Abstract
CD4+ regulatory T cells (Tregs) play an important role in maintaining immune tolerance by suppressing pathologic immune responses. The generation of large numbers of antigen-specific Tregs ex vivo is critical for the development of clinical immunotherapy based on the adoptive transfer of Tregs. Both CD40-activated B cells (CD40-B) and immature dendritic cells (imDCs) have been used as professional antigen-presenting cells (APCs) to generate antigen-specific Tregs. However, the efficiencies of CD40-B and imDCs to generate CD4+ Tregs have not been compared directly and the mechanism driving the generation of these Tregs remains largely unknown. In this study, we found that CD40-B exhibited mature phenotypes and were more able to induce and expand CD4highCD25+ Tregs than imDCs. Moreover, Tregs induced by CD40-B had greater suppressive capacity than those induced by imDCs. The generation of CD4highCD25+ Tregs by CD40-B and imDCs is cell–cell contact dependent and partially relies on the expression of human leukocyte antigen (HLA)-DR and CD80/86. Differences in CD4highCD25+ Treg generation efficiency were largely explained by the production of endogenous IL-2 by CD40-B. Our results suggest that CD40-B is better able to generate large numbers of antigen-specific Tregs than imDCs. Additionally, using CD40-B to generate Tregs may accelerate the clinical use of Treg-based immunotherapy in the treatment of allograft rejection, graft versus host disease (GVHD) and autoimmune diseases.
Keywords: B cells, immature DC, Treg, IL-2
CD4+ regulatory T cells (Tregs) represent a small population of T cells that are able to maintain balance within the immune system and negatively regulate immune reactions caused by endogenous or exogenous stimuli.1 These novel T cells are composed of two major subpopulations, natural Tregs and induced Tregs,1 and have been confirmed to play crucial roles in autoimmune diseases1, 2 and cancer.3, 4 In vitro and in vivo experiments have also suggested the therapeutic potential of Tregs to prevent and treat T-cell-mediated inflammatory diseases, such as promoting transplantation tolerance,5, 6 inhibiting graft versus host disease (GVHD)7, 8 and controlling autoimmune diseases.2 However, the clinical application of nature Tregs, which are formed by negative selection in the thymus,1 has been limited due to their low frequency (only 1–5% in peripheral blood CD4+ T cells) and lack of antigen-specificity.
To overcome these obstacles, several protocols have been established to induce and expand polyclonal induced Tregs in vitro using repeated stimulation with either CD3 and CD28 monoclonal antibodies (mAbs) or artificial antigen-presenting cells (APCs) to achieve activation through CD3 and CD28, together with the administration of exogenous IL-2 and/or TGF-β.9, 10, 11, 12, 13 However, the general suppression of recipients' immune systems caused by these polyclonal Tregs may impair normal host defense against infectious or harmful substances and lead to disastrous consequences. In contrast, antigen-specific Tregs are more efficient and able to prevent specific T-cell-mediated inflammation without the global immune suppression induced by polyclonal Tregs.14, 15 Some dendritic cells (DCs), such as immature DCs (imDCs) and plasmacytoid DCs, exhibit regulatory but not stimulatory functions on CD4+ T cells.16, 17, 18, 19 Therefore, these DCs, especially monocyte-derived imDCs, have been used to induce antigen-specific CD4+ Tregs from naive precursors.
In a previous study,20 we developed a simple and cost-effective method to rapidly induce and expand large numbers of functional human alloantigen-specific CD4+ Tregs from antigenically naive precursors in vitro using allogeneic CD40-activated B cells (CD40-B) as stimulators. Using this approach, naive CD4+CD25− T cells could be expanded eight folds into alloantigen-specific CD4highCD25+ Tregs after 3 weeks of culture in the absence of exogenous cytokines. However, the relative efficiency of CD40-B and monocyte-derived imDCs to generate CD4+ Tregs has not been directly compared, and the mechanism underlying the generation of these Tregs remains largely unknown.
