Abstract
The chemokine thymus-expressed chemokine (TECK), which regulates T-cell development and tissue-specific homing, has been identified as a potential contributor to the pathogenesis and progression of endometriosis. Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD), an air pollutant, and estrogen also appear to be involved in endometriosis. Both endometrial stromal cells (ESCs) and the combination of 17β-estradiol and TCDD increase the secretion of TECK in the endometriosis-associated cells and promote the invasiveness of ESCs by increasing expression of matrix metalloproteinase (MMP)-2 and MMP-9. Anti-TECK neutralizing antibodies can effectively inhibit the invasiveness of ESCs and the expression of MMP-2 and MMP-9 in the cells. Interestingly, the expression of chemokine C receptor 9 (CCR9) and its ligand TECK increases significantly in the endometriotic milieu of patients with endometriosis. Therefore, the over-expressed TECK interacts with CCR9 on the ESCs in the endometriotic milieu, which may contribute to the onset and progression of endometriosis.
Keywords: TECK, CCR9, DSC, invasiveness, endometriosis
Introduction
Endometriosis is a very frequent gynecological disorder in fertile women. Although a series of theories on the pathogenesis of endometriosis exists, no single paradigm can explain all cases of endometriosis.1 Sampson's theory of implantation of endometrial cells and fragments refluxed during the menstrual period is generally accepted as a cause of pathogenesis. Retrograde menstruation occurs in up to 80% of women during their reproductive life; the discrepancy between the incidence of this phenomenon and the occurrence might be explained by the presence of further ‘permissive' factors that promote the ectopic implantation and growth of endometrial cells.2
The involvement of chemokines in endometriosis is becoming more evident as current research progresses. Chemokines have the ability to attract and activate inflammatory cells.3 It is increasingly clear that some chemokines play an important role in many physiological and pathological situations, such as ovulation, menstruation, implantation, cervical ripening, pre-term labor and endometriosis.4, 5
Chemokine C receptor 9 (CCR9) is expressed mainly on immature T cells, such as double-positive (DP) T cells or gut-associated T cells,6 which makes CCR9 a valuable receptor for T-cell development and tissue-specific homing. Additionally, DP T cells undergo extensive apoptosis, thereby becoming single-positive T cells. Therefore, it is possible that CCR9-mediated signaling is associated with T-cell apoptosis. Because several studies have shown that chemokine receptor-mediated signaling activates a battery of protein or lipid kinases that are generally thought to be involved in cell survival or proliferation,7, 8, 9 CCR9 engagement and activation by its ligand, thymus-expressed chemokine (TECK), provides anti-apoptotic signaling to the DP T cells.
The endometriotic tissue is composed mainly of the ectopic endometrium, peritoneal mesothelial cells and macrophages, as well as extracellular matrix (ECM). Because the retrograded endometrial stromal cells (ESC) are responsible for the adherence and implantation of endometrium to the peritoneum in the early stage of endometriosis, we used ESCs rather than endometrial epithelial cells as a member of our coculture units to represent the retrograded endometrium.
The initial phase of endometriosis is an invasive event that requires ECM breakdown.10, 11 It has been clearly shown that besides being responsible for the mass degradation of the ECM as bulldozers, matrix metalloproteinases (MMPs) play sophisticated roles in modulating normal cellular behavior and cell–cell communication, among which is the action of MMPs on cell growth through regulating several growth factors.12 Several studies have shown an increase in the expression of MMP-1, MMP-2, MMP-3, MMP-7 and MMP-9 in endometriotic tissues. Alteration of MMP-9 and MMP-2 is an important factor in the development of endometriosis.13, 14, 15, 16, 17, 18
Recent studies have suggested that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and dioxin-like compounds are important in the development of endometriosis. TCDD is a prototype of the polyhalogenated aromatic hydrocarbons, a family of chemicals that have a common mechanism of action.19 Since endometriosis is an estrogen-dependent disease20, 21, 22, 23 and the inflammatory milieu in the peritoneal cavity with endometriosis has been extensively characterized, altered metabolism of estradiol by TCDD or other dioxin-like halogenated aromatic hydrocarbons and proinflammatory effects of TCDD may be involved in the pathogenesis of endometriosis. Our previous work has shown that the combination of 17β-estradiol with TCDD upregulates CXC chemokine receptor 1 expression in ESCs and promotes secretion of IL-8, a ligand of CXC chemokine receptor 1, in coculture of ESC–human peritoneal mesothelial cells (HPMCs).24 We have also observed that the combination of 17β-estradiol with TCDD increases the secretion of regulated on activation, normal T cell expressed and secreted (RANTES) and macrophage inflammatory protein (MIP)-1α and promotes the invasiveness of ESCs.25
In the present study, we first investigated the effects of 17β-estradiol and TCDD on secretion of TECK in the endometriosis-associated cells in the coculture unit and then studied the expression of MMP-2 and MMP-9 and the invasiveness of ESCs. To better understand the role of TECK and its receptor CCR9 in the progression of endometriosis, we investigated the regulation of TECK and CCR9 expression and the underlying mechanisms of their potential proinvasion activity by ESCs.
Materials and methods
Tissue collection and cell culture
All eutopic endometrial tissues were obtained from patients with endometriosis (mean age 40.2 years; range 31–44) at the time of laparoscopy by uterine curettage in the Obstetrics and Gynecology Hospital of Fudan University (Shanghai, China). The endometriotic tissues included peritoneal implants and ovarian endometriomas. The patients were classified according to the revised American Fertility Society classification and had not received any gonadotrophin-releasing hormone analogue or other hormonal drug in the 6 months prior to the surgical procedure. Before surgery, informed consent was obtained from each patient using protocols approved by the Human Investigation Committee of Fudan University. All the samples were obtained in the proliferative phase of the cycle, which was confirmed histologically. The eutopic tissues were collected under sterile conditions and transported to the laboratory on ice in a 1:1 formula of Dulbecco's modified Eagle's medium (DMEM)/F-12 (Gibco, Grand Island, NY, USA) with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). The minced eutopic endometrium was digested with collagenase type-α (0.1% Sigma, Ronkonkoma, NY, USA) for 30 min at 37 °C with constant agitation. The tissue pieces were filtered through a 200 µmol/l wire sieve to remove debris. Following gentle centrifugation, the supernatant was discarded, and the cells were resuspended in DMEM/F-12. The ESCs were separated from epithelial cells by passing through a 400 µmol/l wire sieve. The filtrated suspension was layered over Ficoll and centrifuged at 2000 r.p.m. for 20 min to further remove leukocytes and erythrocytes. The middle layer of cells was collected and washed with D-Hanks and then placed in a culture flask and allowed to adhere for 20 min. The adherent stromal cells were cultured as monolayers in flasks with DMEM/F-12 containing 10% FCS, 20 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 100 IU/ml penicillin and 100 µg/ml streptomycin and incubated in 5% CO2 at 37 °C. This method supplied a 95% purity of ESCs.
