) and effector memory T cell (TEM) populations in tumor-bearing hosts
The memory T-cell pool functions as a dynamic repository of antigen-experienced T lymphocytes that accumulate over the lifetime of the individual. Thus, our observation of tumor-induced depletion of CD4+ and CD8+ T-cell populations prompted us to further study the status of the memory T-cell population. Recent studies indicate that memory T lymphocytes contain distinct TCM and TEM populations, characterized by distinct homing capacity and effector functions.31 With this in mind, we characterized the effect of tumor burden on memory T-cell populations. Central and memory T cells were characterized by monitoring the surface expression of CD44 and CCR7 among CD4+ and CD8+ cell populations (Figure 2a). Our study revealed decreased percentages of both TCM and TEM cells in the peripheral circulation, lymph nodes and tumor sites of tumor-bearing hosts (Figure 2b and c). Curcumin-fed, tumor-bearing mice, however, demonstrated normal percentages of both TCM (CD44highCCR7high) and TEM (CD44highCCR7low) within the CD4+ and CD8+ T-lymphocyte populations (Figure 2b and c), with P<0.005 in the case of circulatory and lymph-node T cells and P<0.05 in the case of tumor-site T cells.
Figure 2.
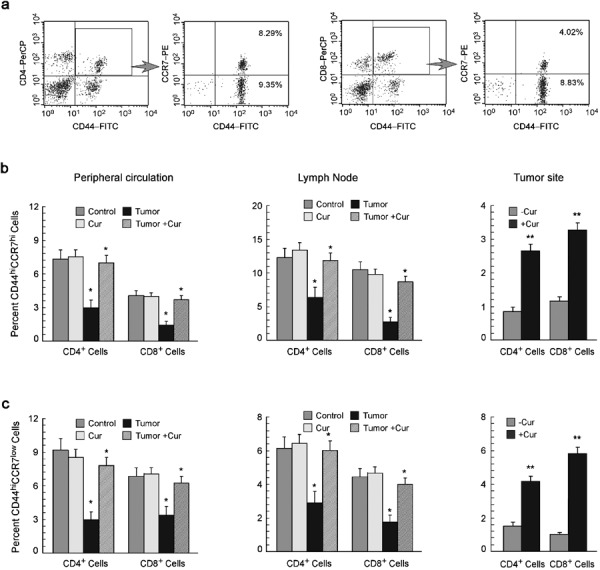
Curcumin replenishes suppression of memory T cells. (a) Dot plot analysis of flow cytometric data representing percentages of CCR7-expressing T cells in CD4+/CD44+- and CD8+/CD44+-gated populations. Graphical representation of flow cytometrically determined percentages of (b) CD44highCCR7high central memory cells and (c) CD44highCCR7low effector memory cells among CD4+ and CD8+ T-cell populations in the peripheral circulation, lymph nodes and tumor sites of untreated or curcumin-treated normal and tumor-bearing mice. The values are mean±SEM of five independent experiments. *P<0.001 and **P<0.005 when compared with respective untreated/normal controls.
Curcumin normalizes Th1/Tc1-type cytokine-producing effector T–cell populations in tumor-bearing hosts
We next analyzed the capacity of lymphocytes of normal and tumor-bearing mice to produce cytokines after phorbol-12-myristate-13-acetate/ionomycin activation (Figure 3a). We observed a reduced proportion of Th1/Tc1-type cytokine (IFN-c)-secreting CD4+ and CD8+ T cells (Figure 3b) and an increased proportion of Th2/Tc2-type cytokine (IL-4)-secreting CD4+ and CD8+ T cells (Figure 3c) within the peripheral circulation, lymph nodes and tumor sites of tumor-bearing mice in comparison to their normal counterparts. Administration of curcumin to the tumor-bearing mice, however, restored the cytokine expression in the circulation, lymph-node and tumor-site lymphocytes to normal levels (Figure 3b and c). Overall, we observed that tumor-bearing hosts have a Th2-type cytokine bias in the peripheral circulation, lymph nodes and tumor sites, which was essentially reversed by curcumin (Figure 3d).
Figure 3.
