Abstract
In eukaryotic cells the nuclear genome is enclosed by the nuclear envelope (NE). In metazoans, the NE breaks down in mitosis and it has been assumed that the physical barrier separating nucleoplasm and cytoplasm remains intact during the rest of the cell cycle and cell differentiation. However, recent studies suggest that nonmitotic NE remodeling plays a critical role in development, virus infection, laminopathies, and cancer. Although the mechanisms underlying these NE restructuring events are currently being defined, one common theme is activation of protein kinase C family members in the interphase nucleus to disrupt the nuclear lamina, demonstrating the importance of the lamina in maintaining nuclear integrity.
Introduction
The nuclear envelope (NE) in animal cells comprises three structures: the nuclear membrane, the nuclear pore complex (NPC), and the lamina. The nuclear membrane is divided into the inner nuclear membrane (INM) and outer nuclear membrane (ONM) based on protein content, but the membranes are contiguous with each other and with the ER. The nuclear membrane covers the chromatin and restricts nuclear–cytoplasmic trafficking to the NPCs. The NPCs extend through both the INM and ONM as well as the lamina (Schermelleh et al., 2008) and regulate the passage of macromolecules with molecular weights exceeding ∼40 kD between the nucleus and the cytoplasm (Wente and Rout, 2010). The nuclear lamina is a dense meshwork of lamin filaments attached to the INM. The two major types of lamin proteins are the B-type, lamins B1 and B2, and the A-type, lamins A and C, which are different isoforms of the same gene (Dechat et al., 2010). The lamin proteins interact with transmembrane INM proteins, like LBR and Lap2, and chromatin-binding proteins, like BAF, at the nuclear periphery to form a stable network that supports the membrane and links the INM to the chromatin (Ellenberg et al., 1997; Moir et al., 2000; Wilson and Foisner, 2010). The expression of lamin and lamin-associated proteins varies widely between cell types, likely due to different requirements for nuclear mechanical stiffness and chromatin organization in cells with different functions (Burke and Stewart, 2013).
NE breakdown during mitosis has been the focus of many studies and is a dramatic example of endomembrane reorganization (Güttinger et al., 2009). Unexpectedly, however, it has been shown that the NE can also undergo extensive remodeling in interphase, despite the importance of nuclear compartmentalization for eukaryotic cell biology. At this time, four main types of nonmitotic NE remodeling have been characterized, and will be the focus of this review. First, NE budding has been identified as an export mechanism for large nuclear particles (see Fig. 1). In this process, INM-derived vesicles bud into the perinuclear space and fuse with the ONM to release enclosed nuclear contents into the cytoplasm with no obvious loss of nuclear integrity or cell viability. Lamina disruption is required for budding. Second, transient NE rupturing is characterized by a sudden loss of compartmentalization, causing mislocalization of both nuclear and cytoplasmic components, followed by the restoration of NE integrity without cell death (see Fig. 2, A and B). Third, NE collapse is similar to NE rupturing in that both involve a rapid loss of nuclear integrity associated with lamina gaps and chromatin herniation. However, the membrane does not repair, and instead ER tubules mislocalize to the chromatin (see Fig. 2 C). Fourth, two kinds of NE fusion have been described; (1) the ONM and INM fuse to make a channel through the NE to accommodate NPC insertion, and (2) the ONM and then INM of two separate nuclei fuse to make one contiguous nucleus (see Fig. 3). Thus, accumulating evidence suggests that much remains to be learned about the NE barrier and its remodeling during interphase in normal and diseased cells.
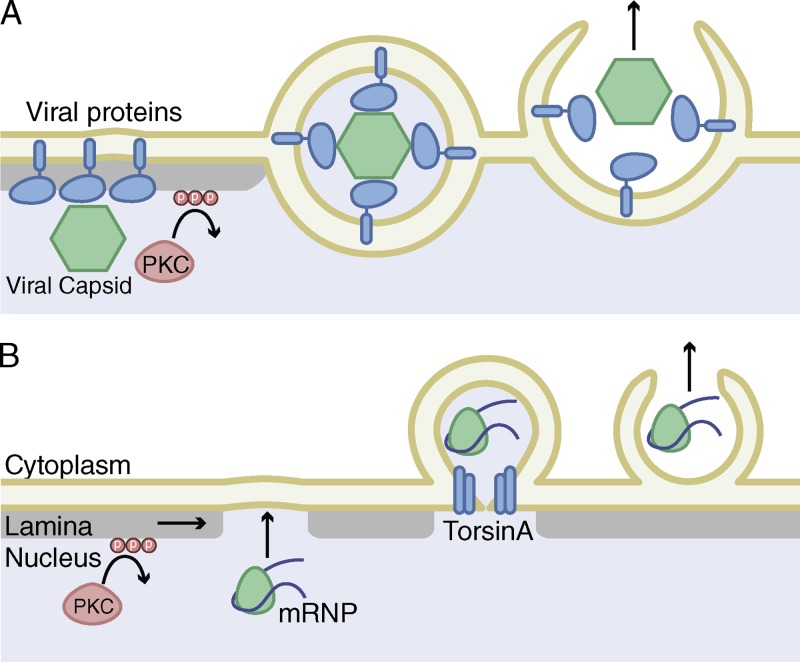
Figure 1.
Nuclear envelope budding of export complexes. (A) Herpes virus capsids bind to viral proteins at the INM that also recruit PKC. Viral capsids then bud through the envelope at sites of lamina disorganization (gray) and are released into the cytoplasm. (B) mRNP export in differentiating muscle cells also requires disorganization of the nuclear lamina by PKC. mRNPs interact with the INM at sites of lamina disorganization and bud into the perinuclear space with the help of torsinA. The perinuclear vesicle fuses with the ONM and the mRNP is released into the cytoplasm.
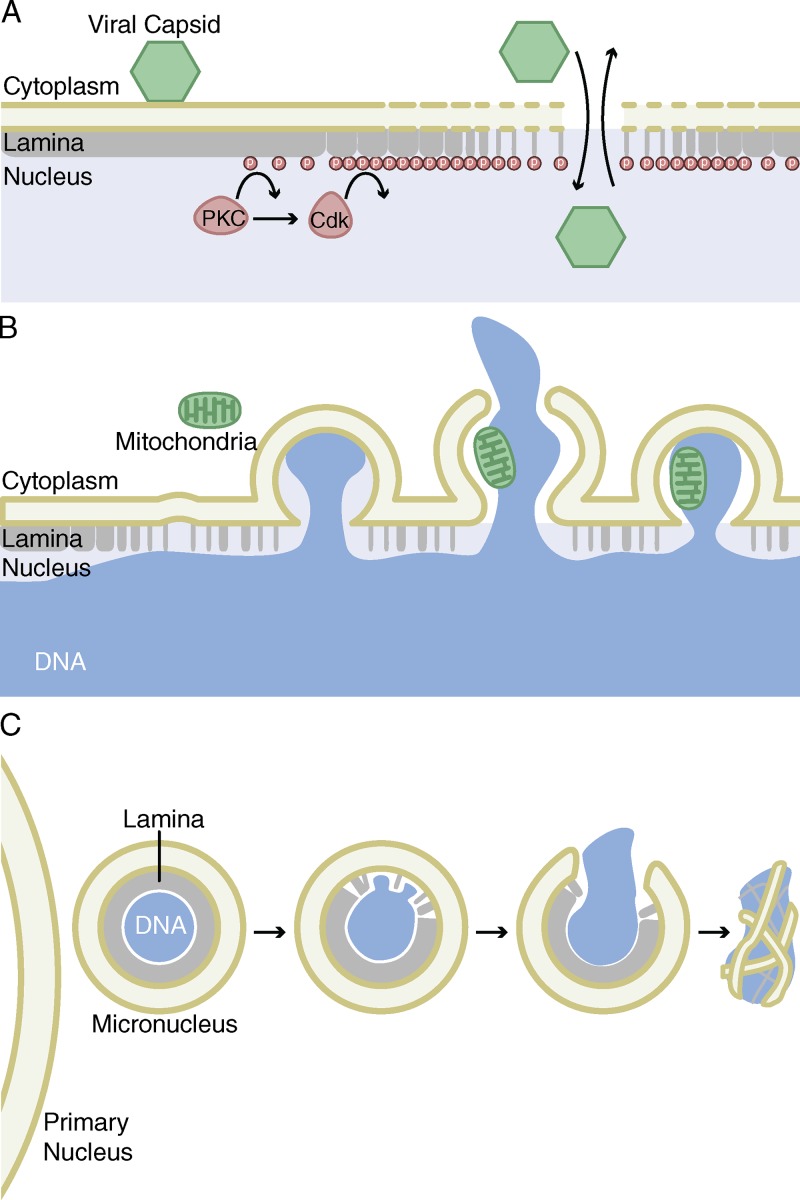
Figure 2.
