Abstract
pRST98 is a chimeric plasmid isolated from Salmonella enterica serovar Typhi (S. typhi) that mediates the functions of drug resistance and virulence. Previously, we reported that Salmonella plasmid virulence (spv) genes were present in S. typhi. In our current study, we investigated whether plasmid pRST98 exhibits significant cytotoxicity in macrophages. pRST98 was transferred into the avirulent Salmonella enterica serovar Typhimurium (S. typhimurium) strain RIA to create the transconjugant pRST98/RIA. The standard S. typhimurium virulent strain SR-11, which carries a 100-kb virulence plasmid, was used as a positive control. The bacterial strains were incubated with a murine macrophage-like cell line (J774A.1) in vitro. Apoptosis of J774A.1 cells was examined by electron microscopy and flow cytometry after annexin-V/propidium iodide labeling, and the survival of Salmonella strains in J774A.1 cells was determined. Results showed that macrophages infected with strain pRST98/RIA displayed greater levels of apoptosis than those infected with RIA and that pRST98 may increase bacterial survival in macrophages. Further studies showed that the pRST98-induced death of macrophages was associated with the loss of mitochondrial membrane potential and that pRST98 may activate caspase-9 and then caspase-3. The research data indicate that the virulence of bacteria that contain the pRST98 plasmid is enhanced; the presence of this plasmid increases the survival of the bacterial pathogen and acts through the mitochondrial pathway to mediate macrophage apoptosis.
Keywords: apoptosis, macrophage, plasmid, Salmonella enterica serovar Typhi
Introduction
Typhoid fever is a classic systemic infection caused by Salmonella enterica serovar Typhi (S. typhi), which remains a serious public health problem in developing countries. Recent data estimate that 22 million (range 16–33 million) cases occur each year, resulting in 216 000 deaths, predominantly in school-age children and young adults.1 In addition, strains of S. typhi have become resistant to chloramphenicol and other recommended antibiotics (ampicillin, cotrimoxazole and even ciprofloxacin). These resistant strains have become prevalent in several areas of the world. Polluted water is the most common source of typhoid transmission. The highest incidence of typhoid usually occurs where large populations are exposed to contaminated water supplies. In China, the morbidity of typhoid fever has been under control since 1990, but there are still localized areas where the disease incidence remains high and there are occasional outbreaks.
A pandemic of multidrug resistant S. typhi occurred in China in the mid to late 1980s, with 13 provinces and cities affected. In Suzhou, 591 strains of S. typhi were isolated from the blood of patients; these strains were examined for antimicrobial susceptibility by our lab. More than 80% of the isolates were multidrug resistant. This drug resistance was caused by a large, conjugative plasmid of 98.6 MDa (150 kb) that is classified in incompatibility group C.2 The S. typhi plasmid was designated pRST98 and is known to mediate bacterial multidrug resistance to chloramphenicol, streptomycin, trimethoprim, sulfonamide, gentamycin, neomycin, kanamycin, cephalosporin, ampicillin, carbenicillin and tetracycline. Patients infected with S. typhi that was positive for pRST98 had severe symptoms and exhibited complications with high mortality rates. However, only one plasmid existed in all of these isolates. This led us to presume that pRST98 may be a mosaic-like plasmid that is responsible for drug resistance and increased virulence in bacteria.
The importance of large plasmids for the virulence of Salmonella has been the subject of much research in recent years. Although the exact contribution of virulence plasmids to pathogenesis is still a matter of debate, progress has been made in identifying regions of the plasmid that are necessary for conferring virulence. Kurita et al.3 identified a highly conserved region of 8 kb, designated the Salmonella plasmid virulence (spv) genes; these genes are present in the plasmids of all other pathogenic Salmonella spp. except S. typhi. The spv genes encompasses five genes, the first gene, spvR, encodes a positive activator for the following four genes, spvABCD. The spv genes appear to promote the rapid growth and survival of Salmonella within the host cells that are important for maintaining systemic Salmonella infection in experimental animals.4 In 2005, we identified the virulence genes on pRST98 by Southern blot and DNA sequence analyses, which demonstrated that 99.8% of the homologous spv genes present on spvR and spvB were also present on pRST98, a plasmid carrying the genes encoding the properties of drug resistance and virulence in S. typhi.5
Although we made the first report that spv homologous genes also existed on pRST98, we were still interested in its phenotype because the mechanism of pRST98-increased bacterial virulence is still relatively poorly illuminated. Salmonella are intracellular bacteria, mainly residing in mononuclear phagocytes of the host. Their capacity to enter, survive and replicate within a cell, resulting in cytotoxic macrophage cells, may play a major role in their ability to cause disease. Some studies have shown that S. typhi and Salmonella enterica serovar Typhimurium (S. typhimurium) can mediate macrophage apoptosis.6, 7, 8, 9, 10 The virulence of Salmonella strains in humans and other animals is frequently serovar specific. S. typhi causes typhoid fever only in humans, but no disease is associated with experimental infections in mice. On the other hand, S. typhimurium possesses broad host specificity, which is usually associated with localized gastroenteritis in humans and a typhoid-like systemic disease in mice. Strains of S. typhimurium also appear to damage mammalian macrophage cells more extensively than do strains of S. typhi.11 In the present study, pRST98 was transferred into an avirulent S. typhimurium strain RIA, and the murine macrophage-like cell line J774A.1 was used to investigate the pathogenicity of S. typhimurium strains harboring pRST98.