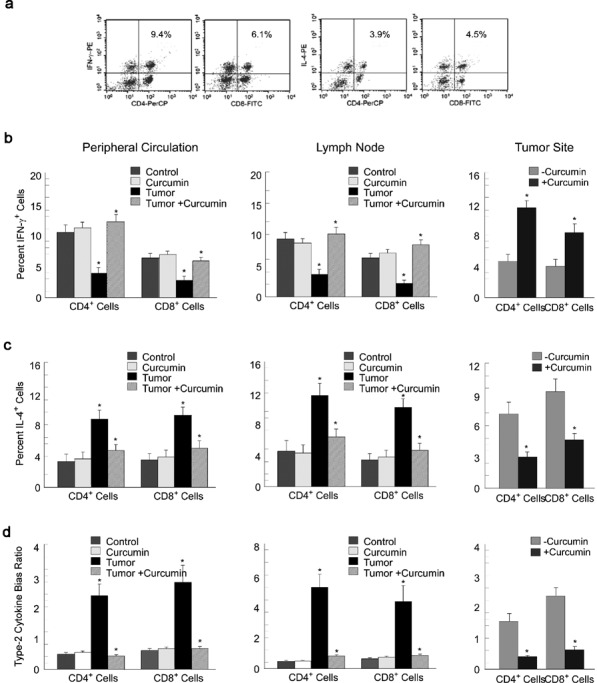
Curcumin normalizes cytokine-producing effector T-cell populations in tumor-bearing hosts. (a) Typical flow cytometric patterns for IFN-γ- and IL-4-producing CD4+ and CD8+ T cells in the peripheral circulation of mice. Graphical representation of flow cytometric data of intracellular IFN-γ (b), IL-4 (c) and type 2 cytokine bias ratio (d) in CD4+ and CD8+ T cells from the peripheral circulation (left panels), lymph-node (middle panels) and tumor-infiltrating lymphocytes (right panels) of the untreated or curcumin-treated tumor-bearing and normal mice. Graphical values are mean±SEM of five independent experiments. *P<0.001 when compared with respective untreated/normal controls. IFN, interferon.
Curcumin prevents tumor-induced downregulation of T-cell proliferation
Our results have demonstrated the loss of effector T-cell activity due to a decrease in the percentage of effector/memory T cells and an increase in Th2-type cytokines in tumor-bearing animals. To determine if tumors also influence the proliferative capacity of effector T cells in response to a T-cell antigen receptor stimulus, CD3+ T cells from normal, tumor-bearing or curcumin-fed tumor-bearing mice were isolated and loaded with 5-(and-6)-carboxyfluorescein succinimidyl ester (CFSE). Data presented in Figure 4 show that T cells from healthy and curcumin-fed mice proliferated in response to anti-CD3 and anti-CD28 antibodies. However, T cells from tumor-bearing mice failed to proliferate in response to anti-CD3 and anti-CD28 antibodies. Interestingly, curcumin prevented tumor-induced downregulation of T-cell proliferation (Figure 4), suggesting that apart from inhibiting tumor-induced loss of effector T-cell activity, curcumin also restored T-cell proliferative capacity.
Figure 4.
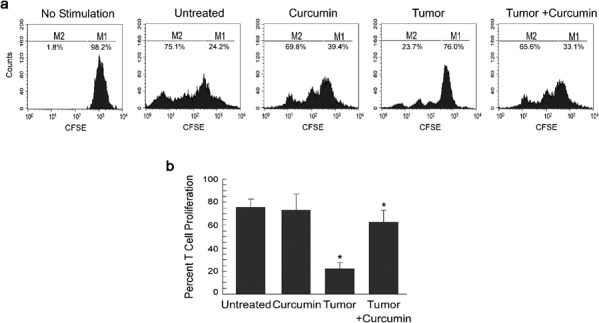
Curcumin attenuates tumor-induced inhibition of T-cell proliferation. (a) Flow cytometric histogram display of CFSE fluorescence (x axis) versus counts (y axis) and (b) respective graphical representation used to determine proliferation of effector T cells isolated from untreated or curcumin-treated normal and tumor-bearing mice following stimulation with CD3 and CD28 antibodies. Values are mean±SEM of five independent experiments. *P<0.001 when compared with respective untreated/normal controls. CFSE, 5-(and-6)-carboxyfluorescein succinimidyl ester.
