Abstract
Helicobacter pylori infection is associated with an inflammatory response in the gastric mucosa, leading to chronic gastritis, peptic ulcers, gastric carcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphomas. Recent studies have shown that apoptosis of gastric epithelial cells is increased during H. pylori infection. Apoptosis induced by microbial infections are factors implicated in the pathogenesis of H. pylori infection. The enhanced gastric epithelial cell apoptosis in H. pylori infection has been suggested to play an important role in the pathogenesis of chronic gastritis and gastric pathology. In addition to directly triggering apoptosis, H. pylori induces sensitivity to tumor-necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in gastric epithelial cells via modulation of TRAIL apoptosis signaling. Moreover, H. pylori infection induces infiltration of T lymphocytes and triggers inflammation to augment apoptosis. In H. pylori infection, there was significantly increased CCR6+CD3+ T-cell infiltration in the gastric mucosa, and the CCR6 ligand, CCL20 chemokine, was selectively expressed in inflamed gastric tissues. These results implicate that the interaction between CCL20 and CCR6 may play a role in recruiting T cells to the sites of inflammation in the gastric mucosa during Helicobacter infection. Through these mechanisms, chemokine-mediated T lymphocyte trafficking into inflamed epithelium is initiated and the mucosal injury in Helicobacter infection is induced. This article will review the recent novel findings on the interactions of H. pylori with diverse host epithelial signaling pathways and events involved in the initiation of gastric pathology, including gastric inflammation, mucosal damage and development of MALT lymphomas.
Keywords: apoptosis, CagA, chemokine, Helicobacter pylori, TRAIL
Introduction
Helicobacter pylori, a common human pathogen, which infects about 50% of the world's population, is associated with duodenal and peptic ulcer diseases. The clinical consequences range from asymptomatic gastritis to peptic ulceration and gastric malignancy.1, 2 The outcome of the infection is determined by interactions among H. pylori virulence factors, host gastric mucosal factors and the environment. However, the mechanisms by which host factors cause disease remain unclear. The H. pylori infection changes the microenvironment of gastric mucosa. The apoptosis of gastric epithelial cells is increased,3, 4, 5, 6, 7 and direct cytotoxicity as well as inflammatory responses occurs in the gastric mucosa cells.6, 8, 9, 10 It has been demonstrated that T helper type 1 cells selectively increased during H. pylori infection.11, 12, 13, 14, 15 T helper type 1 cytokines, such as gamma interferon (IFN-γ) and tumor-necrosis factor alpha (TNF-α), can increase the release of proinflammatory cytokines, augmenting apoptosis induced by H. pylori.10 H. pylori infection could also induce gastric mucosa damage by increasing expression of Fas in gastric epithelial cells, leading to gastric epithelial cell apoptosis through Fas/FasL interaction with infiltrating T cells.9, 16 These findings suggest a role for immune-mediated apoptosis of gastric epithelial cells during H. pylori infection. Recently, several bacterial pathogens have been found to trigger apoptosis in host cells in vitro or in vivo, and several types of mechanisms have been elucidated.17 It was shown that H. pylori directly triggers cell death by cytotoxins after interaction with gastric epithelial cells.18, 19 Meanwhile, recent reports have shown that H. pylori translocates cytotoxin-associated gene A (CagA) into gastric epithelial cell by type IV secretion, inducing intracellular protein phosphorylation and dysregulate the signal transduction pathways within host cells.20, 21, 22, 23
Modulation of TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis by H. pylori