Materials and methods
Bacterial strains and culture
The multidrug resistant S. typhi strains harboring pRST98 and the antibiotic sensitive S. typhi strains were obtained from the blood of patients during a typhoid fever outbreak in Suzhou, China from 1987 to 1992. The S. typhimurium strain SR-11, a virulent wild type strain carrying a 100-kb virulence plasmid,12 was used as a positive control. S. typhimurium strain RIA is avirulent for mice13, 14 and harbors a 136.8-kb resistance plasmid that mediates drug resistance to ampicillin and carbenicillin. Plasmid-free Escherichia coli (E. coli) K12W1485 Rifr F−Lac+ (E. coli K12W1485), which contains a rifampicin resistance gene on its chromosome, and S. typhimurium strain RIA were used as recipients to create the transconjugant strain pRST98/RIA; RIA was used as a negative control. E. coli V517 (54.4, 7.3, 5.6, 5.2, 4.0, 3.0, 2.7 and 2.1 kb) and Shigella flexneri 24570 (159.6, 4.0 and 3.0 kb), both harboring standard plasmids, were used as size markers. S. typhimurium strain SR-11, pRST98/RIA and RIA were grown to mid-logarithmic phase at 37 °C in Luria–Bertani (LB) broth and quantified spectrophotometrically by determining the optical density at 600 nm and using viable plate counts. The S. typhimurium cultures were then centrifuged at 2300g for 5 min and the bacteria were resuspended in RPMI 1640 medium without antibiotics prior to the addition of macrophage cells.
Conjugal transfer of pRST98 and plasmid DNA extraction
The conjugal transfer was divided into two steps. First, pRST98 was transferred from the clinical isolated multidrug resistant S. typhi to E. coli K12W1485; Shigella and Salmonella selective agar plates containing rifampicin (100 µg/ml) and chloramphenicol (20 µg/ml) were used. As E. coli can ferment lactose, they can be easily identified on Shigella and Salmonella agar plates. E. coli K12W1485 receiving pRST98 were transconjugant pRST98/E. coli K12W1485. Second, pRST98 was transferred from pRST98/E. coli K12W1485 to S. typhimurium RIA. Roe et al.'s method15 was modified as follows. Both donor and recipient bacteria were grown for 16 h at 37 °C in LB broth separately, and then the cultures were mixed well by taking 0.1 ml of each to a new LB broth and incubated for 4 h at 37 °C, centrifuged at 2,300g for 5 min and resuspended in normal saline. A portion (0.1 ml) of the suspension was transferred to a casein hydrolysate agar plate and grown for 16 h at 37 °C. The lawn was collected and serial dilutions in test tubes was performed. We transferred 0.1 ml of the suspension to a Shigella and Salmonella agar plate that contained rifampicin (100 µg/ml), chloramphenicol (20 µg/ml) and ampicillin (25 µg/ml). The colonies producing hydrogen sulfide were selected to be cultured a second time on the same selective agar; these colonies were labeled transconjugant pRST98/RIA and reactivated on LB agar plates. Extraction and analysis of bacterial plasmid DNA was performed according to the method recommended by Takahashi et al.16 Specifically, DNA extracts were analyzed by electrophoresis on 0.7% agarose gels and then stained with ethidium bromide (1 µg/ml) for 30 min.
PCR amplification of spv genes
To insure that spv was located on the transconjugant pRST98/RIA, PCR was used to identify the spvR and spvB fragments. S. typhimurium RIA and SR-11 were used as spv-negative and -positive controls, respectively. The spvR primers were (forward) 5′-ATG GAT TTC ATT AAT AAA AAA TTA-3′ and (reverse) 5′-TCA GAA GGT GGA CTG TTT CAG TTT-3′. The spvB primers were (forward) 5′-ATG TTG ATA CTA AAT GGT TTT TCA-3′ and (reverse) 5′-CTA TGA GTT GAG TAC CCT CAT GTT-3′. The conditions for amplification with all primer sets were 95 °C for 10 min, followed by 32 cycles of 95 °C for 20 s, 55 °C for 20 s and 72 °C for 1 min, followed by a single extension cycle at 72 °C for 5 min. The amplified products were electrophoresed on a 1.2% agarose gel, stained with ethidium bromide and observed under UV light.
Cell culture and infection
Murine macrophage-like cell line J774A.1 was propagated in RPMI 1640 medium with 10% fetal calf serum and 5 mM L-glutamine at 37 °C in a humidified incubator containing 5% CO2 and 95% air. Cells from exponentially growing cultures were used in all experiments and seeded in 24-well tissue culture plates at 5×105 cells/well 16–24 h before use. Mid-logarithmic phase growth cultures of S. typhimurium strain SR-11, pRST98/RIA and RIA were added to J774A.1 macrophages at a multiplicity of infection of 100∶1. After incubation at 37 °C for 3 h (0-h time point), infected cells were washed three times with phosphate buffered saline (PBS); then RPMI complete medium containing 100 μg amikacin per ml was added to kill the remaining extracellular bacteria. After 2 h of further incubation at 37 °C, the medium in the 24-well plates was replaced with RPMI containing 10 μg amikacin per ml to prevent extracellular growth of bacteria released from the infected J774A.1 cells. At different time points following infection with Salmonella, J774A.1 macrophages were processed in the following ways. All assays were conducted in triplicate and repeated at least three times. The results are presented as the mean±standard error of the mean. Statistical analyses were conducted using Student's t-test.