HMrSV5 (HPMC, a human peritoneal mesothelial cell line provided by Professor Jian Yao, the First People's Hospital, Shanghai, China) and the human monocyte U937 cell line (purchased from Bank of Cells, Chinese Academy of Sciences, Shanghai, China) were maintained in DMEM (Gibco) with 10% FCS and RPMI 1640 medium (Life Technologies, Carlsbad, California, USA) with 10% bovine calf serum, respectively, containing 20 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 100 IU/ml penicillin and 100 µg/ml streptomycin at 37 °C in a humidified, CO2-controlled (5%) incubator. The medium was changed every other day.
Cell coculture unit, direct coculture of two types of cells
ESCs or HPMCs were cultured in 24-well plates at a concentration of 1×105 cells per well until adhered to the plastic. The medium was removed, and ESC or U937 cells were applied over ESCs or HPMCs, respectively, at the same concentration. The cells were cultured in a final volume of 1 ml fresh DMEM with 2.5% FCS for 48 h. HPMC, ESC and U937 cells cultured alone in the same media were used as controls. Each experiment was carried out in triplicate wells per time and repeated three times.
Direct coculture of three types of cells (U937–ESC–HPMC)
HPMCs were cultured in 24-well plates at a concentration of 1×105 cells per well until adhered to the plastic. The medium was removed, and ESCs were applied over HPMCs at the same concentration. After the ESCs adhered to the plastic and fused with HPMCs, the medium was removed again. U937 cells were applied over ESCs and HPMCs at the same concentration. The cells were cultured in a final volume of 1 ml fresh DMEM with 2.5% FCS for 48 h. Each experiment was carried out in triplicate wells per time and repeated three times.
Indirect coculture of three types of cells
ESC, HPMC and U937 cells at 1×105 cells per well were plated in the lower or upper compartment of Costar transwell cell culture chamber inserts (0.4 µm, 12 mm diameter). There were three models, which were determined by different combinations of these three cells. In the first model, U937/HPMC–ESC, the U937 cells were plated in the upper compartment, and the HPMCs and ESCs were in the lower compartment. In the second model, HPMC/ESC–U937, HPMCs were in the upper compartment, and ESC and U937 cells were in the lower compartment. In the third model, ESC/HPMC–U937, ESCs were in the upper compartment, and HPMC and U937 cells were in the lower compartment. Each experiment was carried out in triplicate wells per time and repeated three times.
Treatment in vitro by TCDD and 17β-estradiol
After serum starvation for 12 h, ESC, HPMC and U937 cells (1×105cells per well) were treated with 1×10–8 mol/l 17β-estradiol (Sigma), 1 nmol/l TCDD (Sigma), or the combination of 17β-estradiol with TCDD for 48 h with vehicle (dimethylsulphoxide) as a control. Each experiment was carried out in triplicate wells per time and repeated three times.
Enzyme-linked immunosorbent assay (ELISA) for determination of TECK
The culture supernatants were harvested, centrifuged to remove cellular debris, and stored at −80 °C until assay by ELISA. Each experiment was carried out in triplicate and repeated three times. The TECK concentration in the culture supernatant was quantified by ELISA kits (R&D Systems, Abingdon, UK) according to the manufacturer's instructions. The limit of detection of TECK was <10 pg/ml.
Matrigel invasion assay for ESCs
The invasion of ESCs across matrigel was evaluated objectively in invasion chambers with 6.4-mm diameter and 8-µm pore size (Corning, Corning, NY, USA). Invasion chambers coated with 6 µl pure matrigel were placed in a 24-well plate. The purified ESCs (2×105 in 200 µl DMEM with 1% FCS) were plated in the upper chamber. There were two groups in terms of the different cells in the lower compartment. In the first group, there were no cells in the lower compartment, and 1×10−8 mol/l 17β-estradiol, 1 nmol/l TCDD, or a combination of 17β-estradiol with TCDD or combined with 2.5 µg/ml anti-TECK neutralizing antibody (R&D Systems) was added to both upper compartments and lower compartments, respectively. The second group was a coculture of HPMC and U937 cells in the lower compartment. 17β-estradiol (1×10−8 mol/l), 1 nmol/l TCDD, or a combination of 17β-estradiol with TCDD or anti-TECK neutralizing antibody was added to both the upper and lower compartments. The cells were then incubated at 37 °C for 48 h.
The inserts were removed and washed in phosphate-buffered saline (PBS), and non-invasive cells together with the matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10 min at room temperature and stained with hematoxylin. The result was observed under an Olympus BX51+DP70 fluorescence microscope (Olympus, Tokyo, Japan). The cells that migrated to the lower surface were counted in five predetermined fields at a magnification of ×200. Each experiment was carried out in triplicate wells per time and repeated three times.
In-cell Western
According to the description by Egorina et al.,26 we used a newly established assay called in-cell Western to determine the in-cell protein level of MMP-9 and MMP-2. The procedure was as follows. Freshly isolated endometriotic stromal cells (2×104 cells per well) were seeded into 96-well plates. After incubation for 24 h, the cells were starved in DMEM-F12 supplemented with 1% fetal bovine serum for 12 h. Then, these cells were incubated with 17β-estrogen (1×10−8 mol/l), TCDD (1 nmol/l) or 17β-estrogen (1×10−8 mol/l) combined with TCDD (1 nmol/l) for another 48 h. The cells were immediately fixed with 4% formaldehyde in 1× PBS for 20 min at room temperature. After being washed with 0.1% Triton, the cells were blocked by adding 100 ml of LI-COR Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) for 90 min at room temperature. The cells were then incubated with mouse antihuman MMP-9 or MMP-2 (R&D Systems) primary antibody. Actin was used as the housekeeping protein, and rabbit antihuman actin (Santa Cruz, Santa Cruz, CA, USA) was added to each well at the same time. After treatment overnight at 48 °C, the wells were then incubated with corresponding secondary IRDyeTM700DX-conjugated affinity-purified (red fluorescence) antimouse or IRDyeTM800DX-conjugated affinity-purified (green fluorescence) antirabbit fluorescent antibody, as recommended by the manufacturer (Rockland, Inc., Gilbertsville, PA, USA). This procedure was performed in the dark to avoid exposure to light. Images of MMP-9 and MMP-2 were obtained using the Odyssey Infrared Imaging System (LI-COR Biosciences GmbH, Bad Homburg, Germany). The protein level of MMP was calculated as the ratio of the intensity of MMP-9 or MMP-2 to that of actin. The experiments were carried out in triplicate wells per time and repeated three times.
Immunostaining
For immunohistochemistry, paraffin sections (5 µm) of human endometrium were dehydrated in Tris-buffered saline (TBS) and incubated with hydrogen peroxide and 1% bovine serum albumin (BSA)/TBS to block endogenous peroxidase. Then, the samples were incubated with mouse antihuman CCR9 antibody (25 µg/ml, MAB179, R&D Systems) or mouse immunoglobulin G (IgG) as the isotype control overnight at 4 °C in a humidified chamber. After being washed three times with TBS, the sections were overlaid with peroxidase-conjugated goat antimouse IgG (04-18-06, KPL, Gaithersburg, MD, USA) and rabbit antigoat (14-13-06, KPL), and the reaction was developed with 3,3-diaminobenzidine and counterstained with hematoxylin. The immunohistochemical results were evaluated by a pathologist. The experiments were repeated five times.