Nuclear envelope rupturing and collapse. (A) Association of parvovirus capsids with the ONM causes breakdown of first the outer and then the inner nuclear membranes. Activation of PKC and Cdk kinases in the nucleus during this time forms large gaps in the lamina, allowing the capsids to enter the nucleoplasm and causing a loss of nuclear integrity. (B) When lamina organization is disrupted by changes in lamina proteins, patches of weak membrane form and chromatin can herniate. This membrane can undergo multiple rounds of NE rupturing and repair, causing mislocalization and entrapment of cytosolic and nuclear components. (C) Micronuclei have a high probability of NE rupturing but fail to undergo NE repair, causing a persistent loss of nuclear integrity. After rupturing, the chromatin forms aberrant associations with ER tubules in a process called NE collapse.
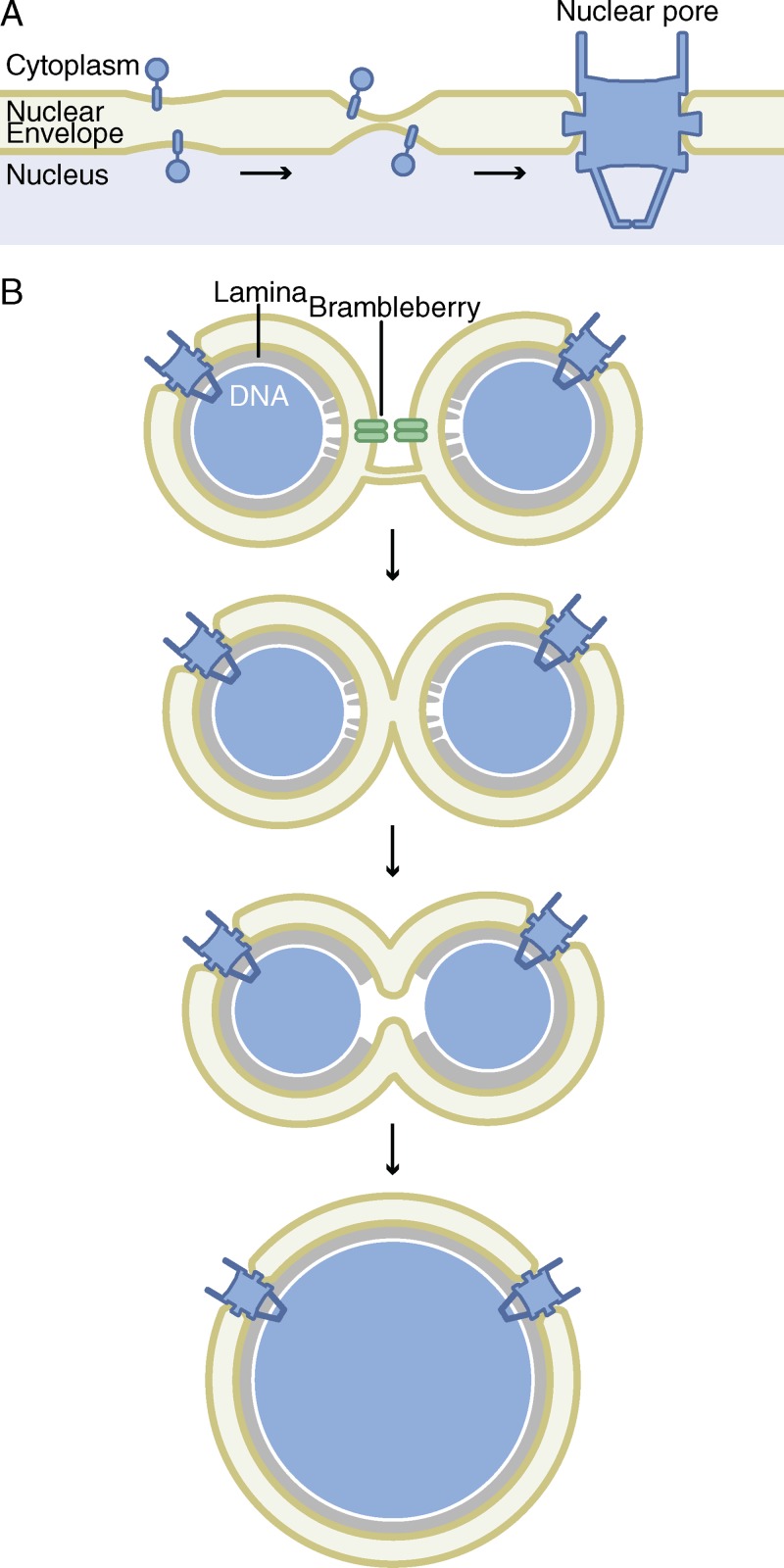
Figure 3.
Nuclear envelope fusion. (A) An early step in interphase nuclear pore assembly is the formation of a channel through the NE by fusion of the inner and outer nuclear membranes. Membrane-associated proteins are thought to bend the membranes toward each other and stabilize the channel before nuclear pore assembly. (B) During fertilization and early embryonic divisions, multiple nuclei can form and must fuse into a mononucleus. Although the nuclear membranes are part of the same endomembrane system, specific proteins, including brambleberry, are required to initiate fusion. Full fusion requires mixing of the outer and inner nuclear membranes and, likely, disorganization of the lamina to expand the fusion pore.
NE budding as an alternate mechanism for nuclear export
Passage through the nuclear pores is the main mechanism of transport between the cytoplasm and the nucleus. Even objects many times larger than the diameter of the NPC, such as certain messenger ribonucleoproteins (mRNPs; Grünwald et al., 2011), exit the nucleus through the nuclear pores by undergoing a complex unfolding program (Mehlin et al., 1992; Kiseleva et al., 1998; Mor et al., 2010). However, the NPC is not the only avenue for nuclear export. Studies on herpes viruses, neutrophils, and neuromuscular junction formation have shown that large complexes, including mRNPs, can also exit the nucleus by budding through the NE.
The first indication of nuclear membrane–based export came from images of herpes virus capsids within the NE perinuclear space (Stackpole, 1969). Herpes viruses replicate their DNA and package it into capsids in the nucleus. These capsids are larger than the size of the NPC and thus must use an alternative route for nuclear egress, namely NE budding (Fig. 1 A). When the capsids form, they associate with viral protein complexes at the INM that recruit kinases to induce lamina disassembly (Muranyi et al., 2002; Park and Baines, 2006; Marschall et al., 2011) and drive vesiculation of the membrane (Klupp et al., 2007). Once in the perinuclear space, the capsid-containing vesicles then fuse with the ONM to release their cargo into the cytoplasm where the capsids undergo further maturation (Johnson and Baines, 2011). During this process the NE remains intact and cells remain viable.
