Abstract
Interferon gamma induced GTPase (IGTP) (also named Irgm3) and interferon gamma inducible protein 47 (IRG-47) (also named Irgd) are interferon (IFN)-inducible p47 GTPases that have been shown to regulate host resistance to intracellular pathogens. Little knowledge has been known about the role of p47 GTPases in host responses against extracellular pathogens. To investigate possible roles of IGTP and IRG-47 in the course of Schistosoma japonicum infection, IGTP and IRG-47 knockout and wild-type (WT) mice were challenged with cercariae of S. japonicum, and host responses were analyzed. At the acute stage of S. japonicum infection, mice that lacked IGTP displayed similar parasite burden and pathological damage to WT mice. Importantly, S. japonicum-infected IRG-47-deficient mice, in contrast to IGTP-deficient mice and WT mice, showed significantly reduced worms and lower egg-burden, but intense granulomatous reaction evoked by schistosome eggs in peripheral parts of liver lobes. In addition, upregulation of inflammation-related gene expression was observed in the spleen of IRG-47-deficient mice using oligonucleotide microarrays, in which multiple pathways of cytokine–cytokine receptor interaction, T-cell receptor signaling, complement, coagulation cascades and cell adhesion molecules were highlighted. Taken together, these data suggest that IGTP and IRG-47 might have distinct features that were differentially required for resistance to S. japonicum.
Keywords: acute infection, immunity, p47 GTPase, Schistosoma japonicum
Introduction
Schistosomes are extracellular worms that are a prime example of a complex multicellular pathogen that flourishes in many mammalian hosts including human. In China, Schistosoma japonicum causes more severe diseases along the Yangtze River than any other Schistosoma species. Although great efforts have been made to determine the nature of the immune response to schistosomes, how to deal with such complex organisms under surveillance of host immune system is still uncertain.
It is well recognized that the strongest and most long-lasting vaccine protection induced by the radiation-attenuated cercariae implicated T-cell-mediated immunity involving interferon (IFN)-γ-dependent mechanisms.1 IFN-γ regulates the expression of more than 1200 genes, the products of which presumably involve multiple biological functions of IFN-γ-mediated effects.2, 3 These IFN-γ-regulated proteins act together to form a barrier to control pathogens effectively. A new family of IFN-γ-induced genes has been identified as IFN-inducible p47 GTPases that have a marked impact on host control of infection at both organism level and cellular level.4, 5 These genes encode a series of 47- to 48-kDa guanosine triphosphate-binding proteins.6, 7 Functional studies have concentrated on six mouse p47 GTPase proteins that can be classified into two groups based on sequence homology: group I including LRG-47 (Irgm1),8 interferon gamma-induced GTPase (IGTP) (Irgm3)6, 7 and GTPI (Irgm2),9 and group II including IRG-47 (Irgd),10 TGTP/Mg21 (Irgb6)11, 12, 13 and IIGP (Irga6).9 At present, studies on this GTPase family have mainly been focused on intracellular pathogens. Individual member has various functions: IGTP-deficient mice manifest high susceptibility to a small group of protozoa14 but resistant to all intracellular bacteria examined to date; LRG-47-deficient mice show decreased resistance against all protozoa and intracellular bacteria that have been tested; IRG-47-deficient mice show slightly decreased or normal resistance to all tested protozoa and bacteria. GTPase family members seem to have essential, pathogen-specific roles in resistance to infections.14, 15, 16, 17, 18, 19, 20, 21 Also, GTPase family represents a new IFN-γ-dependent, nitric oxide synthase 2 (NOS2)-independent pathway in the control of pathogen invasion.16, 17
However, little information is available regarding potential functions of this family in the infection of extracellular pathogens. Our previous research, employing microarray and real-time polymerase chain reaction (PCR) to analyze IFN-responsive pathways in mouse model infected with S. japonicum, found that most IFN-inducible p47 GTPases were upregulated in the early infection, and then downregulated more rapidly as well as significantly than IFN-γ per se with the progression of this disease. The expression of certain p47 GTPases also seemed to be negatively correlated with the schistosome burden in our other studies (data not shown). Moreover, each GTPase gene possessed completely different intensities. These results suggested that GTPase family might be involved in IFN-mediated effects on S. japonicum infection, and each member might function distinctly in immune defense.22 In this study, we used gene-knockout mice that lacked expression of IGTP or IRG-47, representatives of the two groups of the GTPase family, to investigate the potential involvement of p47 GTPases in the protective immunity against S. japonicum infection.
Materials and methods
Animal model
IGTP-deficient (IGTP−/−) and IRG-47-deficient (IRG-47−/−) mice14, 18 were kind gifts from Dr Gregory A. Taylor (Duke University, Durham, NC, USA); wild-type (WT) C57BL/6 mice obtained from Model Animal Research Center of Nanjing University (Nanjing, China) were sex- and age-matched (8–12 weeks) to IGTP−/− and IRG-47−/− mice. All mice were maintained and bred under specific pathogen-free conditions. All experiments were performed with the approval of the Animal Ethics Committee of Nanjing Medical University.