Curcumin prevents tumor-induced augmentation of CD4+CD25+FoxP3+ Treg cell populations in tumor-bearing hosts
It is well known that the loss of Th1-type effector activity is associated with the upregulation of Treg suppressor activity in tumor-bearing animals.32 Tregs were flowcytometrically characterized by the expression of FoxP3 in CD4+CD25+ T cells as shown in Figure 5a. The proportions of CD4+CD25+FoxP3+ (markers of Treg cells in mice)33 Treg cells were found to be elevated in the peripheral circulation, lymph nodes and tumor sites of tumor-bearing mice when compared to that of normal mice (Figure 5b). Administration of curcumin to tumor-bearing mice decreased the proportion of CD4+CD25+FoxP3+ Treg cells in the peripheral circulation, lymph nodes and tumor sites (Figure 5b).
Figure 5.
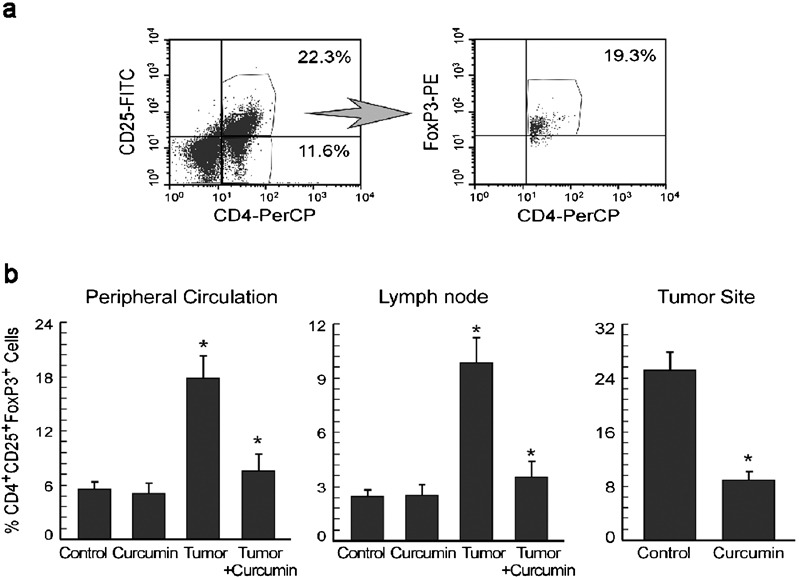
Curcumin prevents tumor-induced augmentation of CD4+CD25+FoxP3+ Treg cells in tumor-bearing hosts. (a) Flow cytometric determination of FoxP3+ cells in CD4+CD25+-gated T cells. (b) Graphical representations of percentages of CD4+CD25+FoxP3+ Treg cell populations in the peripheral circulation (left panel), lymph nodes (middle panel) and tumor sites (right panel) of untreated or curcumin-treated normal and tumor-bearing mice. Values are mean±SEM of five independent experiments. *P<0.001 when compared with respective untreated/normal controls.
Curcumin prevents Treg suppressor activity through downregulation of transforming growth factor (TGF)-β and IL-10 in these cells
Numerous studies have demonstrated that Treg cells mediate their suppressive activity in a cytokine-dependent manner, mainly through the production of TGF-β and IL-10.34, 35 Thus, our previous observations prompted us to measure the levels of intracellular TGF-β and IL-10 in CD4+CD25+ cells of tumor-bearing animals by flow cytometry. Our intracellular cytokine staining experiments showed elevated intracellular TGF-β in Treg cells in the peripheral circulation, lymph nodes and tumor sites of tumor-bearing hosts compared with normal hosts, which was essentially reversed upon administration of curcumin to tumor-bearing animals (Figure 6a, b and c). Further experiments showed that curcumin administration also normalized IL-10 levels in both FoxP3+ and FoxP3− CD4+CD25+ Treg cells (Figure 6a and b).
Figure 6.