In this study, we compared the efficiencies of CD40-B versus monocyte-derived imDCs to generate CD4+ Tregs as well as the functions of CD4+ Tregs induced by these two different APCs. Our results showed that CD40-B were more readily able to induce and expand alloantigen-specific CD4highCD25+ Tregs than imDCs. Additionally, the cells induced by CD40-B had a greater suppressive capacity than those induced by imDCs. The generation of CD4highCD25+ Tregs by CD40-B and imDCs was dependent on cell–cell contact and partially relied on the expression of human leukocyte antigen (HLA)-DR and CD80/86. Endogenous IL-2 produced by CD40-B was primarily responsible for the difference in the efficiency of CD4highCD25+ Treg generation between CD40-B and imDCs.
Materials and methods
Generation of CD40-B
Human peripheral blood was obtained from healthy donors in accordance with local ethical committee approval. B cells from peripheral blood mononuclear cells (PBMCs) were stimulated via CD40 using NIH3T3 cells transfected with the human CD40 ligand (t-CD40-L cells) as described previously.20, 21 Briefly, PBMCs were cocultured with lethally irradiated (96 Gy) t-CD40-L cells in the presence of IL-4 (2 ng/ml; R&D systems, Minneapolis, MN, USA) and cyclosporine A (5.5×10−7 M, Sigma Chemical Co., St. Louis, MO, USA) in Iscove's modified Dulbecco's medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated human AB serum, 50 µg/ml transferrin (Boehringer Mannheim, Indianapolis, IN, USA), 5 µg/ml insulin (Sigma Chemical Co.), and 15 µg/ml gentamicin (Gibco BRL) at 37 °C in 5% CO2. After 14 days of coculture, more than 99% of the viable suspended cells were CD19 positive. B cells were cryopreserved for future use. For coculture with CD4+ T cells, CD40-B were always Ficoll-density centrifuged, followed by two washes with phosphate buffered saline to remove non-viable cells, including the remaining t-CD40-L cells.
Generation of imDCs
imDCs were generated as described by Jonuleit et al.22 In brief, monocytes were isolated from PBMC with anti-CD14 microbeads (Miltenyi Biotec, Auburn, CA, USA) following single-column selection. Selected cells were then cultured in RPMI 1640 medium (Gibco BRL) supplemented with 10% heat-inactivated fetal bovine serum, 800 IU/ml granulocyte macrophage colony-stimulating factor (R&D) and 1000 IU/ml IL-4 (R&D). On day 5, non-adherent imDCs were rinsed off, washed once in phosphate buffered saline, and used for T-cell stimulation.
CD4+ T-cell isolation
Human CD4+ or naive CD4+ T cells were isolated from healthy donor PBMCs by negative selection using a CD4+ T-cell isolation kit or naive CD4+ T-cell isolation kit (Miltenyi Biotec) to deplete cells expressing CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ and CD235a (for CD4+ T cells) or cells expressing CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ, CD235a and CD45RO (for naive CD4+ T cells). CD25+ cells were further depleted by positive selection with directly conjugated anti-CD25 magnetic microbeads (Miltenyi Biotec). The purity of CD4+CD25− or CD4+CD45RO−CD25− cells was routinely more than 99% as determined by flow cytometric analysis.
Induction and expansion of CD4highCD25+ Treg
Purified naive CD4+CD25− T cells were cocultured with allogeneic CD40-B or imDCs from the same donors at a T/B or T/DC ratio of 10/1 in RPMI 1640 medium (Gibco BRL) supplemented with 10% heat-inactivated human AB serum. In some coculture experiments, CD40-B or imDCs were added every 6 days repeatedly. Cell numbers were determined by counting trypan blue negative aliquots at the indicated times.