TRAIL (also called Apo2L), a novel TNF superfamily member with strong homology to FasL, is capable of inducing apoptosis in a variety of transformed cell lines in vitro,24, 25 but usually not in normal primary cells. It was shown that T cells can kill target cells via TRAIL/TRAIL receptor interaction,26, 27, 28, 29, 30, 31 indicating that TRAIL might serve as a cytotoxic effector molecule in activated T cells in vivo. These findings suggest that TRAIL/TRAIL receptor interaction is involved in the interaction between infiltrating T cells and gastric epithelium during H. pylori gastritis. In recent reports, Wu et al. demonstrated that human gastric epithelial cells sensitized to H. pylori confer susceptibility to TRAIL-mediated apoptosis, suggesting a role for immune-mediated apoptosis of gastric epithelial cells by infiltrating T cells during Helicobacter infection.7, 15 The induction of TRAIL sensitivity by H. pylori is independent of expression of H. pylori virulent factors vacuolating cytotoxin gene A (VacA) and CagA, and is dependent on viable bacteria and direct contact with cells.7 The expression of TRAIL receptors did not change after H. pylori infection, indicating the H. pylori-induced enhanced sensitivity to TRAIL-mediated apoptosis in gastric epithelial cells was not due to upregulation of TRAIL death receptors. H. pylori-induced sensitivity to TRAIL-mediated apoptosis in gastric epithelial cells is dependent on activation of caspase 8 downstream pathway to convey the death signal to mitochondria, leading to activation of mitochondrial pathway and breaking the apoptosis resistance. H. pylori induces TRAIL-mediated apoptosis via enhancing the assembly of TRAIL death-inducing signaling complex and activation of caspase 8 and its downstream apoptosis signaling pathway. Thus, in addition to directly triggering apoptosis, H. pylori induces sensitivity to TRAIL in gastric epithelial cells by modulation of death-receptor signal transduction pathways. Modulation of host cell apoptosis by bacterial interaction adds a new dimension to the immune pathogenesis in Helicobacter infection.
Chemokine-mediated lymphocyte trafficking of T lymphocytes in gastric inflammation during H. pylori infection
Previous studies have indicated that T lymphocytes play an important role in the pathogenesis of Helicobacter gastritis.11, 32 Inflammation of the gastric mucosa develops in response to the host immune reaction against the pathogens. The stimulation of epithelial cells with H. pylori contributes to the recruitment of neutrophils and lymphocytes. The activation of macrophages results in the release of cytokines, including IL-1, IL-6, IL-8, IL-12, TNF-α and IFN-γ. As described, the features of H. pylori-induced inflammatory immune response are orchestrated by sequential elaboration of proinflammatory cytokines including IL-10, IFN-γ, TNF-α and IL-1β. Accordingly, factors involved in regulating cytokine responses may confer susceptibility to or protection against H. pylori-associated diseases. All these results indicate that immune reaction and inflammation mediators to H. pylori play an important role in the pathogenesis of H. pylori-associated diseases. Among the T cells in response to H. pylori infection, the gastric infiltrating T cells mostly are CD45RO+CD69+CD4+ T cells, indicating that there was accumulation of activated memory CD4+ T cells during Helicobacter infection.15 Recent reports indicated that the T helper type 1 response is induced during infection with H. pylori,11, 13, 14, 33 and the levels of IFN-γ and TNF-α, are increased in the gastric mucosa during H. pylori infection, augmenting the apoptosis induced by H. pylori.6, 7, 10 These results suggest a role for immune-mediated apoptosis of gastric epithelial cells by infiltrating T cells during Helicobacter infection. Therefore, in addition to bacterial virulence factors, the degree of gastric mucosa damage is also determined by the inflammation response induced during H. pylori infection. However, the induction of immune response and the immunopathogenic mechanism(s) of mucosal inflammation in H. pylori infections are still not clear, and chemokines are thought to play an important role in this process.34, 35, 36, 37 Chemokines are small, 6–14-kDa heparin binding proteins, which play a role in a variety of biological processes, most notably leukocyte chemotaxis.38, 39 Chemokines are involved in acute and chronic inflammatory processes by attracting neutrophils, monocytes and T cells to the site of inflammation via their corresponding chemokine receptors.38, 39 Recent reports have shown that there are specific chemokines that mediate the homing of lymphocytes in the intestines,37, 40, 41 suggesting that some chemokines may be involved in lymphocyte trafficking in the gut. It has been demonstrated that distinct sets of