For immunocytochemical staining, ESCs growing on coverslips were cultured for 48 h. The coverslips were fixed in 4% paraformaldehyde for 20 min at room temperature, washed in PBS and permeabilized for 10 min with 0.25% Triton-100 in PBS. The cells were then incubated with 1% BSA in PBS/Tween for 30 min to block non-specific binding of antibodies. The primary antibodies diluted in PBS/Tween containing 1% BSA were added. Mouse antihuman vimentin monoclonal antibody (1:100, ZA0511, Dingguo, Beijing, China) and cytokeratin-7 antibody (1:100, 18-0234, Zymed Laboratories, South San Francisco, CA, USA) were used as markers for ESCs. The antihuman CCR9 monoclonal antibody was used to detect whether ESCs expressed CCR9. The cells were incubated with primary antibody or isotypic control overnight at 4 °C and then incubated with a peroxidase-conjugated secondary antibody for 60 min at 37 °C. The slides were stained with 3,3-diaminobenzidine and counterstained with hematoxylin. The experiments were repeated five times.
Results
CCR9 and its ligand TECK are upregulated in the endometriotic milieu of patients with endometriosis
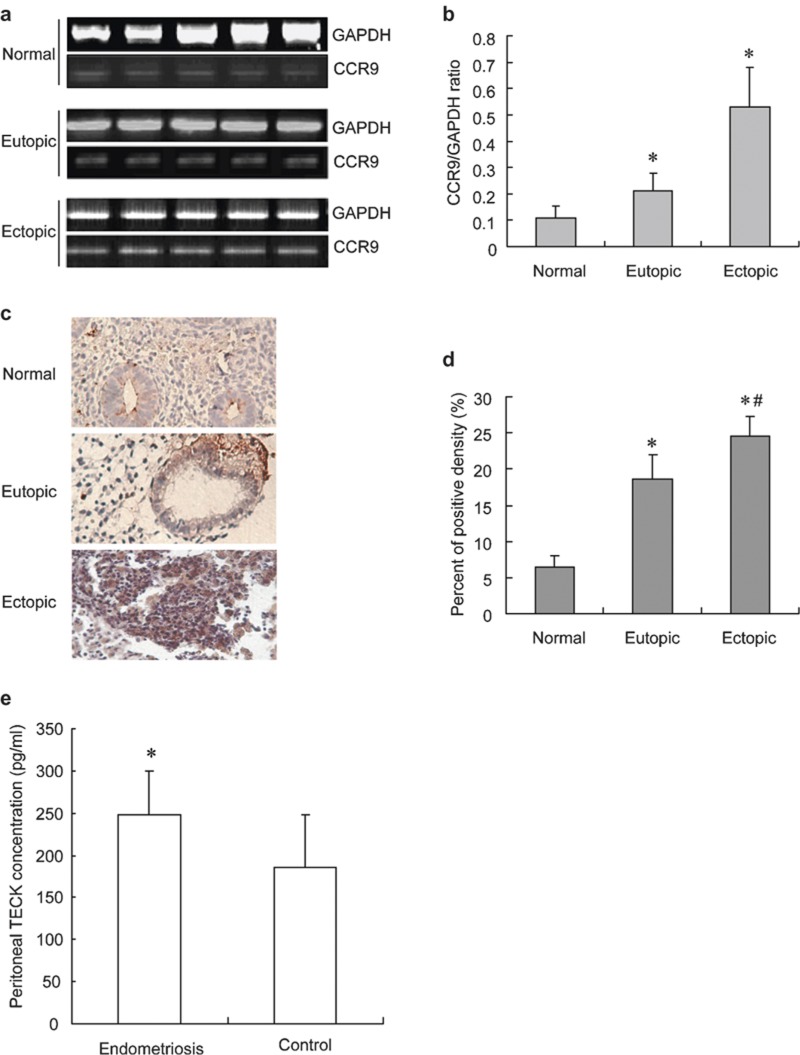
CCR9 transcription in the endometrium was detected by reverse transcription polymerase chain reaction (Figure 1), and the results showed that transcription of CCR9 in the ectopic endometrial stromal cells was significantly higher than that of the eutopic endometrial stromal cells, and the latter was further significantly higher than that of the endometrial stromal cells from the normal fertile phase (P<0.05; Figure 1a).
Figure 1.
The transcription and translation of CCR9 in the normal eutopic endometrium and ectopic tissue of endometriosis. Reverse transcription polymerase chain reaction analysis of CCR9 in the normal eutopic and ectopic tissue (a). Statistical results from eight separate experiments (b). Immunohistochemistry on normal endometrium, eutopic endometrium and ectopic tissue of endometriosis (c). Statistical results of immunohistochemistry from normal eutopic endometrium and ectopic tissue of endometriosis (d). TECK concentration in peritoneal fluid with endometriosis (e). Data are expressed as mean±SD. *P<0.05 compared to the normal control, #P<0.05 compared to the eutopic tissue, *P<0.01 compared to the control. CCR, chemokine C receptor; TECK, thymus-expressed chemokine.
We further analyzed CCR9 protein expression in the endometrium by immunohistochemistry. The results showed positive staining for CCR9 in the stroma and gland of the eutopic endometrium, and CCR9 expression in the ectopic foci was higher significantly than that of the eutopic endometrium, while the latter was significantly higher than that of normal endometrium of the fertile phase (P<0.05; Figure 1c and d). The result by ELISA showed that the TECK concentration was significantly increased in the peritoneal fluid with endometriosis compared to the normal control without endometriosis (P<0.05; Figure 1e).
Effect of 17β-estradiol and/or TCDD on CCR9 expression on eutopic ESCs
We evaluated the expression of cytokeratin and vimentin in primary ESCs by immunocytochemistry. The isolated cells stained positive for vimentin and negative for cytokeratin. The purity of isolated ESCs was >95%.
The expression of CCR9 in the eutopic ESCs as determined by flow cytometry was about 65.11±3.4% (Figure 2). At 100 nM, 17β-estradiol significantly inhibited CCR9 expression on the ESCs (P<0.01), but other concentrations of 17β-estradiol showed no effect on CCR9 expression. The 0.01 nM TCDD alone also significantly inhibited CCR9 expression on the ESCs (P<0.01), and 0.1, 1 and 10 nM TCDD showed no effect on CCR9 expression, but 100 nM TCDD upregulated the molecular expression on the cells. The combination of 1–100 nM 17β-estradiol with TCDD increased CCR9 expression on the ESCs, and the increased level was positively correlated with the concentration of 17β-estradiol (P<0.05; Figure 2).
Figure 2.