Flow cytometric assays
Cells were analyzed by a FACSAria flow cytometer. The following fluorescence-conjugated mAbs were used: anti-CD4-Alexa-405 (Caltag, Burlingame, CA, USA), anti-CD25-allophycocyanin, anti-CD3-FITC, anti-CD19-allophycocyanin, anti-major histocompability complex (MHC)-I-FITC, anti-MHC-II-FITC, anti-CD80-FITC, anti-CD83-FITC, anti-CD86-FITC, anti-immunoglobulin M (IgM)-phycoerythrin (PE)-Cy-5, anti-immunoglobulin D (IgD)-PE, anti-cytotoxic T-lymphocyte-associated antigen (CTLA)-4-PE, anti-glucocorticoid-induced tumor necrosis factor receptor-PE, anti-CD103; these fluorescent-conjugated mAbs and isotype-matched control Abs of irrelevant specificity were purchased from BD Biosciences (San Jose, CA, USA). For Foxp3 staining, a human Foxp3 staining kit (eBiosciences, San Diego, CA, USA) was used as described previously.
Mixed lymphocyte reaction (MLR) assay
The suppressive capacities of cells induced by allogeneic CD40-B or imDCs were studied in an MLR coculture suppression assay as described previously with some modifications.20 Cultured CD4highCD25+ and CD4+CD25− T cells were sorted by FACSAria after 6 days of coculture with allogeneic CD40-B or imDCs from the same donors. The purity of sorted cells was routinely more than 99%. Sorted CD4highCD25+ and CD4+CD25− T cells (referred to as ‘regulators') were titrated and added at the start of MLR cocultures consisting of a total of 5×104 CD4+CD25− T cells (responder) from the same CD4high/CD4+ T cell donor and 5×104 γ-irradiated target PBMCs from the same allogeneic CD40-B/imDC donor. Antigen specificity was examined in cocultures performed with fully class I and II HLA-mismatched third-party stimulator PBMCs. Proliferation was analyzed by [3H]-thymidine incorporation as described previously.20 3H incorporation was expressed as the mean±SEM counts per 1 min of four to six measurements.
Transwell assay
The cell–cell contact dependency of CD4highCD25+ T cell induction was examined using a transwell culture system. Briefly, naive CD4+CD25– cells were plated in the bottom compartment of the cell culture wells while allogeneic CD40-B or imDCs were cultured in the transwell inserts (0.4 µm pore size; Millicell; Millipore, Billerica, MA, USA). At the indicated times, CD4+ T cells were harvested and their absolute number or phenotypes were examined as described above.
Blocking assays
Blocking studies were performed in the presence of neutralizing mAbs against HLA-DR (10 µg/ml, R&D), CD80 (10 µg/ml, R&D), CD86 (10 µg/ml, R&D), IL-2 (1 µg/ml, eBiosciences) or their relevant isotype controls.
Cell death assay
The percentage of live CD40-B cells in coculture was determined using the Live/Dead cell-mediated cytotoxicity kit (Molecular Probes, Eugene, OR, USA) as we described before.23 Briefly, CD40-B cells were labeled with 3,3′-dioctadecyloxacarbocyanine perchlorate before beginning coculture. Cells were harvested and stained with propidium iodide for 15 min and analyzed by flow cytometry at the indicated times during coculture. By back-gating on green fluorescent target cells, propidium-iodide-positive cells were evaluated for the presence of dead cells.
Statistical analysis
Graphs and statistical analyses were performed with Prism 5.00 software for Windows (GraphPad Software, San Diego, CA, USA). P values of 0.05 or less were considered significant.