Recent work from the Budnik group suggests that nuclear export by NE budding might also be important for normal development (Speese et al., 2012). During formation of the neuromuscular junction (NMJ) in Drosophila melanogaster, Wnt signaling mediates maturation of the synapse by promoting mRNP export from muscle cell nuclei (Speese et al., 2012). Unlike other mRNPs, which pass through the NPC, these NMJ-specific mRNPs exit the nucleus through the NE using a similar budding process as herpes viruses (Fig. 1 B; Speese et al., 2012). Because initiation of translation often follows mRNP unfolding at the nuclear pore (Mehlin et al., 1992), it is thought that this export pathway is required to prevent premature translation of mRNAs needed at the NMJ (Strambio-De-Castilla, 2013). Perinuclear vesicles have also been observed during both mouse and rabbit embryogenesis as well as in other developing organs in Drosophila (Gay, 1956; Hadek and Swift, 1962; Hochstrasser and Sedat, 1987; Szöllösi and Szöllösi, 1988), suggesting that NE budding may be routinely used for nuclear export during differentiation. Further research is needed to determine whether mRNP transport by NE budding is limited to early development and whether activation of the NE budding program can occur in all cell types. In addition, it is uncertain whether mRNPs targeted to the NE can also be exported through the NPC and how an export pathway is selected.
An alternative mechanism of NE-based export may be occurring in neutrophils in vivo. Neutrophils are short-lived immune cells that can form neutrophil extracellular traps (NETs), comprised of chromatin and anti-microbial proteins, to trap and kill bacteria (Brinkmann and Zychlinsky, 2012). In most cases NETs form after chromatin decondensation, NE breakdown, and cell lysis (Brinkmann et al., 2004; Fuchs et al., 2007). However a second type of NETosis has been identified where vesicles containing decondensed chromatin appear to bud off from the NE and fuse with the plasma membrane in the absence of NE breakdown or cell death (Pilsczek et al., 2010). This type of NETosis by NE remodeling appears to be important for pathogen containment in vivo (Yipp et al., 2012). In contrast to NE budding, images of this process suggest that the chromatin in the expanded perinuclear space is not in vesicles (Pilsczek et al., 2010). Thus, the chromatin may enter the perinuclear space by a different mechanism than INM budding.
Lamina disassembly during NE budding occurs by a mechanism similar to those that break down the lamina in mitosis and apoptosis. During both mitosis and apoptosis, kinases are activated that phosphorylate the lamins, causing the protein network to fall apart, or targeting the lamins for degradation by caspases (Cross et al., 2000). Members of the PKC kinase family have been shown to be important lamin kinases for mitosis and apoptosis (Hocevar et al., 1993; Goss et al., 1994; Thompson and Fields, 1996; Collas, 1999; Cross et al., 2000), as well as for INM vesicle formation around both mRNPs and herpes virus capsids (Muranyi et al., 2002; Park and Baines, 2006; Leach and Roller, 2010; Speese et al., 2012). Lamin protein phosphorylation by these kinases results in localized lamina disassembly at the site of NE budding (Muranyi et al., 2002; Park and Baines, 2006; Leach and Roller, 2010; Speese et al., 2012). Interestingly, different PKC family members are involved in herpes virus budding than in mRNP budding in Drosophila (Muranyi et al., 2002; Park and Baines, 2006; Milbradt et al., 2010; Speese et al., 2012), suggesting that different signaling pathways regulate lamina gap formation in different contexts. Mitotic activation of PKC results in global disassembly of lamina, thus an important question for interphase PKC activation is how lamina disassembly is restricted to sites of NE budding.
Another interesting question is how vesicle fusion events in the NE are mediated. Analysis of viral protein mutants suggests that vesicle fusion to the ONM is independent of INM envelopment and may be regulated by phosphorylation events (Mettenleiter et al., 2009; Mou et al., 2009; Bakheet et al., 2011). Separation of the two processes is likely crucial to prevent aberrant fusion of the INM and ONM, which would create a membrane channel. Interestingly, overexpression of torsinA negatively regulates vesicle fusion to the ONM from the perinuclear space (Maric et al., 2011). TorsinA is an AAA+ ATPase located in the ER lumen that is known to regulate NE shape and has structural similarities to vesicle fusion enzymes like NSF (Gerace, 2004; Hanson and Whiteheart, 2005). In addition, neurons and muscle cells with torsinA mutations generate perinuclear buds that fail to separate from the INM and are unable to fuse with the ONM (Goodchild et al., 2005; Jokhi et al., 2013). This suggests that proteins that regulate ER dynamics have additional functions in interphase NE remodeling.
Nuclear envelope rupturing associated with viral infection
NE budding as a transport mechanism appears to be limited to nuclear export; examples of cargo import by NE budding have not been identified. This could be due to biochemical asymmetries between INM and ONM that prevent vesicle budding from the ONM or block vesicle fusion to the INM. Consistent with this idea, parvoviruses, the only virus family known to bypass the NPCs for nuclear import, require transient disruption of the NE to access the nucleus. Parvoviruses are a family of small DNA viruses that includes adeno-associated virus. When parvovirus capsids reach the NE they generate large holes in the ONM, then in the INM, and finally in the lamina to allow capsid entry to the nucleus (Fig. 2 A; Cohen and Panté, 2005; Cohen et al., 2006; Porwal et al., 2013). In the absence of either membrane disruption or lamina disassembly, the virus fails to enter the nucleus (Porwal et al., 2013), indicating that breaking down both barriers is required for import. Nuclear integrity is lost a few minutes after infection, but in later stages the NE appears contiguous and compartmentalization is fully restored (Cohen et al., 2011). This indicates that even though parvoviruses generate large breaks in the membrane, the NE is still able to undergo repair.
During NE rupturing by parvovirus infection, as in NE budding of herpes viruses, PKCα activity is required to initiate lamina disruption (Porwal et al., 2013). However, NE rupturing in this system requires activation of an additional kinase, Cdk2, by PKCα and caspase-3 (Cohen et al., 2011; Porwal et al., 2013). Parvovirus infection induces much larger ruptures in the lamina than are observed during NE budding. Thus, additional kinase activation may be required to sustain more extensive lamin phosphorylation.
Infection with HIV can also cause dramatic NE instability. The HIV protein VPR is thought to modulate the cell environment to make it more favorable for viral replication (Andersen et al., 2008). However, VPR expression can induce repeated transient NE rupturing and loss of compartmentalization, causing mislocalization of nuclear and cytosolic cell cycle regulators, including wee-1 and cdc25 (de Noronha et al., 2001). NE rupturing is accompanied by prominent lamina gaps as well as large blebs of herniating chromatin, which are also present in cells infected with HIV (Fig. 2 B; de Noronha et al., 2001). VPR is dispensable for HIV infection (Zufferey et al., 1997), however, indicating that NE rupturing is not required for nuclear import of viral DNA. VPR expression can arrest the cell in G2 by induction of DNA damage (Roshal et al., 2003), and one model suggests that VPR-induced repetitive NE rupturing is the cause of this damage (Planelles and Benichou, 2009). However, it is also possible that by arresting cells in G2, VPR causes premature attachment of microtubules to the NE, resulting in membrane disruption. This would be consistent with the observation that microtubule interactions with the NE, which increase during G2, induce tears in the membrane (Beaudouin et al., 2002; Salina et al., 2002). Further work is needed to address how VPR expression causes lamina disruption, the frequency of NE rupturing in infections in vivo, and what the consequences of nuclear integrity loss are for viral infection.
Nuclear envelope rupturing in laminopathies
Because lamina disruption is a general feature of NE remodeling, a significant rise in interphase NE dynamics is likely to be a feature of lamin-associated human diseases, known as laminopathies. Laminopathies are genetic diseases caused by mutations in lamin A/C or lamin-associated proteins that alter lamina organization and result in tissue-specific cell loss (Worman et al., 2010). A common observation in cells expressing laminopathy mutant proteins is large gaps in the lamina where B-type lamins, NPCs, and other structural INM proteins are absent and large chromatin herniations appear to push out the weakened membrane (Fig. 2 B; Sullivan et al., 1999; Vigouroux et al., 2001). These lamina gaps also occur when lamin B1 is misregulated (Vergnes et al., 2004; Vargas et al., 2012). Regardless of their origin, one consequence of lamina discontinuities is repeated nonlethal transient NE rupturing at these sites (De Vos et al., 2011; Vargas et al., 2012). Transient NE rupturing causes both soluble proteins and cytoplasmic and nuclear organelles, like vesicles, mitochondria, and PML bodies, to become mislocalized. Although soluble proteins can be resorted to the correct compartment, mislocalized organelles become trapped when the NE repairs (de Noronha et al., 2001; De Vos et al., 2011; Vargas et al., 2012). Thus, NE rupturing could be an important contributor to laminopathy pathology.