Preparation of samples for transmission electron microscopy
J774A.1 macrophages were cultured and infected as described above. Time points at 3 and 24 h post-infection were examined. Briefly, the adherent cells were scraped with a cell scraper and pelleted by centrifugation. The cells were then fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, post-fixed in 1% osmium tetroxide, and dehydrated through a series of graded acetone washes. Samples were embedded in epoxy resin, sectioned, and placed onto 200-mesh copper grids. The grids were stained with uranyl acetate and lead citrate, and samples were examined for the presence of apoptotic cells using a Hitachi transmission electron microscope.
Assessment of apoptosis by flow cytometry after annexin-V/propidium iodide labeling
An annexin-V/propidium iodide apoptosis detection kit (Jinmei Biotech, Shanghai, China) was used, in accordance with the manufacturer's instructions, to assess the apoptosis of J774A.1 macrophages. J774A.1 macrophages were pelleted by centrifugation, washed once with ice-cold PBS, and resuspended in binding buffer. Then, 0.1 ml of this cell suspension was transferred to a 5 ml tube and incubated with 5 μl of annexin-V (Ann V) and 5 μl of propidium iodide (PI) for 15 min at 25 °C in the dark. Finally, 0.4 ml of binding buffer was added, and the samples were analyzed within 1 h on a flow cytometer (FC500; Beckman Coulter, Brea, CA, USA). Early apoptotic cells bind annexin-V, a Ca2+-dependent phospholipid-binding protein with a high affinity for externalized phosphatidylserine. Living cells exclude PI, allowing for the specific detection and quantification of apoptosis by fluorescence-activated cell sorting analysis. Samples were gated on the basis of forward versus side scatter for size, and the results are presented as the percentage of cells that were viable (Ann V− PI−) and early apoptotic (Ann V+ PI−).
Measurement of mitochondrial membrane potential
J774A.1 cells were seeded overnight into 24-well tissue culture plates with coverslips. Monolayers were infected and processed as described above. At specific time points after infection with the bacteria, the coverslips were washed twice with PBS and dyed with JC-1 stain (Biyuntian Biotech, Beijing, China) prepared as instructed by the manufacturer. JC-1 is a cationic dye that fluoresces differently in apoptotic and non-apoptotic cells. Briefly, in living cells with normal mitochondrial membrane potential, the dye is taken up into the mitochondria to form red fluorescent aggregates. However, in apoptotic cells with compromised mitochondrial membrane potential, it remains in the monomeric form in the cytosol and fluoresces green. An iCys Research Imaging Laser Scanning Cytometer (CompuCyte Corporation, Cambridge, MA, USA) was set at an original magnification of ×200. JC-1 selectively accumulates within intact mitochondria to form multimeric aggregates emitting fluorescent light at 590 nm. The monomeric form emits light at 527 nm after excitation at 490 nm. Thus, the color of the dye changes from orange to green, depending on the mitochondrial membrane potential. In addition to using a laser scanning cytometer, images were also taken with a laser scanning confocal microscope (Leica Co., Solms, Germany).
Assay of caspase activity
After infection with S. typhimurium strains for 5 h, the J774A.1 cells were collected by centrifugation and washed once with PBS. The cells were then resuspended in lysis buffer at a density of 107 cells/ml and incubated on ice for 10 min. The cell debris was removed by centrifugation at 16 000g for 5 min at 4 °C, and the supernatant was used for the colorimetric assay of caspase-3 and caspase-9 activities using commercial kits (caspase-3 kit from Sigma and caspase-9 kit from Promega). Para-nitroanilide (pNA)-conjugated specific substrates for caspase-3 (DEVD-pNA) or caspase-9 (LEHD-pNA) were used according to the manufacturer's instructions. Cleaved substrates were quantified by reading absorbance at 405 nm with a microplate spectrophotometer (uQuant, Bio-Tek Instruments. Inc., Winooski, VT, USA).
Assessment of bacterial intracellular survival
For viable count determinations, the infected macrophages were washed three times with PBS at the indicated times, and Salmonella were harvested by the addition of 300 μl 0.1% Triton X-100 (prepared in distilled water) to macrophages. After 3 min, cell lysates were collected and serially diluted 10-fold in PBS, and aliquots were plated onto LB agar to enumerate bacterial colony-forming units.
Results
The profile of plasmid pRST98 and identification for its conjugal transfer
To identify the plasmid from multidrug resistant S. typhi, plasmids were extracted and analyzed by electrophoresis. It was demonstrated that the antibiotic resistant strain of S. typhi carried a plasmid with a molecular mass of 150 kb; this plasmid was designated pRST98. It was first transferred from the multidrug resistant S. typhi to E. coli K12W1485, and then it was transferred from E. coli K12W1485 to the avirulent S. typhimurium strain RIA, which harbors a 136.8 kb R plasmid. The recipient bacteria RIA that has received plasmid pRST98 as a result of conjugation is called transconjugant pRST98/RIA. Analysis by electrophoresis confirmed that pRST98 was transferred into RIA. The gel image picture shows that a 150-kb plasmid (pRST98) was found in pRST98/RIA (Figure 1).