Results
CD40-B exhibit mature phenotypes
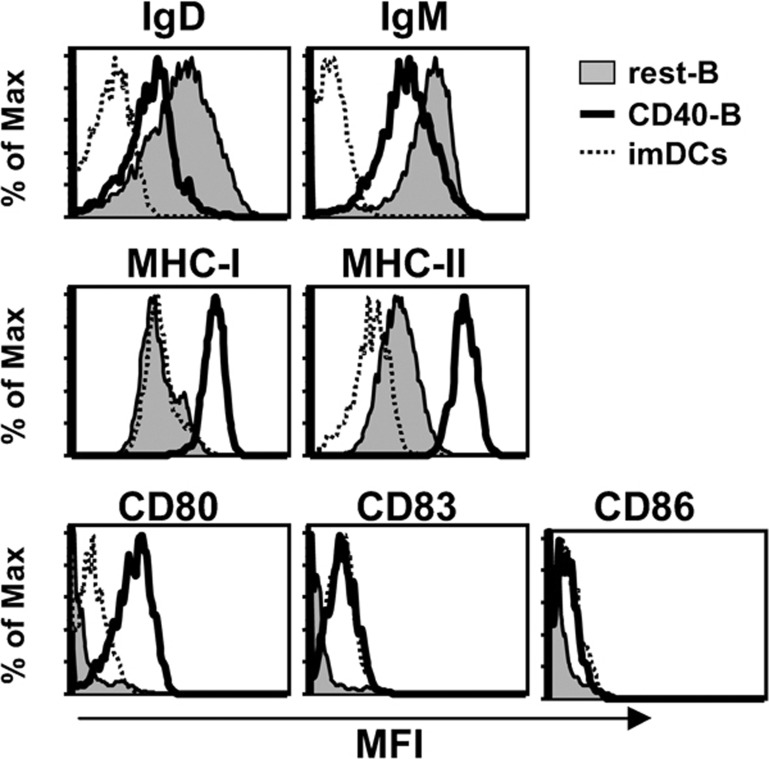
To compare the characteristics of CD40-activated B cells and monocyte-derived imDCs, we examined the expression of surface IgD, IgM, MHC-I and MHC-II, and costimulators CD80, CD83 and CD86 on CD40-B and imDCs. As shown in Figure 1, the expression of surface IgD and IgM on CD40-B was decreased after 14 days of coculture with CD40L-NIH3T3 compared with resting B cells, whereas the expression of MHC-I, MHC-II, CD80, CD83 and CD86 on CD40-B was increased. Compared with imDCs, CD40-B expressed higher levels of MHC-I, MHC-II and CD80. In contrast, there were no significant differences in CD83 and CD86 expression between CD40-B and imDCs (Figure 1).
Figure 1.
Phenotypes of CD40-B cells and imDCs. Surface expression of B-cell maturation markers IgD and IgM (top panel), MHC-I and MHC-II (middle panel), and costimulatory molecules CD80, CD83 and CD86 (bottom panel) in resting B cells, CD40-B and imDCs were examined. The results are representative of four independent experiments. IgD, immunoglobulin D; IgM, immunoglobulin M; imDCs, immature dendritic cells; MHC, major histocompability complex.