Direct evidence of NE rupturing in laminopathy cells has been limited to cultured cells, but indirect evidence of NE rupturing is present in fixed tissues. Gaps in the lamina have been observed in liver nuclei of mice lacking lamin A/C (Sullivan et al., 1999), and disruption of the nuclear membrane is apparent in muscle tissue in flies lacking B-type lamins (Lenz-Böhme et al., 1997). Biopsies from laminopathy patients show even clearer signs of NE rupturing. In post-mitotic cardiomyocytes, both disruption of the nuclear membrane and mislocalization of mitochondria to the nucleus have been observed by electron microscopy (Fidziańska et al., 2008; Gupta et al., 2010). Together, these data demonstrate that NE rupturing can occur in a variety of contexts in vivo.
Although mutation or loss of a lamin protein can induce NE rupturing, lamin depletion by itself is not sufficient to destabilize the membrane. Knockout of either both B-type lamin genes or all three lamin genes in embryonic stem cells does not cause an increase in lamina gaps or chromatin herniation (Kim et al., 2011, 2013). Recent modeling of chromatin herniation suggests that weak points in the membrane form when mismatched protein networks, like lamin B versus lamin A, generate tension on the NE (Funkhouser et al., 2013). Thus, loss of multiple lamins may be less problematic than misregulation of a single lamin because the NE is under less tension. Consistent with this hypothesis, the frequency of membrane rupturing in laminopathy cells decreases when the tension on the NE is reduced by growth on soft substrates (Coffinier et al., 2010; Tamiello et al., 2013). This correlation is also observed in vivo; the most dramatic defects in NE integrity are found in muscle and heart tissue where the nuclei are under increased tension (Lenz-Böhme et al., 1997; Fidziańska et al., 2008; Gupta et al., 2010). Thus, additional factors, including the organization of the cytoplasmic cytoskeleton, likely determine the frequency of nuclear integrity loss in cells with lamina defects.
Tension in the cytoskeleton is transmitted to the nucleus via LINC complexes, which traverse the NE and connect the cytosolic cytoskeleton to the lamina, and defects in LINC complex members have been shown to affect nuclear structure (Tapley and Starr, 2013). Consistent with the hypothesis that increased tension increases NE defects in cells with an altered lamina, depleting the LINC complex member SUN-1 reduced chromatin herniation frequency in laminopathy cells (Chen et al., 2012). However, this was not true for all LINC complex members; interfering with nesprins increased nuclear defects and the severity of laminopathy symptoms (Kandert et al., 2007; Zhang et al., 2007; Puckelwartz et al., 2009, 2010). LINC complex proteins have additional roles in cellular organization and nuclear functions (Rothballer and Kutay, 2013b); thus, more research is required to determine the importance of LINC complex proteins in regulating NE integrity in laminopathy cells.
Recent work suggests that studying senescent cells may also provide valuable information about how lamin misregulation affects NE integrity. One hallmark of senescent cells is a significant decrease in lamin B1 levels (Shimi et al., 2011; Freund et al., 2012), accompanied by small chromatin herniations (Ivanov et al., 2013). Nuclei from senescent cells are more permeable after isolation (Ivanov et al., 2013), but the extent to which NE integrity is altered in these cells is unclear. Determining whether these nuclei are rupturing and, if not, what additional changes prevent this, could provide insight into the mechanism of NE rupturing.
Nuclear rupturing and collapse in cancer cells
Cancer cells often exhibit changes in nuclear morphology and lamin A/C expression similar to those that result in transient NE rupturing in laminopathies (Zink et al., 2004; Prokocimer et al., 2006). Consistent with this, examination of the NE in cultured cancer cell lines demonstrated that they have a significantly higher frequency of chromatin herniation and, unlike nontransformed cells, undergo NE rupturing. Similar to laminopathy cells, the frequency of NE rupturing can be increased by altering the structure of the lamina through decreasing lamin levels (Vargas et al., 2012). At this time, no marker has been developed to positively identify NE rupturing in fixed cancer tissues, but intranuclear mitochondria have been observed in leukemias and lymphomas (Brandes et al., 1965; Oliva et al., 1973), suggesting that NE rupturing and repair can occur in cancer cells in vivo.
Although changes in nuclear morphology are a gold standard for cancer diagnosis, very little is known about why disruption of the NE structure would benefit a cancer cell. It is known that altering the lamina can cause changes in heterochromatin formation and gene expression (Stewart et al., 2007), which could facilitate carcinogenesis (Prokocimer et al., 2006). In this model, NE rupturing would be a passive side effect of lamina disruption. However, NE rupturing could also promote cancer development. Distended chromatin at the site of NE rupturing can undergo changes in both chromatin structure and nuclear functioning (Shimi et al., 2008). In addition, regulation of gene expression might be compromised by mislocalization of transcription factors due to a repeated loss of compartmentalization (De Vos et al., 2011).
When NE rupturing is induced in primary nuclei, either through viral infection or disruption of the lamina, the NE is almost always repaired and compartmentalization restored (de Noronha et al., 2001; Cohen et al., 2011; De Vos et al., 2011; Vargas et al., 2012). However, this repair fails when NE rupturing occurs on micronuclei (MN). Recent work from our laboratory has shown that a large proportion of MN that form from chromosome missegregation rupture during interphase and fail to regain compartmentalization before mitosis. Similar to primary nucleus NE rupturing, MN rupturing also stems from lamin disorganization leading to lamina gaps (Hatch et al., 2013). But instead of resealing over the chromosome, the NE is replaced by ER tubules that invade the chromatin in a process we have termed “NE collapse” (Fig. 2 C).
Unlike NE rupturing in primary nuclei, membrane disruption in micronuclei has clear consequences for genomic instability. First, several nuclear functions important for maintaining chromosome integrity, including DNA damage repair and DNA replication, are impaired in intact micronuclei and abrogated by NE rupturing (Crasta et al., 2012; Hatch et al., 2013). Second, NE rupturing in MN can also trigger massive DNA damage (Hatch et al., 2013). The clustered DNA damage on chromosomes in collapsed MN, which likely arises from the sudden compaction of replicating DNA (Zhang et al., 2013), makes them an ideal substrate for chromothripsis (Crasta et al., 2012). In chromothripsis a single chromosome, or chromosome fragment, is shattered and then stitched together to form a highly rearranged chromosome (Stephens et al., 2011). Since its identification, evidence of chromothripsis has been found in a wide variety of cancers and is generally associated with poor outcomes (Kloosterman et al., 2014). Although a causal link between MN rupturing and chromothripsis remains to be shown, loss of nuclear activity and accumulation of DNA damage is likely to cause significant changes in chromosome function, increasing the likelihood of aneuploidy.
Although NE rupturing in primary nuclei and MN are both rooted in lamina disorganization, it is unclear why MN develop lamina defects more often than primary nuclei. One possibility is that the higher curvature of the NE in MN can induce alterations in the lamina. However, MN with significantly different sizes have the same probability of NE rupturing (Hatch et al., 2013), suggesting that membrane curvature might not play a critical role. In addition, lamin B1 discontinuities in MN can already be observed in early G1 (Hatch et al., 2013), suggesting that problems with NE assembly could contribute to aberrant lamina formation. Lamina construction generally occurs late in NE formation after the membranes have sealed (Newport et al., 1990; Daigle et al., 2001; Haraguchi et al., 2008), although there are examples of lamins binding directly to the chromatin early in anaphase (Moir et al., 2000). Thus, misregulation of early steps in NE assembly could have downstream effects on lamina organization. One hypothesis is that because chromosomes have different overall chromatin properties, not all chromosomes are able to appropriately interact with NE proteins when separated from the main chromatin mass. Consistent with this, specific chromatin sequences or histone modifications are thought to facilitate protein recruitment during NE assembly (Güttinger et al., 2009). Thus, further research on the interaction of NE assembly proteins with chromatin during mitotic exit could elucidate why MN have a high probability of NE rupturing.