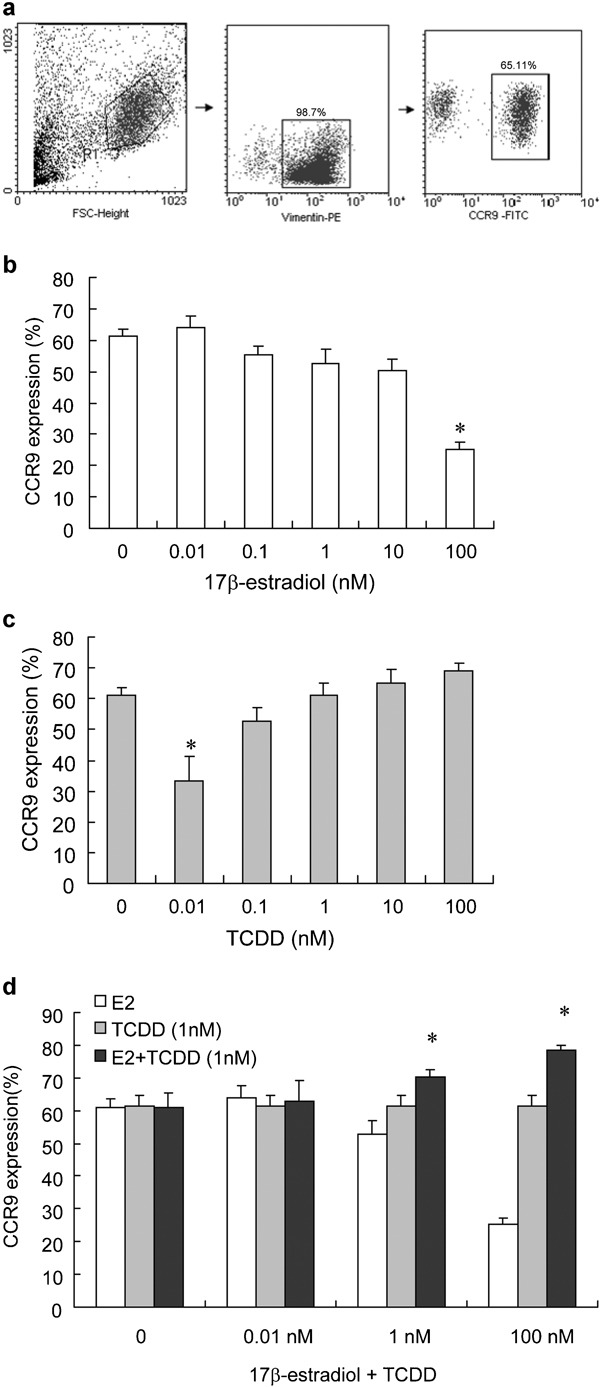
Effect of 17β-estradiol and/or TCDD on CCR9 expression on ESCs. CCR9 expression on ESCs (a). The primary ESC was treated with various concentrations of 17β-estradiol (0.01–100 nM) (b), and TCDD (0.01–100 nM) (c) alone or in combination (d) for 48 h. At the end of the culture period, the cells were digested and analyzed for CCR9 expression on ESCs by flow cytometry. Data are expressed as mean±SEM. *P<0.05 compared to the control. CCR, chemokine C receptor; ESC, endometriotic stromal cell; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Establishment of the coculture units
We have established a series of cell–cell cocultures to elucidate the roles of the endometriosis-associated cells in the endometriotic milieu. Immunocytochemistry showed that HPMCs expressed both vimentin and cytokeratin, and ESCs expressed vimentin, but did not express cytokeratin in the cocultures. The purity of isolated ESCs was >95% (Figure 3).
Figure 3.
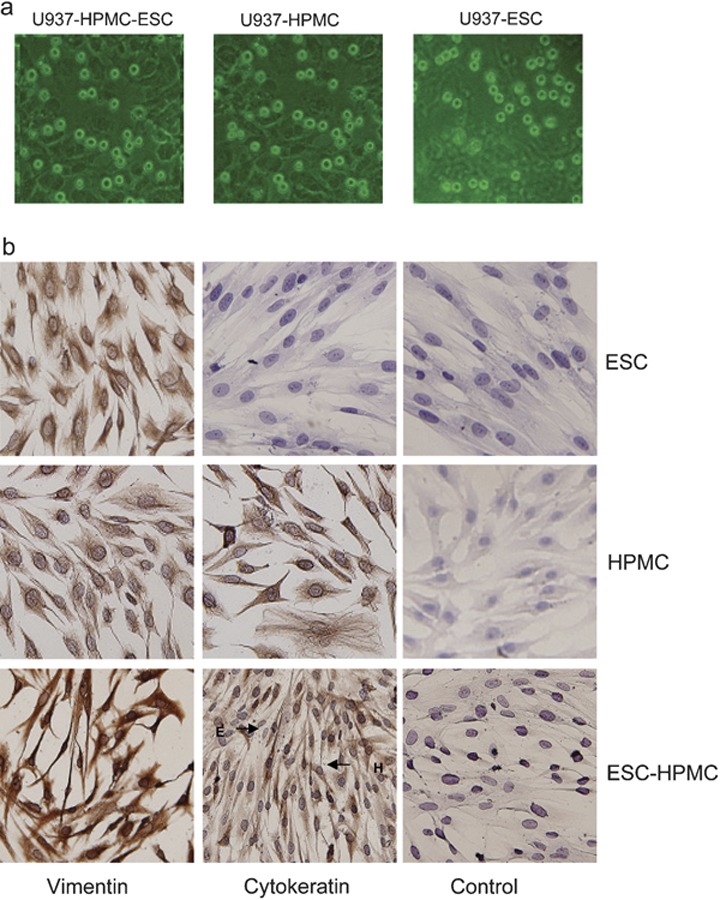
Cells morphous and immunocytochemical characterization of the co-culture units. (a) Cells morphous of co-culture units U937-HPMC-ESC, HPMC and ESC-HPMC were observed by using inverted microscope (×100). (b) Immunocytochemistry shows that HPMCs express both vimentin and cytokeratin, and ESCs express vimentin but do not express cytokeratin. The HPMCs are stained by anti-vimentin mAb and anti-cytokeratin mAb with isotypic control. The ESC-HPMC co-culture is stained by anti-vimentin mAb, anti-cytokeratin mAb and isotypic control (×200). ESC, endometriotic stromal cell; HPMC, human peritoneal mesothelial cell; mAb, monoclonal antibodies.
Effect of direct or indirect coculture on TECK secretion of ESC, HPMC and U937 cells
As shown in Figure 4a, secretion of TECK by ESCs after 48 h was higher than that of either U937 cells or HPMCs. The coculture of ESCs with HPMCs showed no effect on TECK secretion, whereas the coculture of ESC with U937 cells increased TECK production, and the production in the indirect and direct cocultures were 1.2- and 2.82-fold more, respectively, than the production of ESCs cultured alone (P<0.05). The coculture of HPMCs with U937s also showed no effect on TECK secretion. Both the indirect and direct cocultures of the three types of cells showed significant increases in TECK production. Among the three coculture units, the TECK production of the ESC–U937/HPMC coculture was 2.77- and 2.41-fold higher than that of ESC/HPMC–U937 and U937/ESC–HPMC, respectively (P<0.01). The TECK production of the ESC–U937–HPMC coculture was 4.87-fold higher than the production of ESCs cultured alone (P<0.01). The results above showed that direct coculture of the endometriosis-associated cells promoted the secretion of TECK.