Figure 1.

Plasmid electrophoresis profiles and the identification of conjugal transfer of pRST98. Lane 1, E. coli V517, plasmid size marker (54.4, 7.3, 5.6, 5.2, 4.0, 3.0, 2.7 and 2.1 kb); lane 2, S. flexneri 24570, plasmid size marker (159.6, 4.0 and 3.0 kb); lane 3, E. coli K12W1485, plasmid-free; lane 4, multidrug resistant S. typhi carrying a 150-kb plasmid (pRST98); lane 5, E. coli K12W1485 receiving pRST98, the plasmid donor in the second conjugal transfer step; lane 6, S. typhimurium strain SR-11 carrying a 100-kb virulence plasmid; lane 7, avirulent S. typhimurium strain RIA carrying a 136.8-kb R plasmid; and lane 8, transconjugant pRST98/RIA carrying two plasmids (136.8 and 150 kb). E. coli, Escherichia coli; S. flexneri, Shigella flexneri; S. typhi, Salmonella enterica serovar Typhi; S. typhimurium, Salmonella enterica serovar Typhimurium.
PCR amplification of spv genes
For this study, the open reading frames of spvR (894 bp) and spvB (1776 bp) were amplified. The results illustrated that spvR and spvB homologous genetic sequences, which had been found in all other pathogenic Salmonella spp. except for S. typhi, were also present on pRST98 (Figure 2).
Figure 2.

PCR amplification of Salmonella plasmid virulence (spv) genes. The open reading frames of spvR (894 bp) and spvB (1776 bp) were amplified. M, Axygen 100 bp DNA ladder; lanes 1, 4: transconjugant S. typhimurium strain pRST98/RIA; lane 2, 5: S. typhimurium strain SR-11; lane 3, 6: avirulent S. typhimurium strain RIA. S. typhimurium, Salmonella enterica serovar Typhimurium.
Plasmid pRST98 induces morphological features of apoptosis in J774A.1 cells
In comparison with uninfected controls, J774A.1 macrophages infected with S. typhimurium strains pRST98/RIA and SR-11 exhibited morphological features of apoptosis at 3 h post-infection as assessed by transmission electron microscopy, including condensed and marginated nuclear chromatin, cytoplasmic vacuolation, cellular blebbing and the formation of apoptotic bodies. In contrast, cells infected with RIA had a normal appearance within 3 h post-infection, although they contained intracellular bacteria. At 24 h post-infection, cells infected with pRST98/RIA and SR-11 displayed features of necrosis. The appearance of most cells infected with RIA was normal, but there were also some cells showing intense perinuclear chromatin aggregation (Figure 3).
Figure 3.

Transmission electron micrographs of J774A.1 cells infected with S. typhimurium. (a) Uninfected macrophages show normal cellular morphology, including intact plasma and nuclear membranes. (b–e) J774A.1 macrophages at 3 h post-infection. (b) RIA-infected macrophages. (c) pRST98/RIA-infected macrophages. (d) and (e) SR-11-infected macrophages. Black arrows denoted intracellular growth of Salmonella strains in macrophages. Macrophages infected with pRST98/RIA or SR-11 display characteristic features of apoptosis, including condensed and marginated nuclear chromatin (arrowhead), membrane blebbing (white arrows) and cytoplasmic vacuolation. However, cells infected with RIA appeared normal, although they contained intracellular bacteria. (f–h) J774A.1 macrophages at 24 h post-infection. Many of the J774A.1 cells infected with pRST98/RIA (g) and SR-11 (h) underwent necrosis; the ultrastructure was significantly worse than that in macrophages infected with RIA (f). S. typhimurium, Salmonella enterica serovar Typhimurium.
Plasmid pRST98 induces extracellular exposure of phosphatidylserine
The relationship between plasmid pRST98 and macrophage apoptosis was further confirmed by assessing the surface expression of phosphatidylserine using annexin-V in conjunction with propidium iodide for flow cytometry analysis. As shown in Figure 4b, 95.55% of S. typhimurium RIA-infected J774A.1 cells were viable (Ann V− PI−). Only 2.74% of cells were apoptotic (Ann V+ PI−) at 3 h post-infection, and this percentage increased to 4.32% at 24 h post-infection (Figure 4f). In contrast, infections with pRST98/RIA caused an increase in the level of apoptosis from 8.81% (Figure 4c) at 3 h post-infection to 15.02% at 24 h post-infection (Figure 4g). Figure 4B displays the mean percentage of S. typhimurium-infected J774A.1 cells undergoing apoptosis (Ann V+ PI−) in the three separate experiments. Compared with uninfected and S. typhimurium RIA-infected macrophages, pRST98/RIA-infected macrophages displayed an increase in apoptosis similar to that detected in SR-11-infected cells (9.01±1.32% versus 2.81±0.51% at 3 h post-infection; 16.02±3.10% versus 4.37±0.92% at 24 h post-infection; P<0.05; n=9). These results demonstrate that significantly greater apoptosis is induced by bacteria harboring plasmid pRST98.