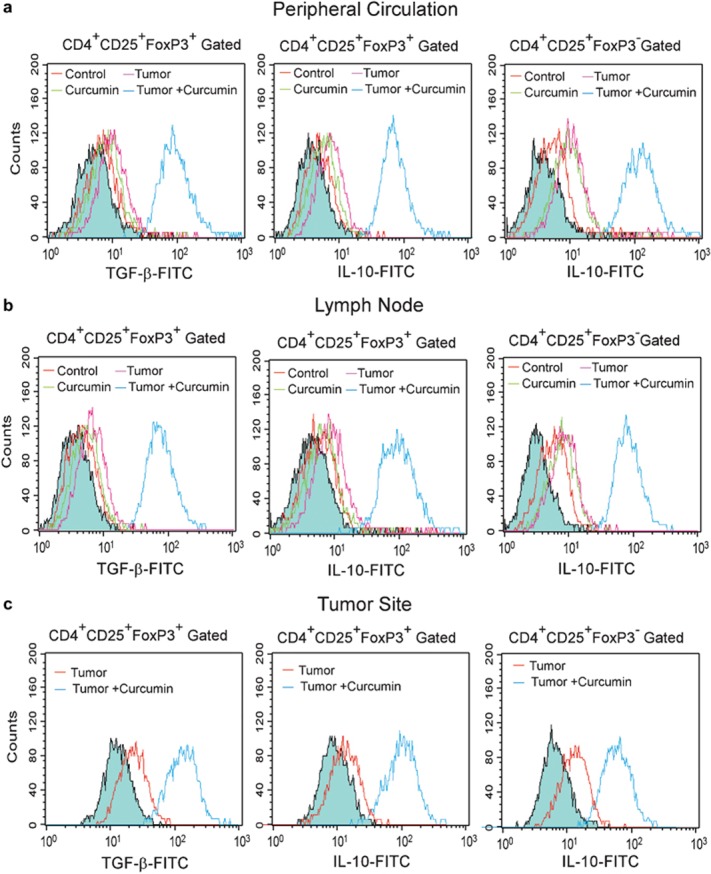
Curcumin normalizes intracellular TGF-β and IL-10 in CD4+CD25+ Treg cells. Histogram overlay represents the intracellular TGF-β (left panels) and IL-10 (middle panels) levels in CD4+CD25+FoxP3+ Treg cell populations from the (a) peripheral circulation, (b) lymph nodes and (c) tumor sites of untreated or curcumin-treated normal and tumor-bearing mice. Right panels of (a–c) represent IL-10 levels in CD4+CD25+FoxP3− Treg cell populations. Filled histograms indicate the isotype controls for intracellular cytokine staining. Values are representatives of three independent experiments. TGF, transforming growth factor.
Curcumin potentiates T cell-mediated tumor-cell killing in vitro
To test whether curcumin-mediated protection of effector T-cell populations influences immune-mediated tumor elimination, peripheral T cells from healthy mice were exposed to cell-free supernatants from tumor cells treated with or without curcumin and then cocultured with EAC cells. After 24 h of incubation, apoptosis of the EAC cells was measured by Annexin-V/7-amino-actinomycin D (7-AAD) binding followed by flow cytometry. We observed that naive T cells were able to kill tumor cells more efficiently than T cells pre-treated with tumor supernatants (Figure 7a). Interestingly, T cells cultured with supernatant from curcumin-treated tumor cells were more efficient at inducing tumor-cell killing than T cells cultured with supernatant from untreated tumor cells (Figure 7a). Since T cell-mediated cancer cell killing is mediated by several proteolytic proteins secreted by CD8+ T cells, we measured the levels of granzyme and perforin in CD8+ T cells. Figure 7b shows that curcumin inhibited the tumor-mediated reduction of granzyme and perforin in CD8+ cytotoxic T cells. These data indicate that curcumin not only prevents tumor-induced loss of effector T-cell populations but also contributes to immune-mediated tumor-cell killing.
Figure 7.