CD40-B are more able to induce and expand CD4highCD25+ Tregs than imDCs
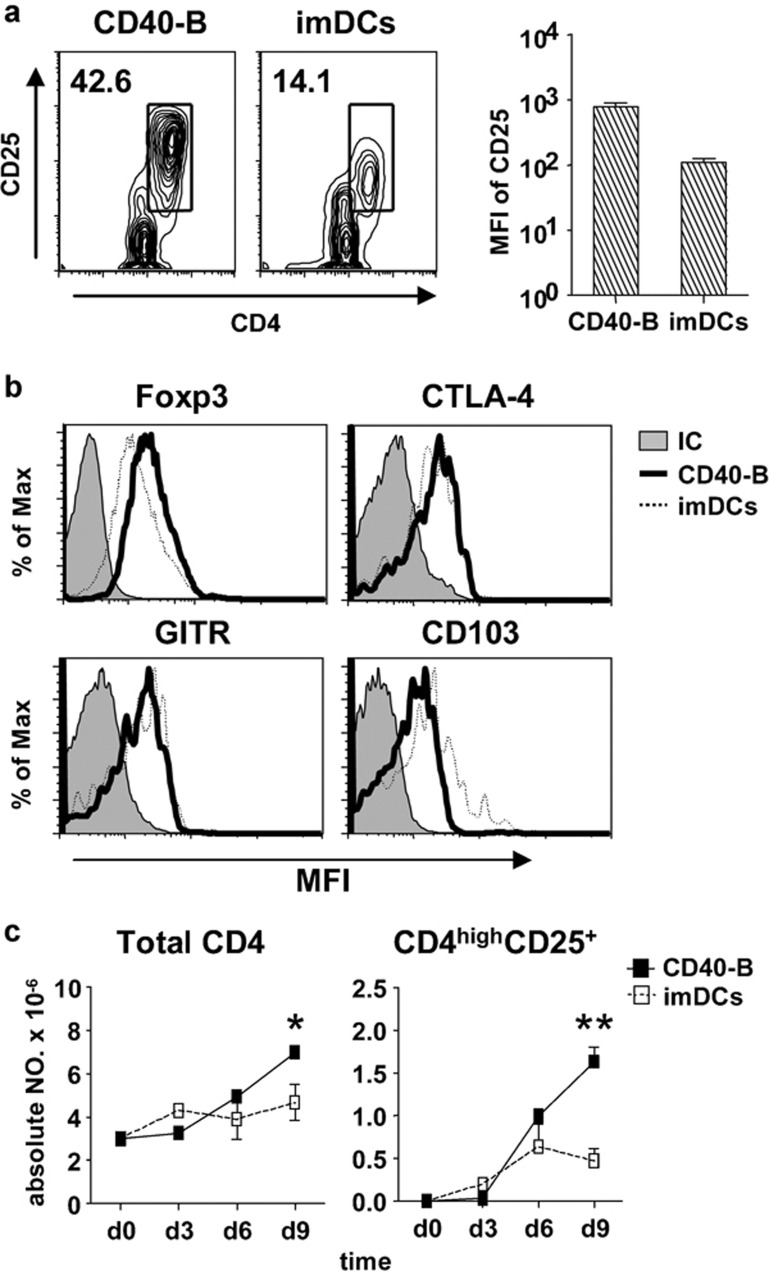
To compare the capability of CD40-B and imDCs to induce and expand CD4highCD25+ Tregs, CD40-B or imDCs from same donor were cocultured with allogeneic naive CD4+CD25− T cells at a T/B or T/DC ratio of 10/1. Although the CD4highCD25+ T-cell subset was induced by both CD40-B and imDCs after 6 days of coculture, the percentage of CD4highCD25+ T cells induced by CD40-B was much higher than that induced by imDCs (Figure 2a). Furthermore, the expression of CD25 in CD4highCD25+ T cells induced by CD40-B was significantly increased compared with that of imDCs (Figure 2a). With regard to the expressions of Treg-related molecules, Foxp3 expression in CD4highCD25+ T cells induced by CD40-B was higher than that in imDC-induced T cells, whereas the expression of CD103 in CD4highCD25+ T cells induced by CD40-B was lower than that induced by imDCs. There were no differences in CTLA-4 and glucocorticoid-induced tumor necrosis factor receptor expression (Figure 2b). During 9 days of coculture, the absolute number of total CD4+ T cells and CD4highCD25+ T cells induced by CD40-B was significantly higher than that induced by imDCs (Figure 2c).
Figure 2.