Figure 4.

Assessment of J774A.1 cells with flow cytometry after annexin-V/propidium iodide labeling. (a, e) uninfected cells; (b, f) J774A.1 cells infected with S. typhimurium strain RIA at 3 and 24 h post-infection, respectively. (c, g) J774A.1 cells infected with S. typhimurium strain pRST98/RIA at 3 and 24 h post-infection, respectively. (d, h) J774A.1 cells infected with S. typhimurium SR-11 at 3 and 24 h post-infection, respectively. The results are presented as the percentage of cells that were viable (Ann V- PI-, represented in the lower left quadrant of the dot plot) and early apoptotic (Ann V+ PI-, the lower right quadrant). Fig. 4B shows the mean percentage of S. typhimurium-infected J774A.1 cells that underwent apoptosis from three separate experiments. In comparison with uninfected and S. typhimurium RIA-infected cells, pRST98/RIA-infected macrophages displayed an increase in apoptosis similar to that detected in SR-11-infected cells (*P<0.05; **P<0.01; n=9). Ann V, annexin-V; PI, propidium iodide; S. typhimurium, Salmonella enterica serovar Typhimurium.
Plasmid pRST98 induces the loss of mitochondrial membrane potential in J774A.1 cells
Infection with the strain pRST98/RIA resulted in a higher number of J774A.1 cells with decreased mitochondrial membrane potential (Δψm) compared with RIA-infected cells (Figure 5a, P<0.05). At 1 h post-infection, the distribution of J774A.1 cells gradually migrated from red to green fluorescence, indicating a decrease in mitochondrial Δψm with increased incubation time. A higher percentage of cells with green florescence were observed with infection of pRST98/RIA than with RIA-infected cells (Figure 5b). Representative fields of view for uninfected cells versus J774A.1 cells infected with S. typhimurium pRST98/RIA or RIA using laser confocal microscopy are shown in Figure 5c. Mitochondria emitted red and green fluorescence with similar intensity in uninfected J774A.1 cells or cells infected with RIA; merging of these two colors resulted in yellow fluorescence. However, for cells infected with S. typhimurium strain pRST98/RIA, the intensity of red fluorescence became lower than that of green fluorescence and merging them still produced green fluorescence, demonstrating that pRST98 induces significant loss of mitochondrial Δψm.
Figure 5.
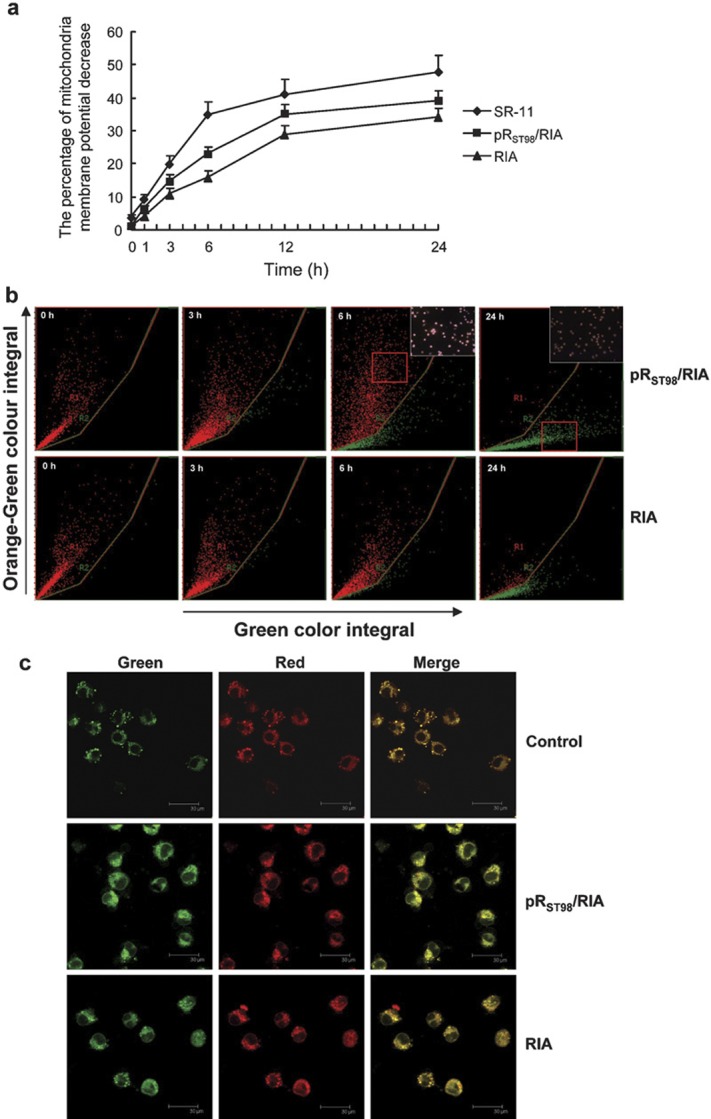
Measurement of mitochondrial membrane potential of J774A.1 cells. (a) The percentage of mitochondria membrane potential (Δψm) decrease in J774A.1 cells. At 1 h post-infection, S. typhimurium strain pRST98/RIA resulted in a higher number of J774A.1 with decreased mitochondrial Δψm than RIA, and a time-dependent decrease in mitochondrial Δψm was observed. (b, c) Fluorescent JC-1 reaction examination of J774A.1 cells undergoing mitochondria Δψm decrease detected with a laser scanning cytometer (×200) and laser scanning confocal microscope, respectively. The corner panels are higher magnifications of the boxed areas. S. typhimurium, Salmonella enterica serovar Typhimurium.