Experimental infection and parasite burden
To determine parasite burden in different mouse groups, mice were infected by percutaneous exposure to 40 cercariae from Oncomelania hupensis snails infected with S. japonicum (strain of Chinese mainland). Mice were killed at 6 weeks after infection. Worm burden was assessed by perfusion of the portal system. Weighed liver samples except left front lobes for hematoxylin and eosin (HE) staining from individual mouse were digested in 5% KOH at 37 °C overnight. The released eggs were microscopically counted. Parasite burden was measured by the total number of worms recovered, released eggs in the liver and eggs per pair counted.
Histological examination
At 6 weeks after infection, the left front liver lobes of mice were fixed in 10% buffered formalin and embedded in paraffin. Sections (4 μm) stained with HE were microscopically examined for qualitative and quantitative alterations. Two parameters were used in HE staining: (i) distribution of eggs and granulomas in liver section; and (ii) cellularity in granulomas formation: average cell numbers of eosinophils, lymphocytes and plasma cells were counted within each granuloma with a single egg. At least 10 single-egg granulomas were counted in each section of mice.
Isolation of splenocytes
Mice were killed at 6 weeks after infection, and spleens were aseptically removed. Spleen cells were prepared by gently forcing spleen tissue through a fine nylon net into incomplete RPMI 1640 which contained 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Erythrocytes were lysed with tris-NH4Cl and the remaining cells were washed twice with incomplete RPMI 1640. The cells were resuspended in complete RPMI 1640 containing 10% heat-inactivated fetal calf serum (JRH bioscience, Lenexa, KS, USA), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, to yield a final concentration of 1.0×107 cells/ml. The viability of splenocytes was >95% as assessed by trypan blue dye exclusion.
Cytokine assay
Sera from IGTP−/−, IRG-47−/− and WT mice were collected at 6 weeks after infection. The cytokine levels of sera were examined by Bio-Plex mouse T helper 1/T helper 2 (Th1/Th2) cytokine assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for interleukin (IL)-12p70, IFN-γ, tumor necrosis factor (TNF)-α, granulocyte macrophage colony-stimulating factor (GM-CSF), IL-2, IL-4, IL-5 and IL-10 according to the manufacturer's instruction. Parameters were read on the Bio-Plex suspension array system, and the data were analyzed by Bio-Plex Manager software with five-parameter logistic regression curve fitting.
Microarray hybridization, data mining and analysis
First, total RNA was extracted from each 6-week-S. japonicum-infected mouse splenocytes using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and purified with RNeasy kit (QIAGEN GmbH, Hilden, Germany). Equal amount of total RNA from five mice per group was mixed, and cDNA was generated using the One-Cycle Target Labeling and Control Reagents (Affymetrix, Santa Clara, CA, USA), and cRNA was made with GeneChip IVT Labeling Kit (Affymetrix). Biotin-labeled, fragmented (200 nt or less) cRNA was hybridized for 16 h at 45 °C to Affymetrix Mouse 430 2.0 arrays (Affymetrix) by the microarray facility. The arrays were washed and stained, afterwards read by GeneChip Scanner 3000. The fluorescence signal was excited at 570 nm, and data were collected on a confocal scanner at 3 μm resolution. Data in sorting and analyzing were acquired by GeneSpring GX7 software (Agilent, Santa Clara CA, USA). After normalization and filtering procedure, the system picked up the differentially expressed genes with two folds. These genes were placed into pathways based on Kyoto Encyclopedia of Genes and Genomes database. Significant pathways with differentially expressed genes were identified (P<0.05).
Quantitative real-time PCR
Total RNA was extracted from isolated spleen cells of 6-week-infected mice using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Aliquots of 0.5 μg of total RNA were transcribed to cDNA using a commercially available reverse-transcription kit (TaKaRa Biotechnology, Shiga, Japan) in a 10 μl reaction mix. The amounts of mRNA including ccl2, il12, il10, igtp, lrg47 and irg47 were measured by quantitative real-time PCR (qRT-PCR). The validated primers for all target genes were listed in Table 1. The PCR reaction was carried out in a 20 μl reaction mixture containing 8 μl of cDNA, 10 μl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), and 1 μl of forward primer and reverse primer respectively (Invitrogen). qRT-PCR was performed with a PE Applied Biosystems model 7300 sequence detector with the following procedures: 50 °C for 2 min, 95 °C for 10 min to activate DNA polymerase, and 40 cycles at 95 °C for 15 s and at 60 °C for 1 min. β-actin was used as an internal control. The relative transcription levels of individual target gene were normalized by the internal control. The identity and the purity of the PCR product were confirmed by melting curve analysis. All data were analyzed using the PE Applied Systems Sequence Detector 1.3 software. The threshold cycle number was used to quantify the target gene transcription for each sample using the comparative threshold cycle method. The results represented the expression of the target gene relative to the expression of β-actin.