chemokines and their receptors are responsible for directing lymphocytes to inflammatory sites.35, 36, 40, 42, 43 A set of proinflammatory chemokines has been shown to be involved in H. pylori gastritis: Gro-α, IL-8, RANTES, IFN-γ-inducible protein-10 (CXCL10), a monokine induced by IFN-γ (CXCL11) and CCL20 (MIP-3α/LARC/exodus).15, 44, 45, 46 It has been demonstrated that the gastrointestinal epithelium senses the invading microorganisms and produces cytokines/chemokines that attract lymphocytes and dendritic cells to the site of inflammation.35, 47, 48 Recently, it was reported that CCR6 mediates dendritic cell localization, lymphocyte homeostasis and immune responses in mucosal tissue.49 CCR6, a specific β-chemokine receptor for CCL20, is selectively expressed on dendritic cells and some memory T cells48, 50, 51, 52 and may play a role in chemokine-mediated lymphocyte trafficking during gastric inflammation. It has also shown that CCL20, the ligand of CCR6, is abundantly expressed in mouse and human inflammatory enteric mucosa.53, 54 The production of CCL20 was upregulated in response to H. pylori in gastric epithelial cells when there was stimulation by the proinflammatory cytokines IL-1β and TNF-α.15, 55, 56, 57 These results implicate that the interaction between CCL20 and CCR6 may play a role in recruiting CD45RO+ memory T cells to the sites of inflammation in the gastric mucosa during Helicobacter infection.
H. pylori CagA protein and the development of mucosa-associated lymphoid tissue (MALT) lymphomas
It has been established that the cagA gene product, CagA, can be directly injected into bacterium-attached host gastric epithelial cells via the bacterial type IV secretion system.20, 21, 22, 23 Infection by cagA-positive H. pylori is associated with gastric carcinomas and gastric MALT lymphomas.1, 58 The development of gastric MALT lymphomas is closely associated with H. pylori infection. The pathogenic role of H. pylori infection in gastric MALT lymphomas was observed in in vitro experiments and clinical evaluations of the effects of eradication on the progression of gastric MALT lymphomas.59, 60 Epidemiological studies further indicated that cagA-positive H. pylori is present in the gastric mucosa of most patients with gastric MALT lymphomas.61, 62 Clinical observations that eradication of H. pylori by antibiotic therapy can lead to the complete remission of MALT lymphomas60 provide evidence that cagA-positive H. pylori plays an important role in the development and/or maintenance of MALT lymphomas. The development of gastric MALT lymphomas is dependent on H. pylori infection. Bacterial colonization of the gastric mucosa triggers lymphocyte infiltration.15, 63 and the formation of acquired MALTs. Previous studies suggested that MALT lymphoma cells preserve B-cell properties and that their growth may be partially driven by antigenic stimulation. H. pylori stimulates lymphoma B cells through tumor-infiltrating T cells, involving CD40 and CD40L costimulatory molecules.64, 65 However, the pathogenesis and how H. pylori induces the development of B-cell MALT lymphomas are still not clear. Much attention has been focused on the role of the cagA gene product, CagA, in the malignant transformation of cells. CagA was directly injected from bacteria into attached gastric epithelial cells by a type IV secretion system, encoded by the cag pathogenicity island,20, 66 and underwent tyrosine phosphorylation.23, 67, 68, 69 In human B lymphocytes, overexpression of cagA via transfection induces activation of extracellular signal-related kinase and their downstream apoptosis regulators, indicating that CagA has effects on the growth and survival of B lymphocytes and may play a role in the development of MALT lymphomas.70, 71 H. pylori infection stimulates immune lymphocytes in the gastric mucosa and induces the formation of MALTs, from which MALT lymphomas of B-cell origin develop. Immune cells constituting MALTs migrate to and infiltrate the site of H. pylori infection in the stomach. In such circumstances, CagA may be injected into lymphocytes as well as gastric epithelial cells. Recent results in our laboratory have demonstrated that CagA could be directly translocated into human B cells from H. pylori. This implies the direct role and importance of CagA in the development of H. pylori-associated MALT lymphomas.