Caspase activation during Salmonella infection of J774A.1 cells
We determined whether caspase-9 and caspase-3 were activated by Salmonella infection using colorimetric substrate assays of lysates from infected cells. As shown in Figure 6, both caspase-9 and caspase-3 activity increased at 5 h following infection with S. typhimurium strain SR-11 and with strain pRST98/RIA; no major differences were found between caspase activation of cells infected with the strains SR-11 and pRST98/RIA. However, cells infected with S. typhimurium strain RIA gave results similar to uninfected cells.
Figure 6.

Caspase-9 and caspase-3 activities in culture supernatants of J774A.1 cells infected with S. typhimurium. Cultures were harvested at 5 h post-infection. Values are expressed as means±standard deviation of seven different observations. *Significantly different (P<0.01) from uninfected control and RIA-infected cells. S. typhimurium, Salmonella enterica serovar Typhimurium.
Plasmid pRST98 enhances bacterial survival in J774A.1 cells
Using the dilution method, infected J774A.1 cells were lysed and plated on agar at 10-fold serial dilutions. Transconjugant pRST98/RIA showed increased viable counts over a 24-h infection time frame, resulting in up to a 1-log increase in intracellular bacteria. On the other hand, S. typhimurium strain RIA demonstrated approximately a 1-log decline in viable counts during the same time frame, as shown in Figure 7. These data demonstrated that pRST98 can enhance survival of Salmonella strains in phagocytes. In addition to the viable count numbers, Giemsa staining of S. typhimurium-infected J774A.1 macrophages visually confirmed the numerical differences between pRST98/RIA and RIA (data not shown).
Figure 7.
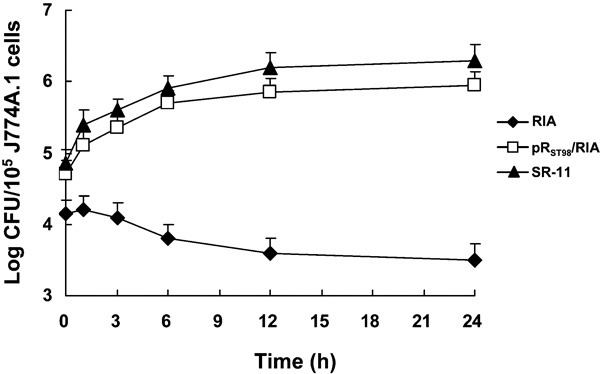
Bacterial survival in J774A.1 cells. Bacterial Log CFU per 105 macrophage cells (y axis) and time after addition of amikacin (x axis) are indicated. The results are presented as the mean±standard error of the mean. CFU, colony-forming unit.
Discussion
The genus Salmonella contains facultative intracellular bacteria that reside mainly in mononuclear phagocytes. The ability of Salmonella to resist and evade the antimicrobial arsenal of phagocytes is a prerequisite for virulence. Macrophages, as professional phagocytes and antigen-presenting cells, are a key link between innate and adaptive immunity and play a critical role in the host immune system. Therefore, during the infection process of Salmonella, it is worth evaluating the fate of the macrophage. Salmonella have been reported to effectively escape being killed by macrophages with a number of defense mechanisms, such as resistance to lysosomes, prevention of the fusion of lysosomes and phagosomes, and the ability to interfere with active oxygen species. In addition to pyroptosis and pyronecrosis,17 S. typhimurium induces apoptosis and autophagy in infected macrophages.18, 19, 20 It seems plausible that both methodological and strain differences could explain these discrepancies in part. In this study, we transferred a chimeric plasmid, pRST98, isolated from S. typhi into an avirulent S. typhimurium strain RIA and investigated whether pRST98 exhibited significant cytotoxicity in a murine macrophage-like cell line J774A.1. Observation of the cell ultrastructure using transmission electron microscopy is the gold standard in the determination of cell apoptosis. In our study, apoptotic changes, including condensed and marginated nuclear chromatin, membrane blebbing and cytoplasmic vacuolation, were obvious in J774A.1 cells infected with S. typhimurium strain pRST98/RIA. The relationship between plasmid pRST98 and macrophage apoptosis was also confirmed cytofluorometrically using the annexin-V/propidium iodide labeling method. The results indicated that J774A.1 cells infected with S. typhimurium strain pRST98/RIA were significantly more apoptotic than those infected with RIA.