Table 1. qRT-PCR primes.
| Gene | Primer sequence (5'→3') | Size (bp) | |
|---|---|---|---|
| β-actin | F: 5'-CCT CTA TGC CAA CAC AGT GC-3' | R: 5'-GTA CTC CTG CTT GCT GAT CC-3' | 211 |
| igtp | F: 5'-GAA AGA AGC CGA GGA CCA C-3' | R: 5'-TCT GTC ACC GCC TTA CCA AT-3' | 301 |
| irg47 | F: 5'-GTT AGA ACC AAG GTG GAT AGT GAC-3' | R: 5'-CGA GAT ATT GGG CAA GAG CA-3' | 261 |
| lrg47 | F: 5'-GGA CTG GAA ACT GAG GCT GAT-3' | R: 5'-TGG TGT CCT GGG CAA CTA AG-3' | 137 |
| ccl2 | F: 5'-CCT GCT GTT CAC AGT TGC C-3' | R: 5'-TTG GTT CCG ATC CAG GTT T-3' | 256 |
| il10 | F: 5'-GAC AAC ATA CTG CTA ACC GAC TC-3' | R: 5'-ATC ACT CTT CAC CTG CTC CAC T-3' | 252 |
| il12 | F: 5'-GAA GCA GAC CCT TAC AGA GTG A-3' | R: 5'-GAG ATG AGA TGT GAT GGG AGA AC-3' | 244 |
Statistical analysis
All data are expressed as mean±SD. Statistical analyses of the results of immunological assays were conducted using one-way analysis of variance or Student's t-test. Differences were considered statistically significant where P<0.05.
Results
Differential parasite burden in IGTP−/− and IRG-47−/− mice infected with S. japonicum
To investigate differences of parasite parameters between groups, we measured the number of eggs in the liver and the total amount of worms and pairs recovered from 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice in two independent experiments. No relevant difference in the number of adult worms and liver eggs was found between infected IGTP−/− and WT mice. However, there was a significant decrease in adult worms and egg counts in the livers from infected IRG-47−/− mice compared with the other two groups. Meanwhile, egg production by each parasite pair in IRG-47−/− mice also reduced. Table 2 summarizes the above findings.
Table 2. Parasite parameters in 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice of two independent experiments.
| Experiment No. | Mice groups | N | No. of total worms recovered | No. of pairs recovered | No. of liver eggs except left front lobe (×103) | Eggs/pairs (×103) |
|---|---|---|---|---|---|---|
| 1 | S. japonicum-infected IGTP−/− | 11 | 24.00±6.50 | 11.27±3.57 | 79.18±44.19 | 7.15±3.30 |
| S. japonicum-infected IRG-47−/− | 11 | 18.36±6.93a | 8.18±3.34a | 43.82±24.34b | 6.29±4.11 | |
| S. japonicum-infected WT | 11 | 22.45±5.13 | 10.36±2.77 | 90.00±42.77 | 8.53±3.78 | |
| 2 | S. japonicum-infected IGTP−/− | 10 | 26.00±7.48 | 12.40±3.41 | 98.97±52.22 | 7.92±3.52 |
| S. japonicum-infected IRG-47−/− | 10 | 19.10±3.35c | 8.90±1.91c | 31.53±16.36c | 3.62±1.89c | |
| S. japonicum-infected WT | 10 | 28.40±2.17 | 13.40±0.97 | 114.86±13.65 | 8.49±1.19 |
The data were presented as mean±SD.
Compared to IGTP−/− mice, P<0.05.
Compared to IGTP−/− and WT mice, P<0.05.
Compared to IGTP−/− and WT mice, P<0.01.
Differential distribution of eggs and granulomatous reaction in the liver of S. japonicum-infected IGTP−/− and IRG-47−/− mice
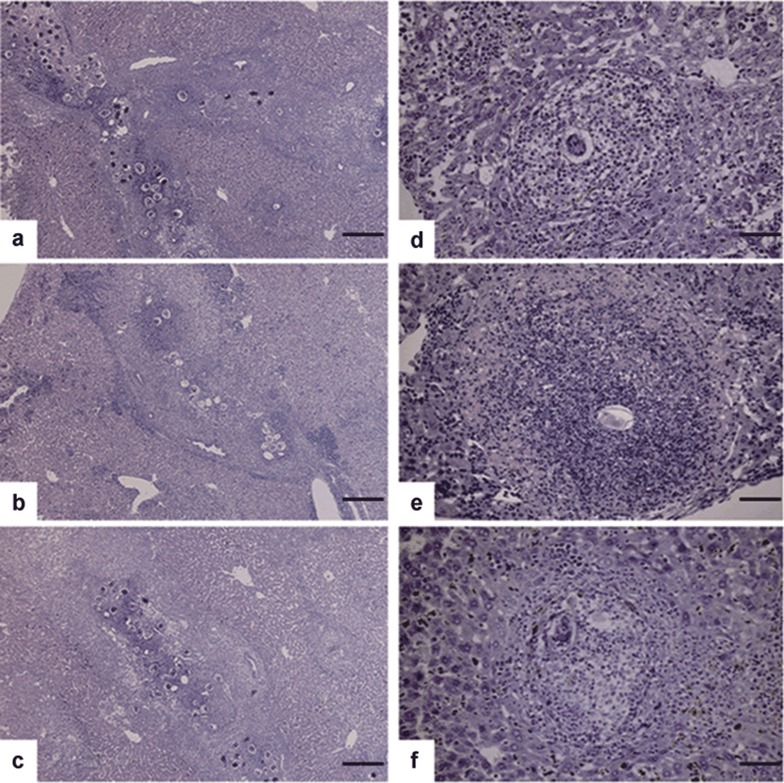
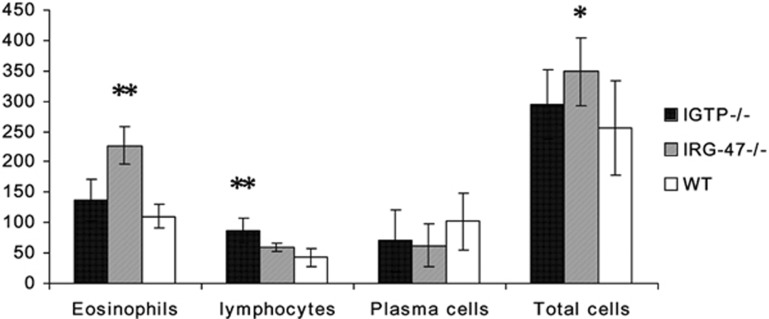