CD40-B cells are more able to induce and expand CD4highCD25+ Tregs than imDCs. (a) The percentage of CD4highCD25+ Tregs in CD4+ T cell populations after 6 days of coculture with naive CD4+CD25− T cells and allogeneic CD40-B or imDCs. Naive CD4+CD25− T cells were isolated from normal PBMCs and cocultured with allogeneic CD40-B or imDCs from the same donors at a T/B or T/DC ratio of 10/1 for 6 days. Surface expression of CD4 and CD25 was examined as described. (b) Expression of Treg related molecules Foxp3 (top left panel), CTLA-4 (top right panel), glucocorticoid-induced tumor necrosis factor receptor (bottom left panel), and CD103 (bottom right panel) in CD4highCD25+ Tregs induced by CD40-B and imDCs. (c) Increase in absolute numbers of CD4highCD25+ Tregs and total CD4+ T cells at indicated times during 9 days of coculture with naive CD4+CD25− T cells and allogeneic CD40-B or imDCs at a T/B or T/DC ratio of 10/1. T-cell number was determined. Data are representative of four independent experiments. Two-tailed unpaired Student's t-tests were used for comparison (*P<0.05, **P<0.01). CTLA, cytotoxic T-lymphocyte-associated antigen; DC, dendritic cell; imDCs, immature DCs; PBMCs, peripheral blood mononuclear cells; Tregs, regulatory T cells.
CD4highCD25+ Tregs induced by CD40-B have greater antigen-specific suppressive capability than those induced by imDCs
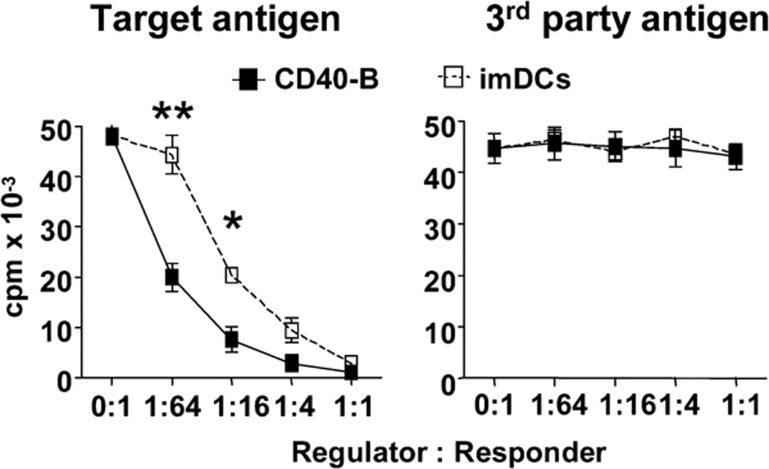
To further compare the functions of CD4highCD25+ Tregs induced by CD40-B versus imDCs, we examined their suppressive capability. CD4highCD25+ T cells were sorted after 6 days of coculture and added into MLR systems. As shown in Figure 3, CD4highCD25+ T cells induced by either CD40-B or imDCs significantly inhibited original target alloantigen-stimulated proliferation but were unable to suppress third-party alloantigen-stimulated proliferation, suggesting that CD4highCD25+ T cells induced by both CD40-B and imDCs are alloantigen-specific Tregs. The suppressive capability of CD4highCD25+ Tregs induced by CD40-B was significantly greater than that of Tregs induced by imDCs at low regulator/responder ratios (1/16 and 1/64).
Figure 3.
CD4highCD25+ Tregs induced by CD40-B have stronger suppressive capability with antigen specificity than that induced by imDCs. CD4highCD25+ subsets were sorted on day 6 of coculture of naive CD4+CD25− T cells and allogeneic CD40-B or imDCs and added into MLR systems as described in the text. Data are representative of four independent experiments. Two-tailed unpaired Student's t-tests were used for comparison (*P<0.05, **P<0.01). imDCs, immature dendritic cells; MLR, mixed lymphocyte reaction; Tregs, regulatory T cells.