S. typhimurium, a close relative of S. typhi, has a broad host range and has been used extensively as an experimental model for typhoid fever. Caspase-3-dependent phagocyte apoptosis during systemic S. typhimurium infection of mice has been reported.9 Mitochondria are an important regulator of apoptosis and undergo major changes during apoptotic cell death that is induced by apoptotic stimuli. Early in the induction of apoptosis, a loss of mitochondrial Δψm can be detected. This loss of mitochondrial Δψm leads to the release of cytochrome-C into the cytoplasm and results in the activation of caspase-9 and, subsequently, the activation of caspase-3. In this study, the change in mitochondrial Δψm was dynamically monitored with a laser scanning cytometer using the JC-1 staining method. A laser scanning cytometer, one of the most advanced instruments currently used in the field of cell biology, enables us to analyze cells using multiple parameters. It functions as both a flow cytometer and a static image cytometer. The results indicated that pRST98/RIA caused decreased levels of Δψm in a higher number of J774A.1 cells than did the RIA strain (P<0.05), suggesting that J774A.1 cell death resulting from pRST98 was associated with the loss of mitochondrial Δψm.
Further study showed that virulent S. typhimurium strains (pRST98/RIA and SR-11) survived in the intracellular environment and multiplied rapidly in J774A.1 cells, compared with the significant declines in viable counts noted for S. typhimurium strain RIA. As bacterial survival rose, more host cells were also damaged, presumably because more cytotoxin was produced. A few recent studies have shed some light on the basis for the cytotoxic effect of Salmonella on murine macrophage cells.11, 21 These reports demonstrate that many Salmonella serovars can trigger cytotoxicity in murine macrophages and that apoptosis is responsible for some of the observed overall host cell death. Furthermore, apoptosis requires a functional Salmonella type III secretion system and actively replicating Salmonella. Thus, higher amounts of protein from virulent strains secreted into the host cells may lead to increased host cell death.
After uptake, Salmonella reside within a unique organelle, the Salmonella-containing vacuole. The type III secretion system encoded by the Salmonella SPI-2 gene releases effectors that function to both interfere with antimicrobial defense mechanisms of the host and modify Salmonella-containing vacuole to provide optimum living conditions for Salmonella.22 It was reported that the Salmonella phoPQ system specifically monitors the inner environment of the macrophage and controls the expression of various genes, such as the spvB gene.23 The SpvB protein has recently been shown to contain an ADP-ribosyltransferase domain in its C-terminus that ADP-ribosylates actin, and this enzymatic activity has been demonstrated to be essential for virulence in mice. Intracellular expression of the SpvB protein induces not only disruption of actin filaments, but also apoptotic cell death in eukaryotic cells.3, 24, 25 We found a gene sequence that is homologous to spvB on the pRST98 plasmid. However, pRST98 is a large chimeric plasmid containing complex sequences of unknown functions. On the basis of our promising results, further investigations should be performed to determine whether the macrophage apoptosis that is induced by pRST98 is directly related to the spvB gene or to a combination of various genes. Moreover, a transition in mitochondrial membrane permeability is not only related to apoptosis but also often associated with autophagy.26 The loss of mitochondrial membrane potential observed in this study could also be a result of the increased autophagy may be mediated by the spv genes on pRST98. Studies of the relationship between autophagy and apoptosis are now being carried out in our lab.
In summary, characterization of the chimeric plasmid pRST98 isolated from S. typhi is potentially important for understanding the virulence mechanism of this pathogen. Our study indicated that the potential of cytotoxicity mediated through the mitochondrial pathway is primarily involved in plasmid pRST98-induced apoptosis. These are the first results to show that the plasmid isolated from S. typhi can enhance the virulence of host bacteria by mediating the apoptosis of the macrophage host cells; this study provides important information on the mechanisms of S. typhi-induced macrophage apoptosis.
Acknowledgments
We are grateful to Professor Roy Curtiss III, The School of Life Sciences, Arizona State University, USA, for kindly supplying S. typhimurium strains SR-11 and RIA. We also thank Professor Jie Yan, Zhejiang University, China, for providing murine macrophage-like cell line J774A.1. This study was supported by the Natural Science Foundation of China (No. 30972768), the Special and General Postdoctoral Science Foundation of China (No. 200902529 and No. 20080430178), the Natural Science Foundation of Jiangsu High Education Institute of China (No. 08KJB310009) and the Social Development Science Foundation of Suzhou City of China (No. SS08025).