S. japonicum, unlike Schistosoma mansoni, releases eggs in conglomeration in large quantity. Eggs mass around the portal area, leading to presinusoidal obstruction and central deposition of multiple eggs in granulomas. Figure 1 shows a representative image of the liver pathology in the three groups. HE staining revealed that a larger number of eggs concentrated around the portal area in 6-week-S. japonicum-infected IGTP−/− and WT mice (Figure 1a and c), while relatively less eggs in infected IRG-47−/− mice (Figure 1b). There was no significant difference in the number of granulomas containing various numbers of eggs in all liver sections of three mouse stains (data not shown). Importantly, the cellularities of single-egg granulomas among the three groups were significantly different. The average number of eosinophils and total number of cells in each single-egg granuloma in IRG-47−/− mice were much higher than those in IGTP−/− and WT mice (Figure 2). Thus, more intense cellular reaction evoked by schistosome eggs was observed in infected IRG-47−/− mice (Figure 1e) than those in the other two groups (Figure 1d and f).
Figure 1.
Liver pathology with HE staining in 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice, respectively. (a–c) A representative portrait of the liver portal area from IGTP−/−, IRG-47−/− and WT mice at a magnification of ×100 (scale bar=400 μm). (d–f) A representative picture of granuloma with a single egg from IGTP−/−, IRG-47−/− and WT mice at a magnification of ×400 (scale bar=100 μm).
Figure 2.
The cellularity in liver granuloma with a single egg in 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice, respectively. Eosinophils, lymphocytes, plasma cells and the total cells were counted in each granuloma. At least 10 granulomas were counted in each section of mice. Asterisks indicate significant differences (*P<0.05, **P<0.01, compared with WT mice).
Differential Th1/Th2 cytokine levels in serum of IGTP−/− and IRG-47−/− mice in response to S. japonicum infection
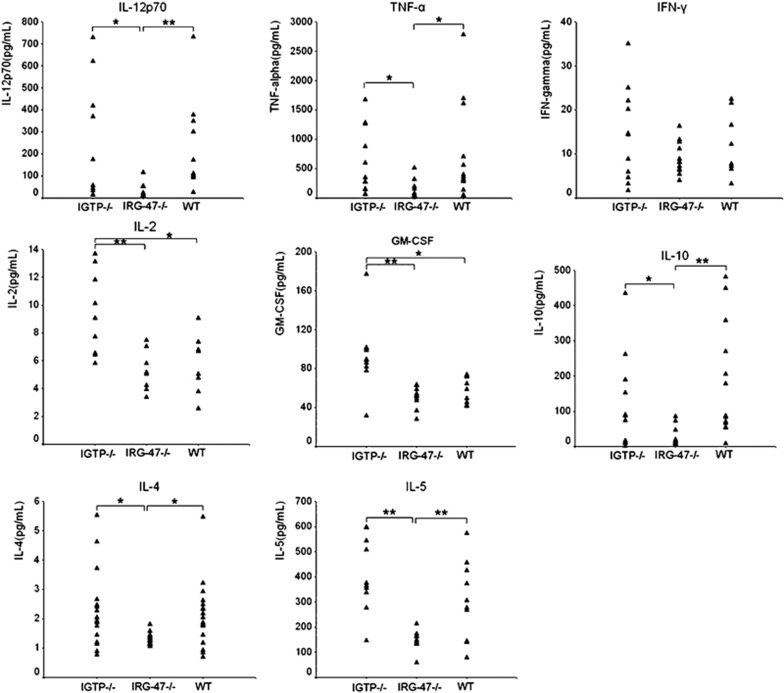
To assess the impact of IGTP- or IRG-47-deficiency on Th1/Th2 immune response, we measured Th1 and Th2 cytokines in 6-week-S. japonicum-infected mouse sera with Bio-Plex technology. For serum detection, most of the cytokine levels in uninfected mice were too low and without significant difference between groups (data not shown). Figure 3 shows Th1/Th2 cytokine profiling in sera from three groups at 6 weeks after infection. Compared with IRG-47−/− mice, S. japonicum-infected IGTP−/− mice produced a high amount of IL-12p70, TNF-α, IL-4, IL-5, and IL-10 as well as WT mice; the GM-CSF and IL-2 productions in IGTP−/− mice were significantly higher than those in WT mice (P<0.05 and P<0.01, respectively). Interestingly, IRG-47−/− mice showed the lowest cytokine levels in sera, whether Th1 or Th2 type such as IL-12p70, IFN-γ, IL-10, IL-4 and IL-2, among three groups.