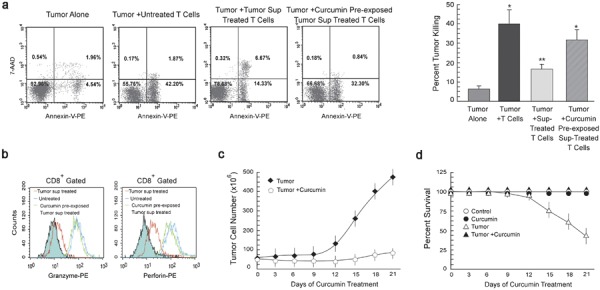
Curcumin potentiates T cell-mediated cancer cell killing. (a) Flow cytometric determination and graphical representation of apoptotic populations amongst the EAC cells cocultured with or without normal T cells. In parallel experiments, T cells, pre-exposed to supernatant from tumor cells cultured with or without 10 µM curcumin, were cocultured with cancer cells (effector/target ratio of 1∶10). Tumor cells were labeled with Annexin-V-PE and 7-AAD and the percentage of apoptosis was analyzed by flow cytometry. Dot plot display shows Annexin-V-PE fluorescence (x axis) versus 7-AAD fluorescence (y axis). Annexin-V/7-AAD positive cells were considered apoptotic. (b) Histogram overlays representing intracellular levels of granzyme (left panel) and perforin (right panel) in CD8+-gated cells among total T lymphocytes exposed to untreated or curcumin pre-treated tumor supernatant. Graphical representation depicting change in (c) tumor cell number and (d) percentage survival with time in both curcumin treated and untreated tumor-bearing mice. Values are mean±SEM of five independent experiments. *P<0.001 and **P<0.005 when compared with respective untreated/normal controls. EAC, Ehrlich's ascites carcinoma; PE, phycoerythrin; 7-AAD, 7-amino-actinomycin D.
Curcumin inhibits tumor growth and increases survival rates of tumor-bearing mice
Our results have indicated that curcumin restores the suppressed immune system found in tumor-bearing hosts. We further attempted to analyze this immunomodulatory capacity of curcumin in terms of tumor regression and survival rates of tumor-bearing animals, using 10 individual mice in each group. As depicted in Figure 7c, we observed that EAC cells grew up to 390 million in untreated mice but the number of EAC cells was reduced to 20 million in curcumin-treated mice. In support of these data, the survival rate in the untreated group was only 50% (Figure 7d), while in the curcumin-treated group the survival rate increased to 96%. Thus, administering curcumin to tumor-bearing mice resulted in both tumor regression and improved survival rates.
Discussion
In order to establish itself, a growing tumor often induces severe immunosuppression, which almost always abolishes effective cell-mediated immune responses of the host. The mechanisms of tumor-induced suppression of cell-mediated immunity include a decline in the percentage of effector T cells (CD4+ and CD8+) accompanied by a loss of effector T-cell activity and downregulation of Th1-type cytokine responses, along with augmentation of Th2-type cytokine responses. This shift from a Th1- to a Th2-type response also contributes to the decreased activity of effector CTLs.36 Another important reason for the failure of tumor immune surveillance is the augmentation of immunosuppressive Treg cells.37 In accordance with previous findings, our results depicted a reduction in the number of effector and memory T cells and a shift from a Th1- to a Th2-type response in tumor-bearing hosts, along with elevated levels of Treg cells. Curcumin, in turn, was found to prevent these tumor-induced alterations of the immune system, thereby strengthening the antitumor response of the tumor-bearing host.
CD4+ T cells appear to be critical for the promotion of memory CD8+ T-cell responses,38 although CD8+ T cells are the effector cells more commonly associated with tumor rejection.39 Tumor insult causes a decrease in the numbers of IL-2- and IFN-γ-secreting CD4+ and CD8+ cells in peripheral circulation and at the tumor site. IL-2 and IFN-γ are two of the most important Th1 cytokines and are pivotal for survival, expansion and activation of both helper T cells and CTLs.12 By contrast, Th2-mediated immunity (IL-4 is the prototypical Th2 cytokine) is thought to favor tumor growth, both by promoting angiogenesis and by inhibiting cell-mediated immunity and subsequent tumor-cell killing.40 We observed that curcumin not only restored the activated/effector CD4+ and CD8+ T-cell populations in the tumor-bearing host but also increased the percentage of these cells at the tumor site. TILs play a critical role in detection and eradication of tumor cells;41 thus, curcumin-induced augmentation of TILs can help to restore appropriate antitumor immune responses in the host. Curcumin administration increased the proportion of IFN-γ-secreting CD4+ and CD8+ T cells in the circulation and at the tumor site of tumor bearers, clearly indicating that it prevents the depletion of Th1 cells in these organs with a corresponding decrease in the type 2 bias. Interestingly, curcumin also reversed tumor-induced inhibition of effector T-cell proliferation.