References
- Siddiqui FJ, Rabbani F, Hasan R, Nizami SQ, Bhutta ZA. Typhoid fever in children: some epidemiological considerations from Karachi, Pakistan. Int J Infect Dis. 2006;10:215–222. doi: 10.1016/j.ijid.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Huang R, Mu RP. Surveillance of the antibiotic resistance and R plasmid of Salmonella typhi. Chin J Infect Dis. 1994;12:204–206. [Google Scholar]
- Kurita A, Gotoh H, Eguchi M, Okada N, Matsuura S, Matsui H, et al. Intracellular expression of the Salmonella plasmid virulence protein, SpvB, causes apoptotic cell death in eukaryotic cells. Microb Pathog. 2003;35:43–48. doi: 10.1016/s0882-4010(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Soto SM, Rodriguez I, Rodicio MR, Vila J, Mendoza MC. Detection of virulence determinants in clinical strains of Salmonella enterica serovar Enteritidis and mapping on macrorestriction profiles. J Med Microbiol. 2006;55:365–373. doi: 10.1099/jmm.0.46257-0. [DOI] [PubMed] [Google Scholar]
- Huang R, Wu SY, Zhang XG, Zhang YY. Molecular analysis and identification of virulence gene on pRST98 from multi-drug resistant Salmonella typhi. Cell Mol Immunol. 2005;2:136–140. [PubMed] [Google Scholar]
- Chanana V, Ray P, Rishi DB, Rishi P. Reactive nitrogen intermediates and monokines induce caspase-3 mediated macrophage apoptosis by anaerobically stressed Salmonella typhi. Clin Exp Immunol. 2007;150:368–374. doi: 10.1111/j.1365-2249.2007.03503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander H, Majumdar S, Sapru S, Rishi P. 55 kDa outer-membrane protein from short-chain fatty acids exposed Salmonella enterica serovar Typhi induces apoptosis in macrophages. Antonie Van Leeuwenhoek. 2006;89:317–323. doi: 10.1007/s10482-005-9033-y. [DOI] [PubMed] [Google Scholar]
- Zhou X, Mantis N, Zhang XR, Potoka DA, Watkins SC, Ford HR. Salmonella typhimurium induces apoptosis in human monocyte-derived macrophages. Microbiol Immunol. 2000;44:987–995. doi: 10.1111/j.1348-0421.2000.tb02594.x. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Sheppard M, Deardon R, Brown SP, Foster G, Bryant CE, et al. Caspase-3-dependent phagocyte death during systemic Salmonella enterica serovar Typhimurium infection of mice. Immunology. 2008;125:28–37. doi: 10.1111/j.1365-2567.2008.02814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Fujii S, Okamoto T, Islam S, Khan S, Ahmed KA, et al. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J Immunol. 2009;182:3746–3756. doi: 10.4049/jimmunol.0803363. [DOI] [PubMed] [Google Scholar]
- Schwan WR, Huang XZ, Hu L, Kopecko DJ. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun. 2000;68:1005–1013. doi: 10.1128/iai.68.3.1005-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig PA, Curtiss Roy., III Cloning and transposon insertion mutagenesis of virulence genes of the 100-kilobase plasmid of Salmonella typhimurium. Infect Immun. 1988;56:3262–3271. doi: 10.1128/iai.56.12.3262-3271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider HA, Zinder ND. Nutrition of the host and natural resistance to infection. V. An improved assay employing genetic markers in the double strain inoculation test. J Exp Med. 1956;103:207–223. doi: 10.1084/jem.103.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M, Stocker BA, Holder IA. Salmonella typhimurium virulence in a burned-mouse model. Infect. Immun. 1989;57:2842–2846. doi: 10.1128/iai.57.9.2842-2846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe DE, Weinberg A, Roberts MC. Mobile rRNA methylase genes coding for erythromycin resistance in Actinobacillus actinomycetemcomitans. J Antimicrob Chemother. 1996;37:457–464. doi: 10.1093/jac/37.3.457. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiology analysis. J Clin Microbiol. 1984;20:608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Takaya A, Suzuki A, Kikuchi Y, Eguchi M, Isogai E, Tomoyasu T, et al. Derepression of Salmonella pathogenicity island 1 genes within macrophages leads to rapid apoptosis via caspase-1- and caspase-3-dependent pathways. Cell Microbiol. 2005;7:79–90. doi: 10.1111/j.1462-5822.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- Das S, Devaraj SN. Effect of Hemidesmus indicus R.Br. root extract against Salmonella enterica serovar Typhimurium-induced apoptosis in murine macrophage cell line (P388D1) Indian J Med Res. 2008;128:647–657. [PubMed] [Google Scholar]
- Birmingham CL, Brumell JH. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy. 2006;2:156–158. doi: 10.4161/auto.2825. [DOI] [PubMed] [Google Scholar]
- Kim GS, Kim DH, Lim JJ, Lee JJ, Han DY, Lee WM, et al. Biological and antibacterial activities of the natural herb Houttuynia cordata water extract against the intracellular bacterial pathogen salmonella within the RAW 264.7 macrophage. Biol Pharm Bull. 2008;31:2012–2017. doi: 10.1248/bpb.31.2012. [DOI] [PubMed] [Google Scholar]
- García-del PF, Núñez-Hernández C, Eisman B, Ramos-Vivas J. Growth control in the Salmonella-containing vacuole. Curr Opin Microbiol. 2008;11:46–52. doi: 10.1016/j.mib.2008.01.001. [DOI] [PubMed] [Google Scholar]
- García-Calderón CB, Casadesús J, Ramos-Morales F. Rcs and PhoPQ regulatory overlap in the control of Salmonella enterica virulence. J Bacteriol. 2007;189:6635–6644. doi: 10.1128/JB.00640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol Med Microbiol. 2008;52:194–201. doi: 10.1111/j.1574-695X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Hochmann H, Pust S, von Figura G, Aktories K, Barth H. Salmonella enterica SpvB ADP-ribosylates actin at position arginine-177-characterization of the catalytic domain within the SpvB protein and a comparison to binary clostridial actin-ADP-ribosylating toxins. Biochemistry. 2006;45:1271–1277. doi: 10.1021/bi051810w. [DOI] [PubMed] [Google Scholar]
- Sy LK, Yan SC, Lok CN, Man RY, Che CM. Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells. Cancer Res. 2008;68:10229–10237. doi: 10.1158/0008-5472.CAN-08-1983. [DOI] [PubMed] [Google Scholar]