Figure 3.
The amounts of Th1/Th2 cytokines in the sera from 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice were assayed by Bio-Plex technology. The symbol ‘▴' presents the absolute amount of each sample. Asterisks indicate significant differences (*P<0.05, **P<0.01).
Upregulation of inflammation-related gene expression profile observed in functional classification of microarray in IRG-47−/− mice
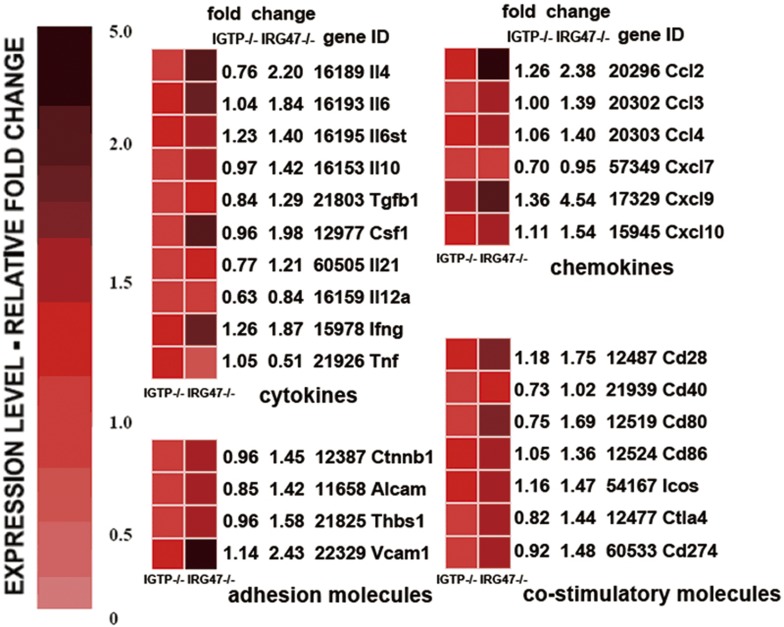
We used high-density oligonucleotide microarrays to compare the changes of functional genes expression in IGTP- or IRG-47-deficient mice with S. japonicum acute infection. To further dissect the underlying molecular events in association with specific gene targeting, we performed a functional classification of the differentially expressed genes that are related to immune response. The most representative families turned out to be that comprising the inflammatory response genes, including cytokines, chemokines, cell adhesion molecules, costimulatory molecules, and so on. Figure 4 summarizes the significantly changed genes in these four families. Interestingly, most genes expressed in IRG-47−/− mice were upregulated markedly, compared with IGTP−/− and WT mice. These genes fell into the following classical categories: cytokines, including il4, il6, il10, fasl, ifng, csf1, and so on; chemokines, including ccl2, ccl3, ccl4, cxcl9, cxcl10, and so on; costimulators, including cd28, cd 80, cd 86, icos, and so on; adhesion molecules, including vascular cell adhesion molecule-1 (vcam-1), activated leukocyte cell adhesion molecule (alcam), and so on. Otherwise, the important cytokine TNF-α was expressed at a lower level in IRG-47-deficient mice, which might be related to decreased egg burden and mild lesion in the liver. Significant candidate genes, chosen from four represented functional families, were validated by qRT-PCR. In addition, NOS2, as expressed in response to proinflammatory cytokines and inducing NO production, showed little difference between three groups.
Figure 4.
Functional classification of 26 selected, differentially expressed genes from microarray data of spleen cells. Genes were divided into immunologically relevant functional families. Each colored box represents the normalized expression level of a given gene in a particular experimental condition and is colored according to the ratio (log2(signal intensity of IGTP−/− or IRG-47−/−)/signal intensity of WT) of a specific gene-expression level in IGTP−/− or IRG-47−/− mice to that in WT mice.