Summary
Human gastric epithelial cells sensitized to H. pylori conferred susceptibility to TRAIL-mediated apoptosis. Although the induction of TRAIL sensitivity by H. pylori in gastric epithelial cells was independent of H. pylori virulent factors CagA and VacA, the degree of apoptosis was linked to the presence of H. pylori and the associated inflammatory response. Therefore, the degree of mucosal damage was also determined by the inflammatory response induced by H. pylori within gastric epithelium. These results suggest a role for immune-mediated apoptosis and mucosa damage by infiltrating T cells during Helicobacter infection (Figure 1). In conclusion, H. pylori enhances susceptibility of gastric epithelial cells to TRAIL-mediated apoptosis. The induction of TRAIL sensitivity by H. pylori is dependent upon direct contact of viable bacteria with gastric epithelial cells. Modulation of host cell apoptosis by bacterial interaction adds a new dimension to the immune pathogenesis in chronic Helicobacter infection. The interplay between H. pylori and immune cells may induce activation of B lymphocytes via direct interaction or indirect immune stimulation leading to the development of H. pylori-associated MALT lymphomas.
Figure 1.
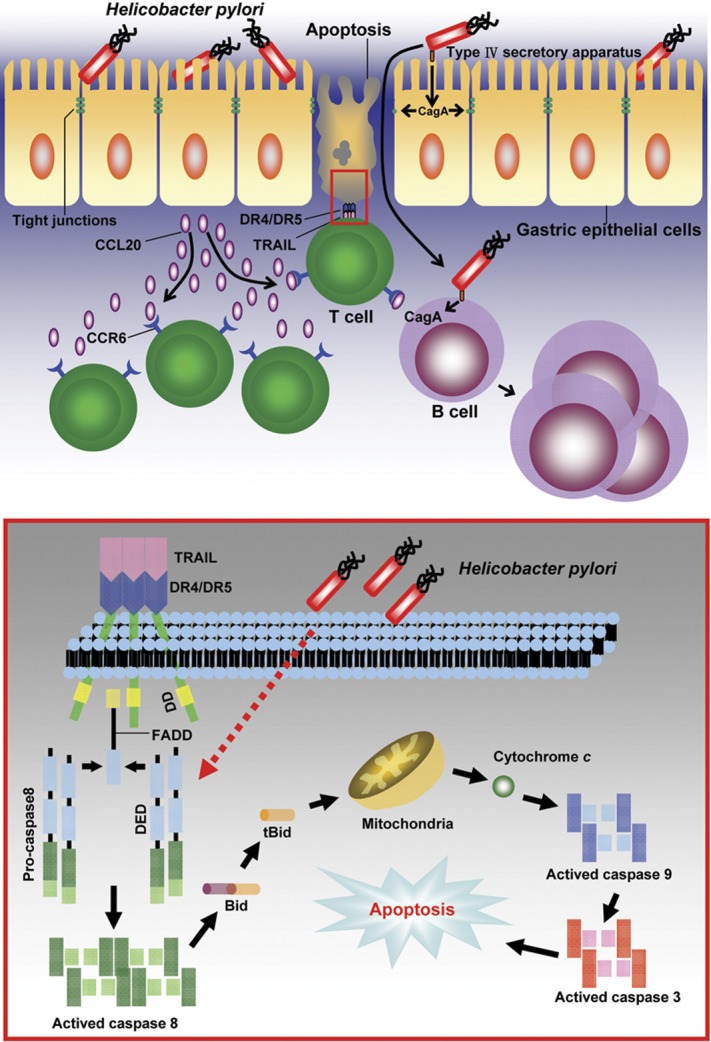
Immune pathogenesis of gastric mucosa damage in H. pylori infection. In the absence of H. pylori infection, there are very few T cells infiltrating into the gastric mucosa, which do not induce apoptosis in gastric epithelial cells. In contrast, in the presence of H. pylori infection, H. pylori induce inflammation and production of chemokine CCL20 to recruit CCR6 expressing activated CD4+ T cells infiltrated to the sites of inflammation in the gastric mucosa. The TRAIL expressing T cells subsequently induce apoptosis in the H. pylori-infected gastric epithelia cells. Meanwhile, immune cells constituting MALTs migrate to and infiltrate the site of H. pylori infection in the gastric mucosa, and in such circumstances, CagA may be injected into lymphocytes as well as gastric epithelial cells. When CagA is transloacted into B lymphocytes, it may induce activation of B lymphocytes to proliferate. The molecular mechanism of H. pylori-induced susceptibility to TRAIL-mediated apoptosis in gastric epithelia cells is shown in the lower inlet of the figure. In the absence of H. pylori infection, TRAIL engagement with death receptors on gastric epithelial cells induces only weak activation of caspase 8, and is not able to activate the caspase 8 downstream signals to trigger cell death. In contrast, in the presence of H. pylori infection, H. pylori enhance the assembly of TRAIL death-inducing signaling complex (DISC) after TRAIL engagement, to augment the activation of caspase 8 and to convey the death signal to mitochondria via cleavage of Bid, leading to activation of mitochondrial pathway and breaking the apoptosis resistance. CagA, cytotoxin-associated gene A; FADD, Fas-associated protein with death domain; MALT, mucosa-associated lymphoid tissue; TRAIL, tumor-necrosis factor-related apoptosis-inducing ligand.
Acknowledgments
This work was supported by grants from the National Health Research Institute (NHRI-EX95-9532SI), National Science Council, Taiwan (NSC90-2314B-075B003 and NSC91-2320B-002) and China Medical University (CMU96-266, CMU97-299).
References
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and risk of gastric carcinoma. N Engl J Med. 1994;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J. Molecular mechanisms for inflammation-promoted pathogenesis of cancer – The Sixteenth International Symposium of the Sapporo Cancer Seminar. Cancer Res. 1997;57:3620–3624. [PubMed] [Google Scholar]
- Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1685–1703. [PMC free article] [PubMed] [Google Scholar]
- Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, et al. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudi J, Kuck D, Strand S, von Herbay A, Mariani SM, Krammer PH, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Tsai HF, Lin WC, Chou AH, Chen HT, Yang JC, et al. Helicobacter pylori enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in human gastric epithelial cells. World J Gastroenterol. 2004;10:2334–2339. doi: 10.3748/wjg.v10.i16.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XJ, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, et al. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NL, Day AS, Jennings HA, Sherman PM. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237–4242. doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, et al. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- Bamford KB, Fan XJ, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- D'Elios MM, Manghetti M, de Carli M, Costa F, Baldari CT, Burroni D, et al. T helper 1 effector cells specific for Helicobacter pylori in gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm C, Quiding-Jalrbrink M, Lonroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Tsai HF, Lin WC, Hsu PI, Shun CT, Wu MS, et al. Upregulation of CCL20 and recruitment of CCR6+ gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Infect Immun. 2007;75:4357–4363. doi: 10.1128/IAI.01660-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fan X, Lindholm C, Bennett M, O'Connoll J, Shanahan F, et al. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect Immun. 2000;68:4303–4311. doi: 10.1128/iai.68.7.4303-4311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LY, Kwaik YA. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le'Negrate G, Ricci V, Hofman V, Mograbi B, Hofman P, Rossi B. Epithelial intestinal cell apoptosis induced by Helicobacter pylori depends on expression of the cag pathogenicity island phenotype. Infect Immun. 2001;69:5001–5009. doi: 10.1128/IAI.69.8.5001-5009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP. Nicholl. et al Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, et al. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–2200. [PubMed] [Google Scholar]
- Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, et al. TRAIL expression by activated human CD4+V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97:2067–2074. doi: 10.1182/blood.v97.7.2067. [DOI] [PubMed] [Google Scholar]
- Kaplan MJ, Ray D, Mo RR, Yung RL, Richardson BC. TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T cell killing of antigen-presenting macrophages. J Immunol. 2000;164:2897–2904. doi: 10.4049/jimmunol.164.6.2897. [DOI] [PubMed] [Google Scholar]
- Dörr J, Waiczies S, Wendling U, Seeger B, Zipp F. Induction of TRAIL-mediated glioma cell death by human T cells. J Neuroimmunol. 2002;122:117–124. doi: 10.1016/s0165-5728(01)00450-7. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Dandekar S, Canfield DR, Solnick JV. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 2000;118:307–315. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- D'Elios MM, Manghetti M, de Carli M, Costa F, Baldari CT, Burroni D, et al. T helper 1 effector cells specific for Helicobacter pylori in gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–420. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- Dwinell MB, Lugering N, Eckmann L, Kagnoff MF. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology. 