Tumor-bearing hosts are known to have a high proportion of CD4+CD25+FoxP3+ Treg cells (FoxP3 is the transcription factor exclusively produced in Treg cells).42 Treg cells produce TGF-β and IL-10, two immunosuppressive cytokines, to downregulate the Th1-type response.43 Apart from their suppressive activity, Treg cells compete with other T cells for IL-2, an important T-cell growth factor, thereby reducing the availability of IL-2 for other T cells.44 Interestingly, our results further demonstrated that curcumin administration not only downregulates Treg suppressive activity but also inhibits its augmentation in tumor-bearing mice.
Reports of the immunomodulatory effects of curcumin indicate that it favors a Th2-type response,45 but there are several reports which indicate that curcumin can augment tumor eradication by reinstating T-cell responses.46 Gertshe et al.47 also showed that curcumin upregulates IFN-γ mRNA expression, indicating that the effect of curcumin on Th1 responses is not solely inhibitory. In line with these observations, our data indicate that curcumin administration enhances the antitumor immune response of T cells in tumor-bearing hosts. Interestingly, normal T cells pre-incubated with curcumin failed to show such immunostimulatory effects. Previously, we have shown that curcumin can induce tumor-cell killing, lower systemic toxicity and neutralize oxidative stress in the immune system, resulting in T-cell protection.28, 29, 30 Here we report that curcumin reduced the levels of TGF-β and IL-10 in Treg cells and reduced the number of Treg cells in the tumor-bearing hosts. Complex interactions between various signals produced by the tumor and by curcumin ultimately result in augmentation of Th1/Tc1-type responses in the tumor-bearing host. The immunoprotective role of curcumin was further strengthened when we found that curcumin potentiates T cell-mediated tumor-cell killing. Thus, the observed protective effects of curcumin on Th1 and Tc1 populations in the tumor-bearing mice may be attributed to the fact that curcumin reduces Treg cell populations and inhibits TGF-β and IL-10 production from Treg cells, finally reducing tumor burden. Taken together, these results and the results of experiments with human samples indicate that curcumin may protect effector T cells in tumor-bearing hosts, thereby supporting the use of curcumin as a pharmacologically and immunologically safe anticancer drug. Curcumin is currently undergoing clinical testing, and although detailed reports of the results in human cancer patients are still unavailable, the current study provides some much needed insight into the effects of this antitumor agent on the immune system.
Materials and methods
Treatment of animals
Swiss albino mice (NCLAS, Hyderabad, India) weighing 20–25 g were maintained in a temperature-controlled room with a normal light–dark cycle. All animal experiments were performed following the Principles of Laboratory Animal Care (NIH Publication No. 85-23, revised in 1985) and Indian laws on ‘Protection of Animals' under the supervision of authorized investigators. Mice were divided into four groups of 10 animals each, including normal (non-tumor-bearing), tumor-bearing (which were intraperitoneally injected with 1×105 exponentially grown EAC), curcumin-treated (non-tumor-bearing) and curcumin-treated tumor-bearing. Curcumin (50 mg/kg body weight every alternate day; treatment started 7 days after tumor inoculation) was fed orally.
Cell culture and experiments
At day 21 of tumor inoculation, the carcinoma cells were isolated aseptically from the peritoneal cavity of tumor-bearing mice.27, 28 The isolated cells were then subjected to negative selection using anti-CD3 and anti-CD16 antibody-coated microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). More than 98% of the CD16-/CD3-negative cells were morphologically characterized as EAC by Wright staining and viability was assessed to be >95% by trypan blue dye exclusion. Peripheral blood, collected from mice, was centrifuged over Ficoll-Hypaque (Amersham Pharmacia, Uppsala, Sweden) density gradient to obtain total lymphocytes. T cells were purified by positive selection from total lymphocytes using microbeads coated with mouse anti-CD3 antibodies. Cells ware maintained in complete RPMI 1640 medium at 37 °C in humidified incubator containing 5% CO2.29, 30
TILs from ascites carcinoma were isolated by positive selection using anti-CD3-coated microbeads. EAC cells were incubated for 48 h in the presence or absence of 10 µM curcumin; supernatant was freed from cellular components and used in a 1∶1 ratio with RPMI 1640 media to study the effect of tumor supernatant on T cells. To determine the effects of curcumin pre-treatment on immune-mediated tumor-cell killing, normal T cells were co-incubated with tumor cells for 24 h (effector/target cell ratio of 1∶10).