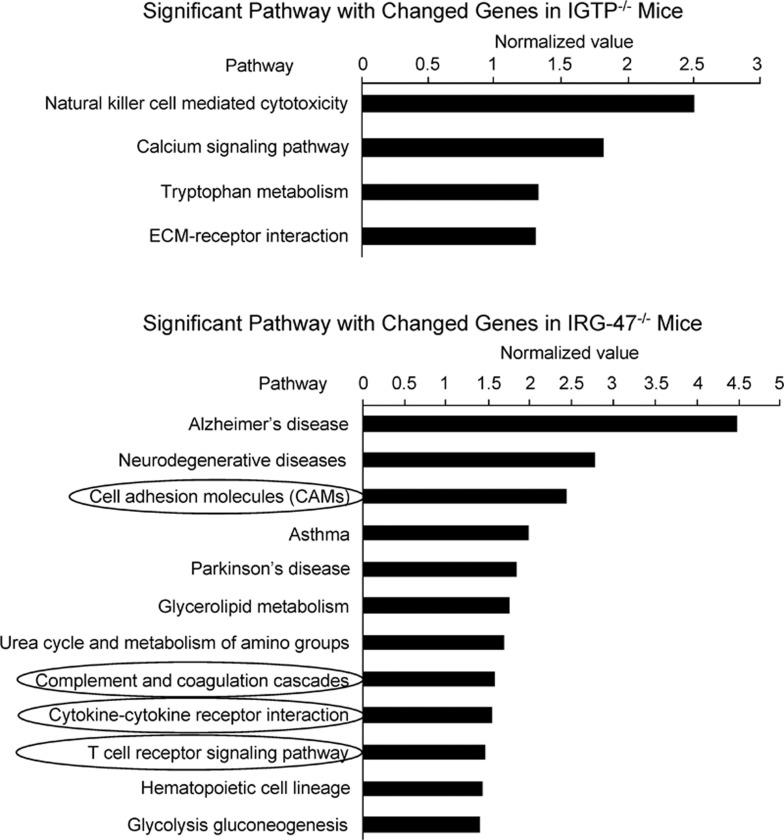
Furthermore, differential gene expression of cytokines, chemokines, cell adhesion molecules and costimulatory molecules in IRG-47-deficient mice significantly influenced the pathways of cytokine–cytokine receptor interaction, T-cell receptor signaling, complement and coagulation cascades and cell adhesion molecules, as shown in Figure 5. Pathway of natural killer cell-mediated cytotoxicity seemed to play a certain role in IGTP−/− mice. Therefore, it was speculated that high expression of inflammatory response genes in IRG-47−/− mice could be related to the decreased parasite burden.
Figure 5.
Significant pathways with differentially changed genes in IGTP−/− and IRG-47−/− mice compared with WT mice. The bioinformatics analysis was carried out using GeneSpring version 7.0 software. Differentially changed genes were placed into Kyoto Encyclopedia of Genes and Genomes pathways database to search the relevant pathways. Significant pathways associated with immune and inflammatory response in IRG-47−/− mice focused on cell adhesion, cytokine–cytokine receptor interaction, T–cell receptor signaling, complement and coagulation.
qRT-PCR-validated microarray data
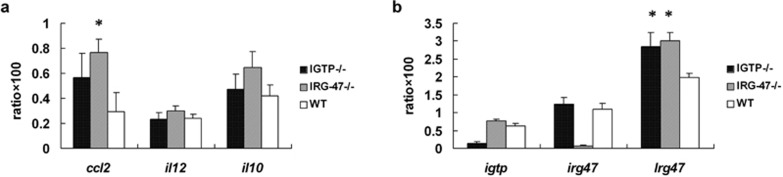
To study the impact of IGTP- or IRG-47-gene deficiency on the expression of other family members of p47 GTPases and also confirm the validity of microarray data, we performed qRT-PCR to identify the mRNA levels of p47 GTPases including igtp, lrg47 and irg47 genes, and some differentially expressed functional genes including ccl2, il12 and il10, in spleen cells from 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice. The relative gene expression of lrg47 was significantly upregulated in both IGTP−/− and IRG-47−/− mice, compared with WT group, while igtp and irg47 were slightly elevated when irg47 or igtp was deficient, but there is no significant difference correspondingly (Figure 6b). These suggested that a certain p47 GTPase deficiency might lead to the increased expression of other members among the same family. qRT-PCR results also showed that the expressions of ccl2, il12 and il10 in IRG-47−/− mice were higher than those in IGTP−/− and WT mice. Especially, ccl2 expression in IRG-47−/− mice presented a significant increase (Figure 6a).
Figure 6.
The relative mRNA levels of ccl2, il12, il10 and three members of p47 GTPase family, igtp, lrg47 and irg47, at 6-week-S. japonicum-infected IGTP−/−, IRG-47−/− and WT mice, were assayed by real-time quantitative PCR. The ratio of target gene to internal control was calculated to represent the relative expression level. (a) The relative mRNA levels of ccl2, il12 and il10 indicated that the expressions of ccl2, il12 and il10 in IRG-47−/− mice were higher than those in IGTP−/− and WT mice. Especially, ccl2 expression in IRG-47−/− mice presented a significant increase (*P<0.05). (b) lrg47 gene was significantly upregulated in both IGTP−/− and IRG-47−/− mice, compared with WT group (*P<0.05).
Discussion
As downstream mediators of IFN-responsive pathway, the p47 GTPases play extensive and profound roles in mediating innate resistance to intracellular pathogens.23, 24 So far, no data have been published showing a role for any p47 GTPase in the host defense against extracellular pathogens. In this study, we employed S. japonicum as a multicellular pathogen model and investigated the different infectious outcomes in IGTP- and IRG-47-deficient mice. IGTP-deficient mice displayed comparable parasite burden, inflammatory reaction in liver and T-cell responses to WT mice during S. japonicum infection. In contrast, IRG-47-deficient mice showed partial resistance to the parasite and alleviated systemic immune responses, displaying reduced parasite burden and suppressed Th1/Th2-cytokine expression in sera compared with IGTP-deficient and WT mice. However, it is worthnoting that more intense granulomatous reaction evoked by schistosome eggs in the periphery of liver was observed in IRG-47-deficient mice. These data indicate that p47 GTPases may play a much broader role in immune responses than previously recognized. They are involved in extracellular infections as well as in intracellular infections.