NE repair
Although countless nuclear injection experiments in a variety of systems depended on the ability of the NE to repair after puncture, the mechanism by which the NE reseals is not understood. The transience of NE rupturing in the primary nucleus, even when membrane disruption is extensive (Cohen et al., 2006), demonstrates that the NE has a much larger capacity for repair than anticipated, but there is little information about potential mechanisms. It is likely that NE repair requires connectivity to the ER, as this connection is required for interphase NE expansion (D’Angelo et al., 2006; Anderson and Hetzer, 2007; Lu et al., 2011). Alternatively, NE repair could be initiated by interactions of INM proteins in the ER with the exposed chromatin, leading to membrane spreading, as occurs during post-mitotic NE closure (Anderson et al., 2009). Transmembrane proteins that localize to the chromatin early in NE assembly do aggregate on MN chromatin after NE collapse, as do ER tubules (Hatch et al., 2013). However, MN do not undergo NE repair, suggesting that this recruitment is not sufficient to reform the nuclear membrane. In contrast, INM proteins are largely depleted from the sites where NE rupturing and repair occurs in the primary nucleus (Sullivan et al., 1999; Vigouroux et al., 2001), suggesting that recruitment of NE assembly proteins to interphase chromatin may inhibit repair. Consistent with this idea, MN efficiently undergo post-mitotic NE assembly (Hatch et al., 2013), suggesting that the MN chromatin is competent for NE formation, but that this process is inhibited during interphase. Alternatively, changes in chromatin state after NE rupturing in MN, including compaction and loss of acetylation (Hatch et al., 2013), could alter the ability of INM proteins and ER membranes to productively interact with the MN chromatin. It will be important to determine whether similar chromatin changes are occurring during primary nucleus rupturing, and whether NE assembly proteins are important for primary nucleus repair to begin to understand this process.
Membrane fusion events involved in NE reorganization
Fusion of the INM and ONM occurs frequently in growing cells when new nuclear pores are assembled into the expanding nuclear membrane (D’Angelo et al., 2006). An early step in interphase NPC assembly is fusion of the ONM and INM to generate a channel where the NPC can go (Goldberg et al., 1997; Doucet et al., 2010). Nucleoporins then associate with the curved membrane channel to stabilize it and initiate pore assembly (Fig. 3 A; Rothballer and Kutay, 2013a; Smoyer and Jaspersen, 2014). Although the mechanism of nuclear membrane fusion is unclear, several proteins are known to function in the remodeling process. First, ER-shaping proteins, such as reticulons, have been shown to be required for membrane fusion before NPC and spindle pole body insertion in yeast (Dawson et al., 2009; Casey et al., 2012). In addition, several NE transmembrane proteins, including Sun-1 and members of the NPC, are required to shape the membrane channel, although it is unclear if these proteins initiate or stabilize membrane fusion (Rothballer and Kutay, 2013a; Smoyer and Jaspersen, 2014). Both NPC subcomplexes and transmembrane proteins have structural similarities to proteins involved in vesicle fusion (Devos et al., 2004, 2006; Brohawn et al., 2009; Rothballer and Kutay, 2013a), but it is unclear how related the two processes are. During NE budding, fusion must also occur between a vesicle derived from the INM and the ONM. Determining what INM proteins are retained in the vesicle and required for fusion may significantly enhance our understanding of ONM and INM fusion in general.
How two nuclei fuse was first addressed using yeast karyogamy as a model (Melloy et al., 2007, 2009; Ydenberg and Rose, 2008), and recent work has begun to elucidate nuclear fusion events in metazoans. In several species, including sea urchins, frogs, zebrafish, and rabbits, NE fusion is an important mechanism to maintain euploidy during embryogenesis. NE fusion occurs at two stages in these animals during early development: pronuclei fuse before the first mitotic division, and multiple nuclei are fused into a mononucleus during early cleavage divisions (Abrams et al., 2012). When the cytoplasm-to-nucleus ratio is very high, as in the oocyte, chromosomes can become separated during mitosis resulting in multinucleation, also called karyomere formation. Starting in telophase, these karyomeres fuse to form a mononucleus. Images of pronuclear fusion in sea urchin describe a model where proximity of the pronuclei causes mixing of the ONM followed by fusion of the INMs and mixing of the nuclear contents (Longo and Anderson, 1968). Although lamina disruption has not been observed, it is likely that some disassembly is required to permit expansion of the fusion pore (Lénárt and Ellenberg, 2003). Recent work in zebrafish identified brambleberry, an NE transmembrane protein, as an important protein for initiating NE fusion in both pronuclei and karyomeres (Fig. 3 B; Abrams et al., 2012). Depletion of brambleberry demonstrated that, although karyomeres can efficiently perform nuclear functions (Lemaitre et al., 1998), NE fusion is required for development, as embryos depleted of brambleberry arrested early in embryogenesis (Abrams et al., 2012). Further analysis of brambleberry interactors and functions will likely provide important insights into the mechanism of nuclear membrane fusion.
Conclusion
A brief survey of interphase NE remodeling and disruption events demonstrates that interphase NE dynamics are important in an increasing number of developmental and disease contexts. As the consequences of these events become clearer it will not only clarify the pathology of viral infections, laminopathies, and cancer, but could also impact new technology development. Currently, the parvovirus AAV (adeno-associated virus) is being used as a delivery mechanism for gene therapy. Thus, understanding the consequences of NE rupturing from virus infection could be important to mitigate side effects of this treatment. In addition, one of the main problems in in vitro fertilization is the high frequency of aneuploidy in early divisions due to multinucleation (Chavez et al., 2012). An ability to initiate NE fusion in these cases may be able to increase the frequency of successful fertilizations. At this time, a number of questions remain about how remodeling in NE budding and fusion occurs and what the consequences of transient NE rupturing are for chromatin structure, gene expression, and other nuclear functions. The world of interphase NE dynamics is just beginning to be explored, but its importance in cell biology is already clear.
Acknowledgments
The authors thank B. Toyama for his assistance with the graphic design of the figures.