Phenotypic analysis of Treg and memory T cells
For the quantification of Treg cells, T cells from the peripheral blood and tumor sites were labeled with peridinin chlorophyll protein complex (PerCP)-conjugated CD4, FITC-conjugated CD25 and phycoerythrin (PE)-conjugated FoxP3 antibodies (BD Bioscience, San Jose, CA, USA). Cells were then analyzed with a FACS Calibur (BD Bioscience) equipped with a 488-nm argon laser light source, a 530-nm band pass filter for FITC fluorescence and a 575-nm band pass filter for phycoerythrin (PE fluorescence. Cells were properly acquired, gated and analyzed using CellQuest Software (BD Bioscience). For the analysis of memory T cells, cells were labeled with PerCP-conjugated CD4 or CD8 monoclonal antibodies and then with PE-conjugated CCR7 and FITC-conjugated CD44 antibodies (for the determination of TCM and TCM populations; BD Bioscience). Cells were then analyzed by FACS.
Proliferation and apoptosis assay
The CD3+ cells were loaded with CFSE (Molecular Probes, Eugene, OR, USA) and proliferation was induced by stimulating CD3+ cells (1×106 cells/ml) with cross-linked anti-CD3 antibodies and soluble anti-CD28 antibodies for 72 h. Dilution of CFSE fluorescence as a marker of cell proliferation was assayed by flow cytometry. For the determination of cell death, tumor cells were labeled with 7-AAD and Annexin-V-PE (BD Bioscience) and analyzed by flow cytometry. Annexin-V+/7-AAD+ cells were considered apoptotic.29, 30
Detection of intracellular cytokines by flow cytometry
T cells were stimulated with phorbol-12-myristate-13-acetate (10 ng/ml) and ionomycin (1 µM), and transport of newly synthesized cytokines from the Golgi apparatus was blocked with 10 µg/ml Brefeldin A (Sigma, St Louis, MO, USA). After incubation for 4 h at 37 °C, cells were washed with phosphate-buffered saline and half of the cells were labeled with PerCP-CD4/FITC-CD8 or PerCP-CD4/FITC-CD25 antibodies. Cells were permeabilized with saponin and intracellular TGF-β, IL-10, IFN-γ, IL-2 and IL-4 (10 µl, dilution 1∶30; BD Bioscience) were labeled with PE-/FITC-tagged antibodies and were analyzed by FACS.
Statistical analysis
Values are shown as standard error of mean (SEM) except when otherwise indicated. Data were analyzed and, when appropriate, significance of the differences between mean values was determined by Student's t-test. Results were considered significant at P<0.05.
Acknowledgments
The authors want to thank Mr Uttam K. Ghosh, Mr Somnath Chakraborty and Mr Ranjan Dutta for technical assistance. This work was supported by grants from the Council for Scientific and Industrial Research and the Indian Council of Medical Research, Government of India.
References
- Ohm JE, Carbone DP. Immune dysfunction in cancer patients. Oncology. 2002;16:11–18. [PubMed] [Google Scholar]
- Kandil A, Bazarbashi S, Mourad WA. The correlation of Epstein–Barr virus expression and lymphocyte subsets with the clinical presentation of nodular sclerosing Hodgkin disease. Cancer. 2001;91:1957–1963. doi: 10.1002/1097-0142(20010601)91:11<1957::aid-cncr1220>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Alexander JP, Kudoh S, Melsop KA, Hamilton TA, Edinger MG, Tubbs RP, et al. T cells infiltrating renal cell carcinoma display a poor proliferative response even though they can produce interleukin 2 and express interleukin 2 receptors. Cancer Res. 1993;53:1380–1387. [PubMed] [Google Scholar]
- Shanker A, Singh SM, Sodhi A. Ascitic growth of a spontaneous transplantable T cell lymphoma induces thymic involution. 1. Alterations in the CD4/CD8 distribution in thymocytes. Tumour Biol. 2000;21:288–298. doi: 10.1159/000030134. [DOI] [PubMed] [Google Scholar]
- Das T, Sa G, Paszkiewicz-Kozik E, Hilston C, Molto L, Rayman P, et al. Renal cell carcinoma tumors induce T cell apoptosis through receptor-dependent and receptor-independent pathways. J Immunol. 2008;180:4687–4696. doi: 10.4049/jimmunol.180.7.4687. [DOI] [PubMed] [Google Scholar]
- Gastman BR, Johnson DE, Whiteside TL, Rabinowich H. Tumor-induced apoptosis of T lymphocytes: elucidation of intracellular apoptotic events. Blood. 2000;95:2015–2023. [PubMed] [Google Scholar]