Different p47 GTPases might make different contributions to the establishment of and/or host resistance to specific infectious agent, also called ‘pathogen-specific resistance'. IGTP could mediate host complete resistance to Toxoplasma gondii, but it did not significantly alter the course of Trypanosoma cruzi infection. During T. gondii infection, IGTP- and LRG-47-deficient mice were killed uniformly and rapidly during the acute phase of the infection; in contrast, IRG-47-deficient mice displayed only partially decreased resistance that was not manifested until the chronic phase. Our study on this extracelluar worm infection also suggested that IGTP and IRG-47 might play distinct roles in resistance to S. japonicum, and IRG-47 could compromise the protective immunity. Unlike intracellular infection in which some p47 GTPases assist in regulating the processing of parasitophorous vacuoles and ultimately driving elimination of the pathogen, in S. japonicum infection, these p47 GTPases might be positioned to exhibit various abilities to dispose or regurgitate exogenous antigens from schistosomes, and involved in handling exocytotic release of soluble lymphocyte cofactors, such as cytokines or chemokines. As liver pathology showed, more inflammatory cells, including eosinophils and lymphocytes, were chemotaxed and accumulated around schistosome eggs to construct relatively bigger granulomas in periphery of the liver in IRG-47−/− mice.
Accordingly, significant upregulation of inflammatory response genes from microarray data was noted in IRG-47-deficient mice, which formed characteristic milieu against schistosomes. These endogenous signals could be grouped into the following three categories: signals that mediated the inflammatory response, including tnf, il6, ifn and various chemokines; signals that functioned as costimulators of T-cell activation, including cd80, cd86, icos, cd28, cd40, and so on; signals that controlled the induction of effector functions and kept in equilibrium, including il4, il10, il12, transforming growth factor-β (tgfb) and ifng. Chemokines here, such as ccl2, ccl3, ccl4, cxcl9, and cxcl10, directed the migration of antigen-specific cells, mainly including T cells, monocytes, neutrophils and eosinophils, along with other effector cells to the site of invading pathogen either by inducing the expression of adhesion molecules on endothelial cells, or by stimulating chemotaxis.
The deficiency of IRG-47 seemed to result in more intense chemotoxicity aiming to specific schistosome antigen. If this phenomenon did occur in schistosomulum stage of early infection, a number of inflammatory cells gathering could lead to destruction of more schistosomula through the damage of tegument or other killing mechanisms, resulting in a reduced number of adult worms and parasite eggs. Thus, the possible mechanism underlying the partial resistance of IRG-47 deficiency might be associated with forming an unfavorable survival environment and cytokine field for S. japonicum.
In addition, NO as an important effector of macrophage-based killing should be considered here. NOS2 is expressed in response to proinflammatory cytokines (such as IFN-γ and TNF-α) and/or microbial products (such as lipopolysaccharide), and results in the prolonged production of a large amount of NO.25, 26 The function of NO in the immune response varies depending on the biological milieu.26, 27, 28, 29 NO may be host-protective, acting as an effector molecule of macrophage cytotoxicity against tumor cells and invading pathogens and as a regulator of inflammatory responses.27, 30 The role of NO during schistosomiasis has been investigated previously and was found to function at early stages of acute schistosomiasis.31 We attempted to determine whether parasite burden caused by the absence of IGTP or IRG-47 was associated with NO-mediated mechanism. Our microarray data showed that there was no significant difference in the expression of NOS2 among IGTP-deficient, IRG-47-deficient and WT mice. Combined with our previous observations that there was no difference in the concentration of NO in the culture supernatant of activated IGTP−/−, IRG-47−/− or WT macrophages, it was indicated that NOS2 activity and NO production were unimpaired in the absence of IGTP and IRG-47. A similar conclusion was drawn from studies of the role of LRG-47 in the microbicidal activity of macrophages for Mycobacterium tuberculosis.5, 32 Therefore, these findings suggest that p47 GTPase and NO function independently in mediating parasite control.
The findings in this study support previous studies indicating that IFN-γ-inducible signaling has pleiotropic effects on immune defense and revealing that p47 GTPases function differentially depending on invading pathogen species. The functions of IGTP and IRG-47 seemed to be different against S. japonicum. More importantly, it argues the protective role of p47 GTPase family in this shared IFN-γ-mediated pathway. Future studies should define the nature and relevant molecular mechanisms of IGTP and IRG-47 in response to S. japonicum infection.
Acknowledgments
We thank Dr Wenbin Huang (Nanjing First Municipal Hospital) for liver pathological analysis. This work was supported by the National Basic Research Program of China (973 Program 2007CB513106) and the National Science Foundation of China (NSFC) (Project No. 30430600 and No. 30872368).