M. Hetzer was supported by National Institutes of Health (NIH) grant R01GM098749, the Glenn Aging Foundation, American Cancer Society Award number P30CA014195, and the Ellison Medical Foundation. E. Hatch is supported by a postdoctoral fellowship, PF-12-137-01-CSM, from the American Cancer Society.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- INM
- inner nuclear membrane
- MN
- micronuclei
- mRNP
- messenger ribonucleoprotein
- NE
- nuclear envelope
- NPC
- nuclear pore complex
- ONM
- outer nuclear membrane
References
- Abrams E.W., Zhang H., Marlow F.L., Kapp L., Lu S., Mullins M.C. 2012. Dynamic assembly of brambleberry mediates nuclear envelope fusion during early development. Cell. 150:521–532 10.1016/j.cell.2012.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J.L., Le Rouzic E., Planelles V. 2008. HIV-1 Vpr: mechanisms of G2 arrest and apoptosis. Exp. Mol. Pathol. 85:2–10 10.1016/j.yexmp.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.J., Hetzer M.W. 2007. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 9:1160–1166 10.1038/ncb1636 [DOI] [PubMed] [Google Scholar]
- Anderson D.J., Vargas J.D., Hsiao J.P., Hetzer M.W. 2009. Recruitment of functionally distinct membrane proteins to chromatin mediates nuclear envelope formation in vivo. J. Cell Biol. 186:183–191 10.1083/jcb.200901106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakheet S.A., Attia S.M., Al-Rasheed N.M., Al-Harbi M.M., Ashour A.E., Korashy H.M., Abd-Allah A.R., Saquib Q., Al-Khedhairy A.A., Musarrat J. 2011. Salubrious effects of dexrazoxane against teniposide-induced DNA damage and programmed cell death in murine marrow cells. Mutagenesis. 26:533–543 10.1093/mutage/ger013 [DOI] [PubMed] [Google Scholar]
- Beaudouin J., Gerlich D., Daigle N., Eils R., Ellenberg J. 2002. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 108:83–96 10.1016/S0092-8674(01)00627-4 [DOI] [PubMed] [Google Scholar]
- Brandes D., Schofield B.H., Anton E. 1965. Nuclear mitochondria? Science. 149:1373–1374 10.1126/science.149.3690.1373 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Zychlinsky A. 2012. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198:773–783 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science. 303:1532–1535 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Brohawn S.G., Partridge J.R., Whittle J.R., Schwartz T.U. 2009. The nuclear pore complex has entered the atomic age. Structure. 17:1156–1168 10.1016/j.str.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Stewart C.L. 2013. The nuclear lamins: flexibility in function. Nat. Rev. Mol. Cell Biol. 14:13–24 10.1038/nrm3488 [DOI] [PubMed] [Google Scholar]
- Casey A.K., Dawson T.R., Chen J., Friederichs J.M., Jaspersen S.L., Wente S.R. 2012. Integrity and function of the Saccharomyces cerevisiae spindle pole body depends on connections between the membrane proteins Ndc1, Rtn1, and Yop1. Genetics. 192:441–455 10.1534/genetics.112.141465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez S.L., Loewke K.E., Han J., Moussavi F., Colls P., Munne S., Behr B., Reijo Pera R.A. 2012. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun. 3:1251 10.1038/ncomms2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-Y., Chi Y.-H., Mutalif R.A., Starost M.F., Myers T.G., Anderson S.A., Stewart C.L., Jeang K.-T. 2012. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell. 149:565–577 10.1016/j.cell.2012.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C., Jung H.-J., Li Z., Nobumori C., Yun U.J., Farber E.A., Davies B.S., Weinstein M.M., Yang S.H., Lammerding J., et al. 2010. Direct synthesis of lamin A, bypassing prelamin a processing, causes misshapen nuclei in fibroblasts but no detectable pathology in mice. J. Biol. Chem. 285:20818–20826 10.1074/jbc.M110.128835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Panté N. 2005. Pushing the envelope: microinjection of Minute virus of mice into Xenopus oocytes causes damage to the nuclear envelope. J. Gen. Virol. 86:3243–3252 10.1099/vir.0.80967-0 [DOI] [PubMed] [Google Scholar]
- Cohen S., Behzad A.R., Carroll J.B., Panté N. 2006. Parvoviral nuclear import: bypassing the host nuclear-transport machinery. J. Gen. Virol. 87:3209–3213 10.1099/vir.0.82232-0 [DOI] [PubMed] [Google Scholar]
- Cohen S., Marr A.K., Garcin P., Panté N. 2011. Nuclear envelope disruption involving host caspases plays a role in the parvovirus replication cycle. J. Virol. 85:4863–4874 10.1128/JVI.01999-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collas P. 1999. Sequential PKC- and Cdc2-mediated phosphorylation events elicit zebrafish nuclear envelope disassembly. J. Cell Sci. 112:977–987 [DOI] [PubMed] [Google Scholar]
- Crasta K., Ganem N.J., Dagher R., Lantermann A.B., Ivanova E.V., Pan Y., Nezi L., Protopopov A., Chowdhury D., Pellman D. 2012. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 482:53–58 10.1038/nature10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross T., Griffiths G., Deacon E., Sallis R., Gough M., Watters D., Lord J.M. 2000. PKC-delta is an apoptotic lamin kinase. Oncogene. 19:2331–2337 10.1038/sj.onc.1203555 [DOI] [PubMed] [Google Scholar]
- D’Angelo M.A., Anderson D.J., Richard E., Hetzer M.W. 2006. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 312:440–443 10.1126/science.1124196 [DOI] [PubMed] [Google Scholar]
- Daigle N., Beaudouin J., Hartnell L., Imreh G., Hallberg E., Lippincott-Schwartz J., Ellenberg J. 2001. Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J. Cell Biol. 154:71–84 10.1083/jcb.200101089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T.R., Lazarus M.D., Hetzer M.W., Wente S.R. 2009. ER membrane-bending proteins are necessary for de novo nuclear pore formation. J. Cell Biol. 184:659–675 10.1083/jcb.200806174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Noronha C.M., Sherman M.P., Lin H.W., Cavrois M.V., Moir R.D., Goldman R.D., Greene W.C. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 294:1105–1108 10.1126/science.1063957 [DOI] [PubMed] [Google Scholar]
- De Vos W.H., Houben F., Kamps M., Malhas A., Verheyen F., Cox J., Manders E.M., Verstraeten V.L., van Steensel M.A., Marcelis C.L., et al. 2011. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum. Mol. Genet. 20:4175–4186 10.1093/hmg/ddr344 [DOI] [PubMed] [Google Scholar]
- Dechat T., Adam S.A., Taimen P., Shimi T., Goldman R.D. 2010. Nuclear lamins. Cold Spring Harb. Perspect. Biol. 2:a000547 10.1101/cshperspect.a000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D., Dokudovskaya S., Alber F., Williams R., Chait B.T., Sali A., Rout M.P. 2004. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2:e380 10.1371/journal.pbio.0020380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D., Dokudovskaya S., Williams R., Alber F., Eswar N., Chait B.T., Rout M.P., Sali A. 2006. Simple fold composition and modular architecture of the nuclear pore complex. Proc. Natl. Acad. Sci. USA. 103:2172–2177 10.1073/pnas.0506345103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet C.M., Talamas J.A., Hetzer M.W. 2010. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 141:1030–1041 10.1016/j.cell.2010.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg J., Siggia E.D., Moreira J.E., Smith C.L., Presley J.F., Worman H.J., Lippincott-Schwartz J. 1997. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 138:1193–1206 10.1083/jcb.138.6.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidziańska A., Bilińska Z.T., Tesson F., Wagner T., Walski M., Grzybowski J., Ruzyłło W., Hausmanowa-Petrusewicz I. 2008. Obliteration of cardiomyocyte nuclear architecture in a patient with LMNA gene mutation. J. Neurol. Sci. 271:91–96 10.1016/j.jns.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Freund A., Laberge R.-M., Demaria M., Campisi J. 2012. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell. 23:2066–2075 10.1091/mbc.E11-10-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser C.M., Sknepnek R., Shimi T., Goldman A.E., Goldman R.D., Olvera de la Cruz M. 2013. Mechanical model of blebbing in nuclear lamin meshworks. Proc. Natl. Acad. Sci. USA. 110:3248–3253 10.1073/pnas.1300215110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay H. 1956. Nucleocytoplasmic relations in Drosophila. Cold Spring Harb. Symp. Quant. Biol. 21:257–269 10.1101/SQB.1956.021.01.021 [DOI] [PubMed] [Google Scholar]