Figure 4.
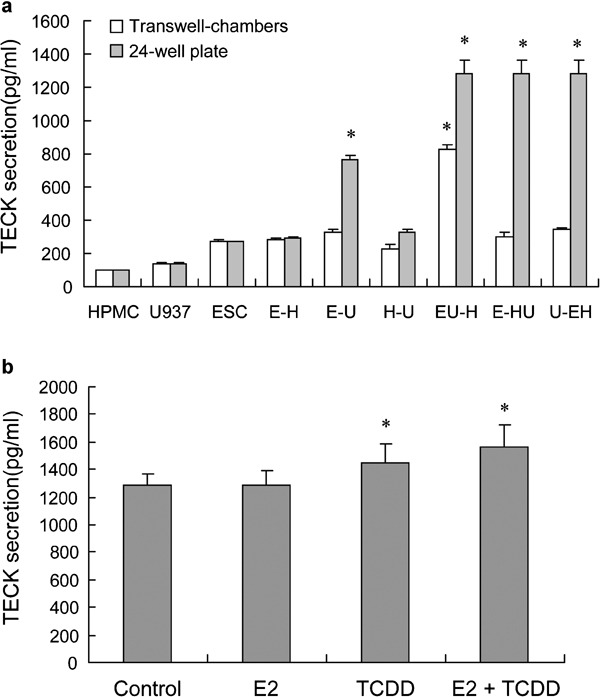
The combination of 17β-estradiol with TCDD enhances secretion of TECK in the endometriotic milieu. The endometriotic cells were cultured or cocultured (a), and the ESC–HPMC–U937 coculture was treated with 17β-estradiol (1 nM), TCDD (1 nM) or 17β-estradiol and TCDD in combination for 48 h (b). At the end of the culture, the supernatant was collected and analyzed for TECK secretion by enzyme-linked immunosorbent assay. Data are expressed as mean±SEM and *P<0.01 compared to the control. ESC, endometriotic stromal cell; HPMC, human peritoneal mesothelial cell; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TECK, thymus-expressed chemokine.
Effect of 17β-estradiol and/or TCDD on TECK secretion in the coculture of the endometriosis-associated cells
Compared to the control, 1 nM TCDD significantly stimulated TECK secretion in the coculture of ESCs with HPMCs, and 1 nM 17β-estradiol had no obvious effect on TECK production in the culture. However, when combined with TCDD, 17β-estradiol showed a synergistic stimulatory effect on TECK secretion (Figure 4b). In our previous work, neither estradiol nor TCDD had an effect on TECK in the non-coculture system.
The combination of 17β-estradiol with TCDD promotes invasiveness of ESCs with increasing secretion of TECK
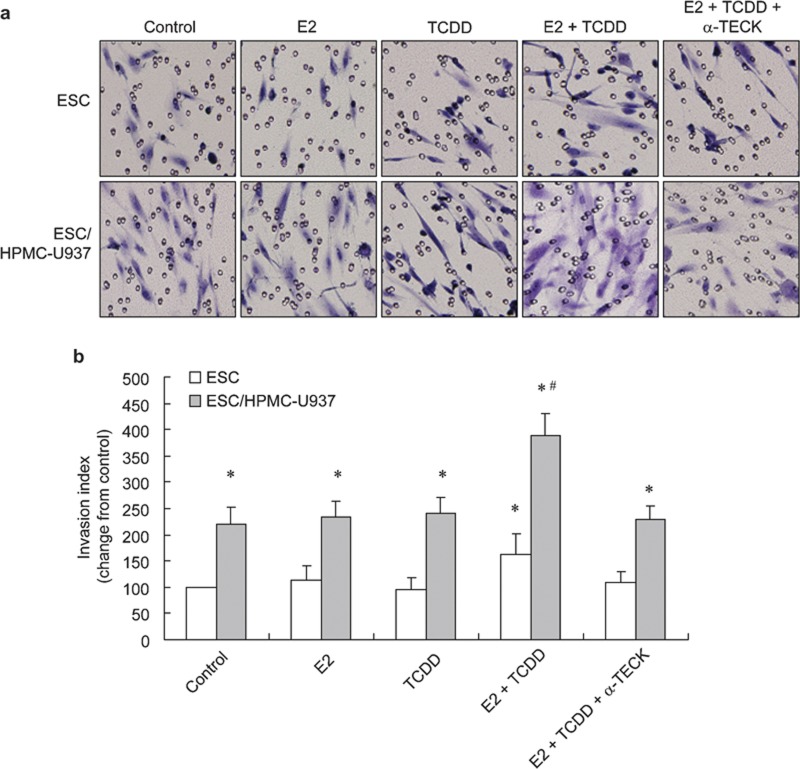
To verify the effects of 17β-estradiol and TCDD on the invasiveness of ESCs, a matrigel-based transwell assay was carried out in two groups. The freshly isolated ESCs were added to the upper chamber in both groups. No cells were added to the lower chamber in the first group. In the second group, HPMC and U937 cells were added to the lower chamber and then treated with estradiol, TCDD or the combination of estradiol with TCDD. The number of cells migrating to the lower surface was counted after 48 h of incubation. As shown in Figure 5, neither 17β-estradiol nor TCDD alone could increase the invasion of human ESCs, but the combination of 17β-estradiol with TCDD promoted invasiveness of ESCs.
Figure 5.
The combination of 17β-estradiol with TCDD enhances invasiveness of ESCs in the ESCs cultured alone or ESC/HPMC–U937 coculture through increasing secretion of TECK. The invasive index of cells under different conditions was normalized to the control. The invasion of ESCs in the ESCs cultured alone or in the ESC/HPMC–U937 coculture through the matrigel-coated membranes was assessed by microscopic morphology at ×200 magnification. The ESCs were incubated in the presence of 1×10−8 mol/l 17β-estradiol, 1 nmol/l TCDD, 17β-estradiol and TCDD in combination or 10 µg/ml anti-TECK neutralizing antibody combined with 17β-estradiol and TCDD (×200). The results of the invasive index show that estradiol and TCDD in combination improved invasion of ESCs in the coculture, and anti-TECK neutralizing antibody abolished the elevated invasion of ESCs (a). Statistical results from above (b). Data are expressed as mean±SEM. #P<0.01 compared to the coculture. ESC, endometriotic stromal cell; HPMC, human peritoneal mesothelial cell; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TECK, thymus-expressed chemokine.
We further investigated the invasion of ESCs after treatment with anti-TECK neutralizing antibody. The anti-TECK neutralizing antibody could decrease, but not abolish completely the increased invasiveness of ESCs induced by the combination of 17β-estradiol and TCDD (Figure 5). It was concluded that the 17β-estradiol combined with TCDD stimulates the invasiveness of human ESCs partly via increasing the TECK secretion.