References
- Wilson RA, Coulson PS, Mountford AP. Immune responses to the radiation-attenuated schistosome vaccine: what can we learn from knock-out mice. Immunol Lett. 1999;65:117–123. doi: 10.1016/s0165-2478(98)00134-5. [DOI] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, et al. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194:1123–1140. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruh K, Ahn K, Peterson PA. Inhibition of MHC class I antigen presentation by viral proteins. J Mol Med. 1997;75:18–27. doi: 10.1007/s001090050082. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Feng CG, Sher A. p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol. 2004;4:100–109. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- MacMicking JD. Immune control of phagosomal bacteria by p47 GTPases. Curr Opin Microbiol. 2005;8:74–82. doi: 10.1016/j.mib.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Jeffers M, Largaespada DA, Jenkins NA, Copeland NG, Woude GF. Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon gamma. J Biol Chem. 1996;271:20399–20405. doi: 10.1074/jbc.271.34.20399. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Stauber R, Rulong S, Hudson E, Pei V, Pavlakis GN, et al. The inducibly expressed GTPase localizes to the endoplasmic reticulum, independently of GTP binding. J Biol Chem. 1997;272:10639–10645. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- Sorace JM, Johnson RJ, Howard DL, Drysdale BE. Identification of an endotoxin and IFN-inducible cDNA: possible identification of a novel protein family. J Leukoc Biol. 1995;58:477–484. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- Boehm U, Guethlein L, Klamp T, Ozbek K, Schaub A, Fütterer A, et al. Two families of GTPases dominate the complex cellular response to IFN-gamma. J Immunol. 1998;161:6715–6723. [PubMed] [Google Scholar]
- Gilly M, Wall R. The IRG-47 gene is IFN-gamma induced in B cells and encodes a protein with GTP-binding motifs. J Immunol. 1992;148:3275–3281. [PubMed] [Google Scholar]
- Carlow DA, Marth J, Clark-Lewis I, Teh HS. Isolation of a gene encoding a developmentally regulated T cell-specific protein with a guanine nucleotide triphosphate-binding motif. J Immunol. 1995;154:1724–1734. [PubMed] [Google Scholar]
- Lafuse WP, Brown D, Castle L, Zwilling BS. Cloning and characterization of a novel cDNA that is IFN-gamma-induced in mouse peritoneal macrophages and encodes a putative GTP-binding protein. J Leukoc Biol. 1995;57:477–483. doi: 10.1002/jlb.57.3.477. [DOI] [PubMed] [Google Scholar]
- Carlow DA, Teh SJ, Teh HS. Specific antiviral activity demonstrated by TGTP, a member of a new family of interferon-induced GTPases. J Immunol. 1998;161:2348–2355. [PubMed] [Google Scholar]
- Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Woude GF, et al. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med. 2001;194:181–188. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza AP, Tang B, Tanowitz HB, Factor SM, Shtutin V, Shirani J, et al. Absence of interferon-γ-inducible gene IGTP does not significantly alter the development of chagasic cardiomyopathy in mice infected with Trypanosoma cruzi (Brazil strain) J Parasitol. 2003;89:1237–1239. doi: 10.1645/GE-3185RN. [DOI] [PubMed] [Google Scholar]
- Feng CG, Collazo-Custodio CM, Eckhaus M, Hieny S, Belkaid Y, Elkins K, et al. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol. 2004;172:1163–1168. doi: 10.4049/jimmunol.172.2.1163. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, et al. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci USA. 2000;97:751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol. 2004;25:601–609. doi: 10.1016/j.it.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Bernstein-Hanley I, Coers J, Balsara ZR, Taylor GA, Starnbach MN, Dietrich WF. The p47 GTPases Igtp and Irgb10 map to the Chlamydia trachomatis susceptibility locus Ctrq-3 and mediate cellular resistance in mice. Proc Natl Acad Sci USA. 2006;103:14092–14097. doi: 10.1073/pnas.0603338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga R, Hamano S, Kuwata H, Atarashi K, Ogawa M, Hisaeda H, et al. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J Immunol. 2006;177:7059–7066. doi: 10.4049/jimmunol.177.10.7059. [DOI] [PubMed] [Google Scholar]
- Ji MJ, Su C, Wu HW, Zhu X, Cai XP, Li C, et al. Gene expression profile of CD4+ T cells reveals an interferon signaling suppression associated with progression of experimental Schistosoma japonicum infection. Cell Immunol. 2003;224:55–62. doi: 10.1016/j.cellimm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Feng CG, Sher A. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Taylor GA. IRG proteins: key mediators of interferon-regulated host resistance to intracellular pathogens. Cell Microbiol. 2007;9:1099–1107. doi: 10.1111/j.1462-5822.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee T, et al. Cloning and characteri- zation of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make. J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons CR. The role of nitric oxide in inflammation. Adv Immunol. 1995;60:323–371. doi: 10.1016/s0065-2776(08)60589-1. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. The multiplex function of nitric oxide in (auto)immunity. J Exp Med. 1998;187:1361–1365. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- Brunet LR, Beall M, Dunne DW, Pearce EJ. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol. 1999;163:4976–4984. [PubMed] [Google Scholar]
- Santiago HC, Feng CG, Bafica A, Roffe E, Arantes RM, Cheever A, et al. Mice deficient in LRG-47 display enhanced susceptibility to Trypanosoma cruzi infection associated with defective hemopoiesis and intracellular control of parasite growth. J Immunol. 2005;175:8165–8172. doi: 10.4049/jimmunol.175.12.8165. [DOI] [PubMed] [Google Scholar]