- Gerace L. 2004. TorsinA and torsion dystonia: Unraveling the architecture of the nuclear envelope. Proc. Natl. Acad. Sci. USA. 101:8839–8840 10.1073/pnas.0402441101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M.W., Wiese C., Allen T.D., Wilson K.L. 1997. Dimples, pores, star-rings, and thin rings on growing nuclear envelopes: evidence for structural intermediates in nuclear pore complex assembly. J. Cell Sci. 110:409–420 [DOI] [PubMed] [Google Scholar]
- Goodchild R.E., Kim C.E., Dauer W.T. 2005. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 48:923–932 10.1016/j.neuron.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Goss V.L., Hocevar B.A., Thompson L.J., Stratton C.A., Burns D.J., Fields A.P. 1994. Identification of nuclear beta II protein kinase C as a mitotic lamin kinase. J. Biol. Chem. 269:19074–19080 [PubMed] [Google Scholar]
- Grünwald D., Singer R.H., Rout M. 2011. Nuclear export dynamics of RNA-protein complexes. Nature. 475:333–341 10.1038/nature10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Bilinska Z.T., Sylvius N., Boudreau E., Veinot J.P., Labib S., Bolongo P.M., Hamza A., Jackson T., Ploski R., et al. 2010. Genetic and ultrastructural studies in dilated cardiomyopathy patients: a large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res. Cardiol. 105:365–377 10.1007/s00395-010-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güttinger S., Laurell E., Kutay U. 2009. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 10:178–191 10.1038/nrm2641 [DOI] [PubMed] [Google Scholar]
- Hadek R., Swift H. 1962. Nuclear extrusion and intracisternal inclusions in the rabbit blastocyst. J. Cell Biol. 13:445–451 10.1083/jcb.13.3.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.I., Whiteheart S.W. 2005. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 6:519–529 10.1038/nrm1684 [DOI] [PubMed] [Google Scholar]
- Haraguchi T., Kojidani T., Koujin T., Shimi T., Osakada H., Mori C., Yamamoto A., Hiraoka Y. 2008. Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J. Cell Sci. 121:2540–2554 10.1242/jcs.033597 [DOI] [PubMed] [Google Scholar]
- Hatch E.M., Fischer A.H., Deerinck T.J., Hetzer M.W. 2013. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 154:47–60 10.1016/j.cell.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar B.A., Burns D.J., Fields A.P. 1993. Identification of protein kinase C (PKC) phosphorylation sites on human lamin B. Potential role of PKC in nuclear lamina structural dynamics. J. Biol. Chem. 268:7545–7552 [PubMed] [Google Scholar]
- Hochstrasser M., Sedat J.W. 1987. Three-dimensional organization of Drosophila melanogaster interphase nuclei. II. Chromosome spatial organization and gene regulation. J. Cell Biol. 104:1471–1483 10.1083/jcb.104.6.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A., Pawlikowski J., Manoharan I., van Tuyn J., Nelson D.M., Rai T.S., Shah P.P., Hewitt G., Korolchuk V.I., Passos J.F., et al. 2013. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 202:129–143 10.1083/jcb.201212110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.C., Baines J.D. 2011. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 9:382–394 10.1038/nrmicro2559 [DOI] [PubMed] [Google Scholar]
- Jokhi V., Ashley J., Nunnari J., Noma A., Ito N., Wakabayashi-Ito N., Moore M.J., Budnik V. 2013. Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope. Cell Rep. 3:988–995 10.1016/j.celrep.2013.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandert S., Lüke Y., Kleinhenz T., Neumann S., Lu W., Jaeger V.M., Munck M., Wehnert M., Müller C.R., Zhou Z., et al. 2007. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum. Mol. Genet. 16:2944–2959 10.1093/hmg/ddm255 [DOI] [PubMed] [Google Scholar]
- Kim Y., Sharov A.A., McDole K., Cheng M., Hao H., Fan C.-M., Gaiano N., Ko M.S.H., Zheng Y. 2011. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 334:1706–1710 10.1126/science.1211222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Zheng X., Zheng Y. 2013. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 23:1420–1423 10.1038/cr.2013.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva E., Goldberg M.W., Allen T.D., Akey C.W. 1998. Active nuclear pore complexes in Chironomus: visualization of transporter configurations related to mRNP export. J. Cell Sci. 111:223–236 [DOI] [PubMed] [Google Scholar]
- Kloosterman W.P., Koster J., Molenaar J.J. 2014. Prevalence and clinical implications of chromothripsis in cancer genomes. Curr. Opin. Oncol. 26:64–72 10.1097/CCO.0000000000000038 [DOI] [PubMed] [Google Scholar]
- Klupp B.G., Granzow H., Fuchs W., Keil G.M., Finke S., Mettenleiter T.C. 2007. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc. Natl. Acad. Sci. USA. 104:7241–7246 10.1073/pnas.0701757104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach N.R., Roller R.J. 2010. Significance of host cell kinases in herpes simplex virus type 1 egress and lamin-associated protein disassembly from the nuclear lamina. Virology. 406:127–137 10.1016/j.virol.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre J.-M., Géraud G., Méchali M. 1998. Dynamics of the genome during early Xenopus laevis development: karyomeres as independent units of replication. J. Cell Biol. 142:1159–1166 10.1083/jcb.142.5.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lénárt P., Ellenberg J. 2003. Nuclear envelope dynamics in oocytes: from germinal vesicle breakdown to mitosis. Curr. Opin. Cell Biol. 15:88–95 10.1016/S0955-0674(02)00011-X [DOI] [PubMed] [Google Scholar]
- Lenz-Böhme B., Wismar J., Fuchs S., Reifegerste R., Buchner E., Betz H., Schmitt B. 1997. Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J. Cell Biol. 137:1001–1016 10.1083/jcb.137.5.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F.J., Anderson E. 1968. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J. Cell Biol. 39:339–368 10.1083/jcb.39.2.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Ladinsky M.S., Kirchhausen T. 2011. Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly. J. Cell Biol. 194:425–440 10.1083/jcb.201012063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric M., Shao J., Ryan R.J., Wong C.-S., Gonzalez-Alegre P., Roller R.J. 2011. A functional role for TorsinA in herpes simplex virus 1 nuclear egress. J. Virol. 85:9667–9679 10.1128/JVI.05314-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall M., Feichtinger S., Milbradt J. 2011. Regulatory roles of protein kinases in cytomegalovirus replication. Adv. Virus Res. 80:69–101 10.1016/B978-0-12-385987-7.00004-X [DOI] [PubMed] [Google Scholar]
- Mehlin H., Daneholt B., Skoglund U. 1992. Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell. 69:605–613 10.1016/0092-8674(92)90224-Z [DOI] [PubMed] [Google Scholar]
- Melloy P., Shen S., White E., McIntosh J.R., Rose M.D. 2007. Nuclear fusion during yeast mating occurs by a three-step pathway. J. Cell Biol. 179:659–670 10.1083/jcb.200706151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloy P., Shen S., White E., Rose M.D. 2009. Distinct roles for key karyogamy proteins during yeast nuclear fusion. Mol. Biol. Cell. 20:3773–3782 10.1091/mbc.E09-02-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T.C., Klupp B.G., Granzow H. 2009. Herpesvirus assembly: an update. Virus Res. 143:222–234 10.1016/j.virusres.2009.03.018 [DOI] [PubMed] [Google Scholar]
- Milbradt J., Webel R., Auerochs S., Sticht H., Marschall M. 2010. Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus. J. Biol. Chem. 285:13979–13989 10.1074/jbc.M109.063628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir R.D., Yoon M., Khuon S., Goldman R.D. 2000. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151:1155–1168 10.1083/jcb.151.6.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A., Suliman S., Ben-Yishay R., Yunger S., Brody Y., Shav-Tal Y. 2010. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat. Cell Biol. 12:543–552 10.1038/ncb2056 [DOI] [PubMed] [Google Scholar]
- Mou F., Wills E., Baines J.D. 2009. Phosphorylation of the U(L)31 protein of herpes simplex virus 1 by the U(S)3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J. Virol. 83:5181–5191 10.1128/JVI.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranyi W., Haas J., Wagner M., Krohne G., Koszinowski U.H. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science. 297:854–857 10.1126/science.1071506 [DOI] [PubMed] [Google Scholar]