The combination of 17β-estradiol with TCDD and coculture of ESC, HPMC and U937 cells upregulates protein production of MMP-9 and MMP-2 in ESCs
As shown in Figure 6, the protein productions of both MMP-9 and MMP-2 in ESCs and the ESC–HPMC–U937 coculture were increased significantly after being treated with the combination of 17β-estradiol and TCDD. Recombinant human TECK significantly increased the protein translation of MMP-9 and MMP-2 in the ESCs, and the anti-TECK neutralizing antibody completely abolished the translation increase, which suggested that the combination of 17β-estradiol and TCDD may promote MMP-9 and MMP-2 protein expression in ESCs via stimulating TECK secretion.
Figure 6.
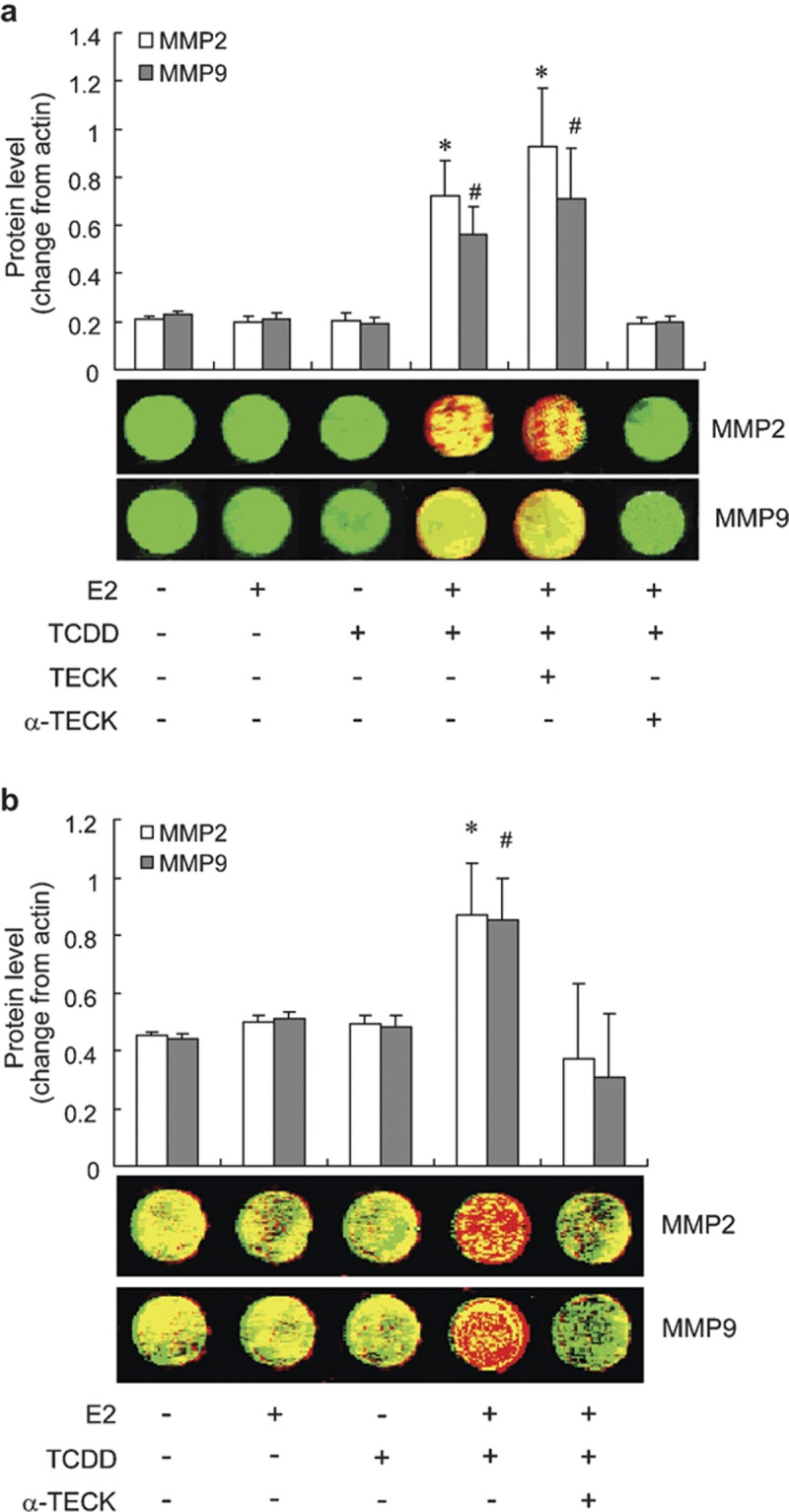
The combination of 17β-estradiol with TCDD upregulates protein expression of MMP-9 and MMP-2 in the endometrial stromal cells and the ESC–HPMC–U937 cells through promoting TECK secretion. The endometrial stromal cells (a) or the ESC–HPMC–U937 cells (b) plated on 96-well plates were starved with 1% fetal bovine serum for 12 h then treated with vehicle, 1 nmol/l 17β-estradiol, 1 nmol/l TCDD, 17β-estradiol and TCDD in combination, or 10 µg/ml anti-TECK neutralizing antibody combined with 17β-estradiol and TCDD for 48 h. *#P<0.01 compared to the control. ESC, endometriotic stromal cell; HPMC, human peritoneal mesothelial cell; MMP, matrix metalloproteinase; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TECK, thymus-expressed chemokine.
Discussion
CCR9 has been found to be expressed mainly on T and B lymphocytes and is a unique receptor for the CC chemokine TECK, which is primarily expressed on thymic dendritic cells and mucosal epithelial cells. In the present study, we have provided evidence that CCR9 is transcribed and translated at significantly higher levels in the ectopic tissue than in the eutopic endometrium, and TECK concentration in peritoneal fluid with endometriosis is higher than the control, which was also proven in the in vitro coculture of the endometriosis-associated cells.
TECK plays a key role in the segregation and compartmentalization of the mucosal immune system through recruitment of immune cells to specific locations.27 CCR9 mediates chemotaxis in response to CCL25, i.e., TECK,28, 29, 30, 31 and is expressed on a minor subset of CD8+ lymph node T cells.32, 33 CD69+ thymocytes enhance the CCL25-induced migration compared with CD69− thymocytes, and thymocyte migration in response to CCL25 is augmented by TCR signaling. Approximately half of all γδ TCR+ thymocytes and peripheral γδ T cells express CCR9, and these cells migrate upon exposure to CCL25. The expression of CCR9 on γδ T cell subsets (e.g., Vγ2+, but not Vγ3+) indicates that CCR9 may also function in the development and/or trafficking of γδ T cells. Finally, pre-pro-B cells in the bone marrow respond to CCL25, raising a possibility that CCR9 regulates the early stages of B-cell development.34 Although there has not yet been any direct evidence that CCR9/TECK is involved in the pathogenesis of endometriosis, we hypothesized their involvement in the disease according to our findings in the ectopic tissue. On the basis of what we have stated here, in the present study, we investigated the cellular and biological actions mediated by CCR9 and TECK, which are involved in endometriosis.