- Sa G, Das T, Moon C, Hilston CM, Rayman PA, Rini BI, et al. GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res. 2009;69:3095–3104. doi: 10.1158/0008-5472.CAN-08-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Sa G, Hilston C, Kudo D, Rayman P, Biswas K, et al. GM1 and TNFα, overexpressed in renal cell carcinoma, synergize to induce T cell apoptosis. Cancer Res. 2008;68:2014–2023. doi: 10.1158/0008-5472.CAN-07-6037. [DOI] [PubMed] [Google Scholar]
- Wang Q, Redovan C, Tubbs R, Olencki T, Klein E, Kudoh S, et al. Selective cytokine gene expression in renal cell carcinoma tumor cells and tumor-infiltrating lymphocytes. Int J Cancer. 1995;61:780–785. doi: 10.1002/ijc.2910610607. [DOI] [PubMed] [Google Scholar]
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- Cao Q, Wang L, Du F, Sheng H, Zhang Y, Wu J, et al. Downregulation of CD4+CD25+ regulatory T cells may underlie enhanced Th1 immunity caused by immunization with activated autologous T cells. Cell Res. 2007;17:627–637. doi: 10.1038/cr.2007.46. [DOI] [PubMed] [Google Scholar]
- Parmiani G, Rivoltini L, Andreola G, Carrabba M. Cytokines in cancer therapy. Immunol Lett. 2000;74:41–44. doi: 10.1016/s0165-2478(00)00247-9. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167:6497–6502. doi: 10.4049/jimmunol.167.11.6497. [DOI] [PubMed] [Google Scholar]
- Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, et al. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–4489. [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr DC, Cardona AC, Ulukpo O, Rigby M, Pelaez A, Ramirez A, et al. Dynamics of human regulatory T cells in lung lavages of lung transplant recipients. Transplantation. 2009;88:521–527. doi: 10.1097/TP.0b013e3181b0e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favorable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer Res. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, et al. Tumor-induced oxidative stress perturbs nuclear factor-κB activity-augmenting tumor necrosis factor-α-mediated T-cell death: protection by curcumin. Cancer Res. 2007;67:362–370. doi: 10.1158/0008-5472.CAN-06-2583. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Mandal D, Saha B, Sen GS, Das T, Sa G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007;282:15954–15964. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J, Wilson C, Palmer B, Richter D, Banerjee A, McCarter M. Melanoma induces immunosuppression by up-regulating FOXP3+ regulatory T cells. J Surg Res. 2007;141:72–77. doi: 10.1016/j.jss.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Subleski JJ, Kopf H, Howard OM, Männel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- Cao X. Regulatory T cells and immune tolerance to tumors. Immunol Res. in press. [DOI] [PubMed]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Matsui S, Ahlers JD, Vortmeyer AO, Terabe M, Tsukui T, Carbone DP, et al. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol. 1999;163:184–193. [PubMed] [Google Scholar]
- Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe. Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- Vose BM, Moore M. Human tumor-infiltrating lymphocytes: a marker of host response. Semin Hematol. 1985;22:27–40. [PubMed] [Google Scholar]
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbs C, Slotosch C, Löffler H, Pfutzner W, Hertl M. Cellular and humoral mechanisms of immune tolerance in immediate-type allergy induced by specific immunotherapy. Int Arch Allergy Immunol. 2008;147:171–178. doi: 10.1159/000142039. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim KH, Lee SH. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res. 2000;89:169–175. doi: 10.1006/jsre.2000.5826. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Guttinger M, Heilmann J, Sticher O. Curcumin differentially modulates mRNA profiles in Jurkat T and human peripheral blood mononuclear cells. Bioorg Med Chem. 2003;11:1057–1063. doi: 10.1016/s0968-0896(02)00461-3. [DOI] [PubMed] [Google Scholar]