- Newport J.W., Wilson K.L., Dunphy W.G. 1990. A lamin-independent pathway for nuclear envelope assembly. J. Cell Biol. 111:2247–2259 10.1083/jcb.111.6.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva H., Valle A., Flores L.D., Rivas M.C. 1973. Intranuclear mitochondriae in Hodgkin’s disease. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 12:189–194 [DOI] [PubMed] [Google Scholar]
- Park R., Baines J.D. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80:494–504 10.1128/JVI.80.1.494-504.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsczek F.H., Salina D., Poon K.K.H., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H.Y., Surette M.G., Sugai M., et al. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185:7413–7425 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- Planelles V., Benichou S. 2009. Vpr and its interactions with cellular proteins. Curr. Top. Microbiol. Immunol. 339:177–200 [DOI] [PubMed] [Google Scholar]
- Porwal M., Cohen S., Snoussi K., Popa-Wagner R., Anderson F., Dugot-Senant N., Wodrich H., Dinsart C., Kleinschmidt J.A., Panté N., Kann M. 2013. Parvoviruses cause nuclear envelope breakdown by activating key enzymes of mitosis. PLoS Pathog. 9:e1003671 10.1371/journal.ppat.1003671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokocimer M., Margalit A., Gruenbaum Y. 2006. The nuclear lamina and its proposed roles in tumorigenesis: projection on the hematologic malignancies and future targeted therapy. J. Struct. Biol. 155:351–360 10.1016/j.jsb.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Puckelwartz M.J., Kessler E., Zhang Y., Hodzic D., Randles K.N., Morris G., Earley J.U., Hadhazy M., Holaska J.M., Mewborn S.K., et al. 2009. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum. Mol. Genet. 18:607–620 10.1093/hmg/ddn386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckelwartz M.J., Kessler E.J., Kim G., Dewitt M.M., Zhang Y., Earley J.U., Depreux F.F., Holaska J., Mewborn S.K., Pytel P., McNally E.M. 2010. Nesprin-1 mutations in human and murine cardiomyopathy. J. Mol. Cell. Cardiol. 48:600–608 10.1016/j.yjmcc.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshal M., Kim B., Zhu Y., Nghiem P., Planelles V. 2003. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J. Biol. Chem. 278:25879–25886 10.1074/jbc.M303948200 [DOI] [PubMed] [Google Scholar]
- Rothballer A., Kutay U. 2013a. Poring over pores: nuclear pore complex insertion into the nuclear envelope. Trends Biochem. Sci. 38:292–301 10.1016/j.tibs.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Rothballer A., Kutay U. 2013b. The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma. 122:415–429 10.1007/s00412-013-0417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina D., Bodoor K., Eckley D.M., Schroer T.A., Rattner J.B., Burke B. 2002. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 108:97–107 10.1016/S0092-8674(01)00628-6 [DOI] [PubMed] [Google Scholar]
- Schermelleh L., Carlton P.M., Haase S., Shao L., Winoto L., Kner P., Burke B., Cardoso M.C., Agard D.A., Gustafsson M.G.L., et al. 2008. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 320:1332–1336 10.1126/science.1156947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi T., Pfleghaar K., Kojima S., Pack C.G., Solovei I., Goldman A.E., Adam S.A., Shumaker D.K., Kinjo M., Cremer T., Goldman R.D. 2008. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 22:3409–3421 10.1101/gad.1735208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi T., Butin-Israeli V., Adam S.A., Hamanaka R.B., Goldman A.E., Lucas C.A., Shumaker D.K., Kosak S.T., Chandel N.S., Goldman R.D. 2011. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 25:2579–2593 10.1101/gad.179515.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoyer C.J., Jaspersen S.L. 2014. Breaking down the wall: the nuclear envelope during mitosis. Curr. Opin. Cell Biol. 26:1–9 10.1016/j.ceb.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Speese S.D., Ashley J., Jokhi V., Nunnari J., Barria R., Li Y., Ataman B., Koon A., Chang Y.-T., Li Q., et al. 2012. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 149:832–846 10.1016/j.cell.2012.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole C.W. 1969. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J. Virol. 4:75–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P.J., Greenman C.D., Fu B., Yang F., Bignell G.R., Mudie L.J., Pleasance E.D., Lau K.W., Beare D., Stebbings L.A., et al. 2011. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 144:27–40 10.1016/j.cell.2010.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.L., Roux K.J., Burke B. 2007. Blurring the boundary: the nuclear envelope extends its reach. Science. 318:1408–1412 10.1126/science.1142034 [DOI] [PubMed] [Google Scholar]
- Strambio-De-Castilla C. 2013. Jumping over the fence: RNA nuclear export revisited. Nucleus. 4:95–99 10.4161/nucl.24237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T., Escalante-Alcalde D., Bhatt H., Anver M., Bhat N., Nagashima K., Stewart C.L., Burke B. 1999. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147:913–920 10.1083/jcb.147.5.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllösi M.S., Szöllösi D. 1988. ‘Blebbing’ of the nuclear envelope of mouse zygotes, early embryos and hybrid cells. J. Cell Sci. 91:257–267 [DOI] [PubMed] [Google Scholar]
- Tamiello C., Kamps M.A.F., van den Wijngaard A., Verstraeten V.L.R.M., Baaijens F.P.T., Broers J.L.V., Bouten C.C.V. 2013. Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus. 4:61–73 10.4161/nucl.23388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley E.C., Starr D.A. 2013. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr. Opin. Cell Biol. 25:57–62 10.1016/j.ceb.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L.J., Fields A.P. 1996. betaII protein kinase C is required for the G2/M phase transition of cell cycle. J. Biol. Chem. 271:15045–15053 10.1074/jbc.271.25.15045 [DOI] [PubMed] [Google Scholar]
- Vargas J.D., Hatch E.M., Anderson D.J., Hetzer M.W. 2012. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 3:88–100 10.4161/nucl.18954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes L., Péterfy M., Bergo M.O., Young S.G., Reue K. 2004. Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl. Acad. Sci. USA. 101:10428–10433 10.1073/pnas.0401424101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux C., Auclair M., Dubosclard E., Pouchelet M., Capeau J., Courvalin J.C., Buendia B. 2001. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J. Cell Sci. 114:4459–4468 [DOI] [PubMed] [Google Scholar]
- Wente S.R., Rout M.P. 2010. The nuclear pore complex and nuclear transport. Cold Spring Harb. Perspect. Biol. 2:a000562 10.1101/cshperspect.a000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K.L., Foisner R. 2010. Lamin-binding proteins. Cold Spring Harb. Perspect. Biol. 2:a000554 10.1101/cshperspect.a000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H.J., Ostlund C., Wang Y. 2010. Diseases of the nuclear envelope. Cold Spring Harb. Perspect. Biol. 2:a000760 10.1101/cshperspect.a000760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ydenberg C.A., Rose M.D. 2008. Yeast mating: a model system for studying cell and nuclear fusion. Methods Mol. Biol. 475:3–20 10.1007/978-1-59745-250-2_1 [DOI] [PubMed] [Google Scholar]
- Yipp B.G., Petri B., Salina D., Jenne C.N., Scott B.N.V., Zbytnuik L.D., Pittman K., Asaduzzaman M., Wu K., Meijndert H.C., et al. 2012. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18:1386–1393 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.-Z., Leibowitz M.L., Pellman D. 2013. Chromothripsis and beyond: rapid genome evolution from complex chromosomal rearrangements. Genes Dev. 27:2513–2530 10.1101/gad.229559.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bethmann C., Worth N.F., Davies J.D., Wasner C., Feuer A., Ragnauth C.D., Yi Q., Mellad J.A., Warren D.T., et al. 2007. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16:2816–2833 10.1093/hmg/ddm238 [DOI] [PubMed] [Google Scholar]
- Zink D., Fischer A.H., Nickerson J.A. 2004. Nuclear structure in cancer cells. Nat. Rev. Cancer. 4:677–687 10.1038/nrc1430 [DOI] [PubMed] [Google Scholar]
- Zufferey R., Nagy D., Mandel R.J., Naldini L., Trono D. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871–875 10.1038/nbt0997-871 [DOI] [PubMed] [Google Scholar]