The establishment of endometriosis has been attributed to the attachment and invasion of retrograded endometrial fragments into the peritoneum, their entry into a blood supply and the triggering of a suboptimal immune response that does not adequately clear the implants, resulting in their continued survival and growth.35 However, interactive molecules, including steroid exposure, immune disturbances, genetic predisposition, and environmental toxin exposure are probably involved in the development of endometriosis36 Endometriosis is actually a chronic inflammation that recruits a series of immune cells.37 Therefore, we constructed the coculture unit of endometriosis-associated cells, including ESCs with HPMCs, ESC with U937 cells, and HPMC with U937 cells, in the present study. We have found that the coculture of ESC with U937 cells can apparently induce TECK secretion; however, the cocultures of ESC–HPMC and HPMC–U937 only slightly increased secretion of TECK. By contrast, TECK secretion is further increased in the coculture of ESC–HPMC–U937. Either indirect or direct coculture can induce TECK secretion, which suggests that ESCs in the shed endometrium represent a foreign entity, initiating an acute inflammatory reaction that in turn recruits monocytes, and the crosstalk between the endometrium and the peritoneal macrophages increased the production of TECK.
To test the hypothesis that the ESCs may be major players in the pathogenesis of endometriosis, we established the coculture system of ESC–HPMC, ESC–U937 and ESC/HPMC–U937 cells to investigate the roles of ESCs. Different models represent different situations of the ectopic tissues. We found that TECK secretion in the direct coculture unit of ESC–HPMC–U937 and the indirect coculture unit of ESC/HPMC–U937 (HPMC and U937 cells in contact with each other) was significantly higher than in the other two models in which HPMCs do not directly contact U937 cells, which suggests that the peritoneum plays an important role in the peritoneal cavity in endometriosis and secretes more TECK in a paracrine and/or autocrine manner. This event is followed by an increased migration of immune cells (T cells and monocytes) into the loci, resulting in their ability to secrete cytokines that further stimulate the expression of TECK. The endpoint of such a process is a high level of TECK in the peritoneal cavity and may mediate processes that contribute to endometriosis progression. TECK may create a positive loop to amplify the signal for the establishment and growth of the endometriotic tissue in the ectopic milieu. Some research has shown that other chemokines, such as IL-8, methyl-accepting chemotaxis protein-1, RANTES, eotaxin and growth-regulated oncogene-α, are implicated directly or indirectly in the formation, maintenance and proliferation of the endometriotic implants.38 They exert effects by binding to their cell membrane receptors, and the levels of the receptors partly modulate their actions,39 which led us to speculate that the over-expressed chemokine receptors in the endometriosis foci might play a key role in the progression of endometriosis by cooperating with their corresponding chemokines. Our observations suggest that the over-expression of CCR9 in the endometriotic tissues and a higher concentration of TECK in peritoneal fluid support an important role of CCR9 engagement by TECK in the pathogenesis of endometriosis.
Our results have demonstrated that TECK may act in paracrine and indirect mechanisms and also directly on the ESCs to support endometriosis progression. One mechanism by which TECK may affect endometriosis progression is its capacity to upregulate the expression of MMPs in ESCs. In our matrigel invasion assay, the invasiveness of ESCs treated with the combination of 17β-estradiol and TCDD was higher than that of treatment with either one, which was also proven in the In-cell Western, and TECK neutralizing antibody abolished the increased invasiveness of ESCs. The combination of 17β-estradiol with TCDD or recombinant human thymus-expressed chemokine enhanced the expression of MMP-9 and MMP-2 in the ESCs. 17β-estradiol and TCDD may promote the invasiveness of ESCs by increasing secretion of TECK. However, other cytokines and chemokines induced by 17β-estradiol and TCDD may also be involved in the progress. IL-8 increases the MMP activity and the invasiveness of ESCs.40 Other cytokines, IL-1β and tumour-necrosis factor-α, can stimulate MMP-1, MMP-2 and MMP-3 mRNA levels and promote MMP-1 and MMP-3 protein secretion.41 Our previous work has shown that 17β-estradiol with TCDD increases the secretion of RANTES and MIP-1α and promotes the invasiveness of ESCs, which may occur at the same time as the increase in TECK because using a RANTES neutralizing antibody cannot block the effect of 17β-estradiol with TCDD on the secretion of MIP-1α. On the contrary, using the MIP-1α neutralizing antibody cannot block the effect of 17β-estradiol with TCDD on the secretion of RANTES.25 Therefore, the combination of 17β-estradiol with TCDD may upregulate the invasiveness of ESCs via a series of cytokines and mechanisms at the same time.
In conclusion, our research in vitro provides possible contributors to the progression of endometriosis. Therefore, we propose a hypothetical model to illustrate the complex set of interactions by which the retrograde endometrium expressing higher levels of CCR9 promotes endometriosis-associated cells to recruit immune cells into ectopic foci. Interaction of the endometriosis-associated cells increases the secretion of TECK. High levels of 17β-estrogen in the endometriotic milieu and TCDD increase the TECK secretion further, and then TECK may act to induce monocyte and T-cell migration into the ectopic milieu. In addition, the increased TECK expression gives rise to invasion-promoting activities of ESCs by upregulating secretion of MMPs or other factors or mediators of angiogenesis, which leads to the onset and progression of endometriosis.
Acknowledgments
This work was supported by the National Basic Research Program of China (2006CB944007, to DJ Li), the National and Shanghai Leading Academic Discipline Project (211XK22, to DJ Li) and the Program for Outstanding Medical Academic Leader of Shanghai (to DJ Li). The human peritoneal mesothelial cell line (HMrSV5) was generously provided by Professor Jian Yao (The First People's Hospital, Shanghai, China)
References
- Witz CA. Current concepts in the pathogenesis of endometriosis. . Clin Obstet Gycol. 1999;42:566–585. doi: 10.1097/00003081-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Melega C, Balducci M, Bulletti C, Galassi A, Jasonni VM, Flamigni C. Tissue factors influencing growth and maintenance of endometriosis. . Ann N Y Acad Sci. 1991;622:256–265. doi: 10.1111/j.1749-6632.1991.tb37869.x. [DOI] [PubMed] [Google Scholar]