2001;120:49–59. doi: 10.1053/gast.2001.20914. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- Shibahara T, Wilcox JN, Couse T, Madara JL. Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology. 2001;120:60–70. doi: 10.1053/gast.2001.20904. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Eck M, Schmausser B, Scheller K, Toksoy A, Kraus M, Menzel T, et al. CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin Exp Immunol. 2000;122:192–199. doi: 10.1046/j.1365-2249.2000.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Felley CP, Bouzourene H, Reimers M, Michetti P, Pan-Hammarstrom Q. Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans. J Immunol. 2004;172:2595–2606. doi: 10.4049/jimmunol.172.4.2595. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y, Kita M, Kodama T, Sawai N, Tanahashi T, Kashima K, et al. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893–14898. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med. 1997;186:825–835. doi: 10.1084/jem.186.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis BD, Franz-Bacon K, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–G719. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Imai T, Baba M, Ishikawa I, Uehira M, Nomiyama H, et al. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29:633–642. doi: 10.1002/(SICI)1521-4141(199902)29:02<633::AID-IMMU633>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nishi T, Okazaki K, Kawasaki K, Fukui T, Tamaki H, Matsuura M, et al. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect Immun. 2003;71:2153–2162. doi: 10.1128/IAI.71.4.2153-2162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimori K, Uema E, Teruya H, Ishikawa C, Okudaira T, Senba M, et al. Helicobacter pylori induces CCL20 expression. Infect Immun. 2007;75:5223–5232. doi: 10.1128/IAI.00731-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yoshida A, Isomoto H, Hisatsune J, Nakayama M, Nakashima Y, Matsushim K, et al. Enhanced expression of CCL20 in human Helicobacter pylori-associated gastritis. Clin Immunol. 2009;130:290–297. doi: 10.1016/j.clim.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- Eck M, Schmausser B, Haas R, Greiner A, Czub S, Müller-Hermelink HK. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- Peng H, Ranaldi R, Diss TC, Isaacson PG, Bearzi I, Pan L. High frequency of CagA+Helicobacter pylori infection in high-grade gastric MALT B-cell lymphomas. J Pathol. 1998;185:409–412. doi: 10.1002/(SICI)1096-9896(199808)185:4<409::AID-PATH121>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Fan XJ, Chua A, Shahi CN, McDevitt J, Keeling PW, Kelleher D. Gastric T lymphocyte response to Helicobacter pylori in patients with H. pyloricolonisation. Gut. 1994;35:1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner A, Knörr C, Qin Y, Sebald W, Schimpl A, Banchereau J, et al. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol. 1997;150:1583–1593. [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Moese S, Hauck CR, Meyer TF, Backert S. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971–980. doi: 10.1046/j.1365-2958.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- Poppe M, Feller SM, Römer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26:3462–3472. doi: 10.1038/sj.onc.1210139. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang C, Huang J, Ge Z, Dong Q, Zhong X, et al. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cell Microbiol. 2007;9:952–961. doi: 10.1111/j.1462-5822.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337–8342. doi: 10.1038/sj.onc.1207028. [DOI] [PubMed] [Google Scholar]