- Visser CE, Tekstra J, Brouwer-Steenbergen JJ. Chemokines produced by mesothelial cells: huGRO-alpha, IP-10, MCP-1 and RANTES. . Clin Exp Immunol. 1998;112:270–275. doi: 10.1046/j.1365-2249.1998.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Arici A. Chemokines and human reproduction. . Fertil Steril. 1999;71:983–993. doi: 10.1016/s0015-0282(99)00120-x. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. . Am J Reprod Immunol. 2002;147:213–221. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts Al, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. . J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Coro AJ, Rodriguez-Frade JM, Martin De AA, Moreno-Ortiz MC, Martinez-A C, Mellado M. The chemokine SDF-1α triggers CXCR4 receptor dirmerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- Majka M, Janowska-Wieczorek A, Ratajczak J, Kowalska MA, Vilaire G, Pan ZK, et al. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. . Blood. 2000;96:4142–4151. [PubMed] [Google Scholar]
- Ganju RK, Dutt P, Wu L, Newman W, Avraham H, Avraham S, et al. Beta-chemokine receptor CCR5 signals via the novel tyrosine kinase RAFTK. . Blood. 1998;91:791–797. [PubMed] [Google Scholar]
- Spuijbroek MDEH, Dunselman GAJ, Menheere PPCA, Evers JLH. Early endometriosis invades the extracellular matrix. . Fertil Steril. 1992;58:929–933. doi: 10.1016/s0015-0282(16)55437-5. [DOI] [PubMed] [Google Scholar]
- Burner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. . J Clin Invest. 1997;99:2851–2857. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. . Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Kokorine I, Nisolle M, Donnez J, Eeckhout Y, Courtoy PJ, Marbaix E. Expression of interstitial collagenase (matrix metalloproteinase-1) is related to the activity of human endometriotic lesions. . Fertil Steril. 1997;68:246–251. doi: 10.1016/s0015-0282(97)81510-5. [DOI] [PubMed] [Google Scholar]
- Wenzl RJ, Heinzl H. Localization of matrix metalloproteinase-2 in uterine endometrium and ectopic implants. . Gynecol Obstet Invest. 1998;45:253–257. doi: 10.1159/000009978. [DOI] [PubMed] [Google Scholar]
- Cox KE, Piva M, Sharpe-Timms KL. Differential regulation of matrix metalloproteinase-3 gene expression in endometriotic lesions compared with endometrium. . Biol Reprod. 2001;65:1297–1303. doi: 10.1095/biolreprod65.4.1297. [DOI] [PubMed] [Google Scholar]
- Rodgers WH, Osteen KG, Matrisian LM, Navre M, Giudice LC, Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. . Am J Obstet Gynecol. 1993;168:253–260. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- Chung HW, Hur SE, Park MH. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-1 expression in ectopic and eutopic endometrium. . Fertil Steril. 2002;78:787–795. doi: 10.1016/s0015-0282(02)03322-8. [DOI] [PubMed] [Google Scholar]
- Collette T, Maheux R, Mailloux J, Akoum A. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. . Hum Reprod. 2006;21:3059–3067. doi: 10.1093/humrep/del297. [DOI] [PubMed] [Google Scholar]
- Rier S, Foster WG. Environmental dioxins and endometriosis. . Toxicol Sci. 2002;70:161–170. doi: 10.1093/toxsci/70.2.161. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, et al. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–1908. [PubMed] [Google Scholar]
- Dizerega GS, Barber DL, Hodgen GD. Endometriosis: role of ovarian steroids in initiation, maintenance and suppression. . Fertil Steril. 1980;33:649–653. doi: 10.1016/s0015-0282(16)44780-1. [DOI] [PubMed] [Google Scholar]
- Kiesel L, Rennebaum B. Mechanisms involved in the hormonal treatment of endometriosis. . Prog Clin Biol Res. 1990;323:179–196. [PubMed] [Google Scholar]
- Sensky TE, Liu DT. Endometriosis: association with menorrhagia, infertility and oral contraceptives. . Int J Gynaecol Obstet. 1980;17:573–578. doi: 10.1002/j.1879-3479.1980.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Shi YL, Luo XZn, Zhu XY, Hua KQ, Zhu Y, Li DJ. Effects of combined 17β-estradiol with TCDD on secretion of chemokine IL-8 and expression of its receptor CXCR1 in endometriotic focus-associated cells in coculture. . Hum Reprod. 2006;21:870–879. doi: 10.1093/humrep/dei414. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Zhou WH, Wang L, He YY, Li DJ. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. . Hum Reprod. 2008;23:1614–1626. doi: 10.1093/humrep/den125. [DOI] [PubMed] [Google Scholar]
- Egorina EM, Sovershaev MA, Osterud B. In-cell western assay: a new approach to visualize tissue factor in human monocytes. . J Thromb Haemost. 2006;4:614–620. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- Meurens F, Whale J, Brownlie R, Dybvig T, Thompson DR, Gerdts V. Expression of mucosal chemokines TECK/CCL25 and MEC/CCL28 during fetal development of the ovine mucosal immune system. . Immunology. 2008;120:544–555. doi: 10.1111/j.1365-2567.2006.02532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaballos A, Gutiérrez J, Varona R, Ardavin C, Márquez G. Identification of the orphan chemokine receptor GPR-96 as CCR9, the receptor for the chemokine TECK. . J Immunol. 1999;162:5671. [PubMed] [Google Scholar]
- Youn BS, Kim CH, Smith FO, Broxmeyer HE. TECK, an efficacious chemoattractant for human thymocytes, uses GPR-9 -6/CCR9 as a specific receptor. . Blood. 1999;94:2533–2536. [PubMed] [Google Scholar]
- Yu CR, Peden KWC, Zaitseva MB, Golding H, Farber JM. CCR9A and CCR9B: two receptors for the chemokine CCL25/TECK/Ckβ-15 that differ in their sensitivities to ligand. . J Immunol. 2000;164:1293–1305. doi: 10.4049/jimmunol.164.3.1293. [DOI] [PubMed] [Google Scholar]
- Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. . J Immunol. 2000;164:639–648. doi: 10.4049/jimmunol.164.2.639. [DOI] [PubMed] [Google Scholar]
- Carramolino L, Zaballos A, Kremer L, Villares R, Martín P, Ardavín C, et al. Expression of CCR9 β-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8+ T cells from secondary lymphoid organs. . Blood. 2001;97:850–857. doi: 10.1182/blood.v97.4.850. [DOI] [PubMed] [Google Scholar]
- Uehara S, Song K, Farber JM, Love PE. Characterization of CCR9 expression and CCL25/TECK responsiveness during T cell development: CD3highCD69+ thymocytes and γ8TCR+ thymocytes preferentially respond to CCL25. . J Immunol. 2002;168:134–142. doi: 10.4049/jimmunol.168.1.134. [DOI] [PubMed] [Google Scholar]
- Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, et al. Butcher. Development switches in chemokine response profiles during B cell differentiation and maturation. . J Exp Med. 2000;191:1303–1318. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. . Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Osteen KG, Yeaman GR, Bruner-Tran KL. Matrix metalloproteinases and endometriosis. . Semin Reprod Med. 2003;21:155–164. doi: 10.1055/s-2003-41322. [DOI] [PubMed] [Google Scholar]
- Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, et al. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. . Mol Hum Reprod. 2002;8:1102–1110. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Mahutte NG, Arici A. Chemokines and human reproduction. . Fertil Steril. 1999;71:983–993. doi: 10.1016/s0015-0282(99)00120-x. [DOI] [PubMed] [Google Scholar]
- Ulukus M, Ulukus EC, Seval Y, Zheng W, Arici A. Expression of interleukin-8 receptors in endometriosis. . Hum Reprod. 2005;20:794–801. doi: 10.1093/humrep/deh675. [DOI] [PubMed] [Google Scholar]
- Mulayim N, Savlu A, Guzeloglu-Kayisli O, Kayisli UA, Arici A. Regulation of endometrial stromal cell matrix metalloproteinase activity and invasiveness by interleukin-8. . Fertil Steril. 2004;81:904–911. doi: 10.1016/j.fertnstert.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Bradundmeier AG, Nowak RA. Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer. . Am J Reprod Immunol. 2006;56:201–214. doi: 10.1111/j.1600-0897.2006.00418.x. [DOI] [PubMed] [Google Scholar]