Generation of CD4highCD25+ Tregs by CD40-B or imDCs depends on cell–cell contact
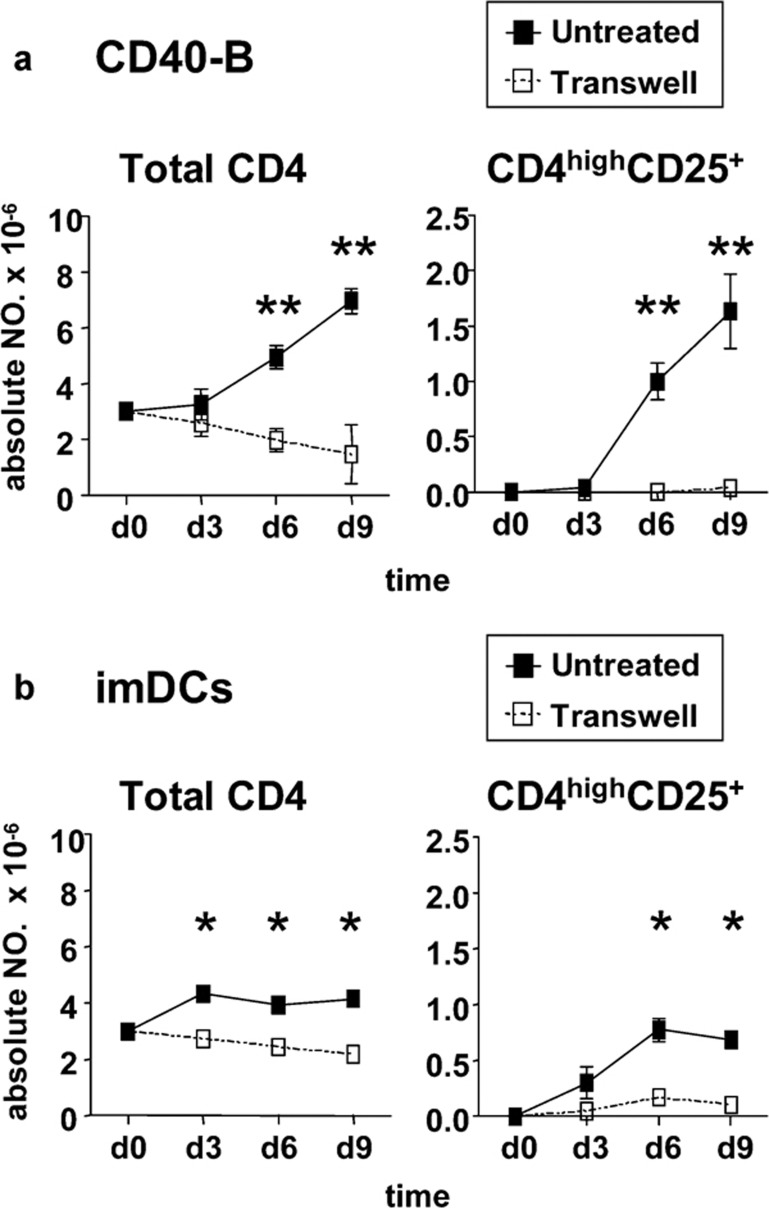
To test the requirement of cell–cell contact for CD4highCD25+ Treg induction, Transwell systems were used. As shown in Figure 4, no CD4highCD25+ Tregs could be induced when naive CD4+CD25− T cells were separated from CD40-B, and the total number of CD4+ T cells in the coculture with Transwell systems was decreased significantly compared with cocultures allowing direct cell–cell contact. Similar results were also found in cocultures of naive CD4+CD25− T cells and imDCs.
Figure 4.
Generation of CD4highCD25+ Tregs by CD40-B or imDCs depends on cell–cell contact. Naive CD4+CD25− T cells isolated from normal PBMCs were cocultured with allogeneic CD40-B (a) or imDCs (b) at a T/B or T/DC ratio of 10/1 with or without a transwell system for 9 days. Absolute numbers of total CD4+ T cells and CD4highCD25+ Tregs at indicated times were determined. Data are representative of four independent experiments. Two-tailed unpaired Student's t-tests were used for comparison (*P<0.05, **P<0.01). DC, dendritic cell; imDCs, immature DCs; PBMCs, peripheral blood mononuclear cells; Tregs, regulatory T cells.
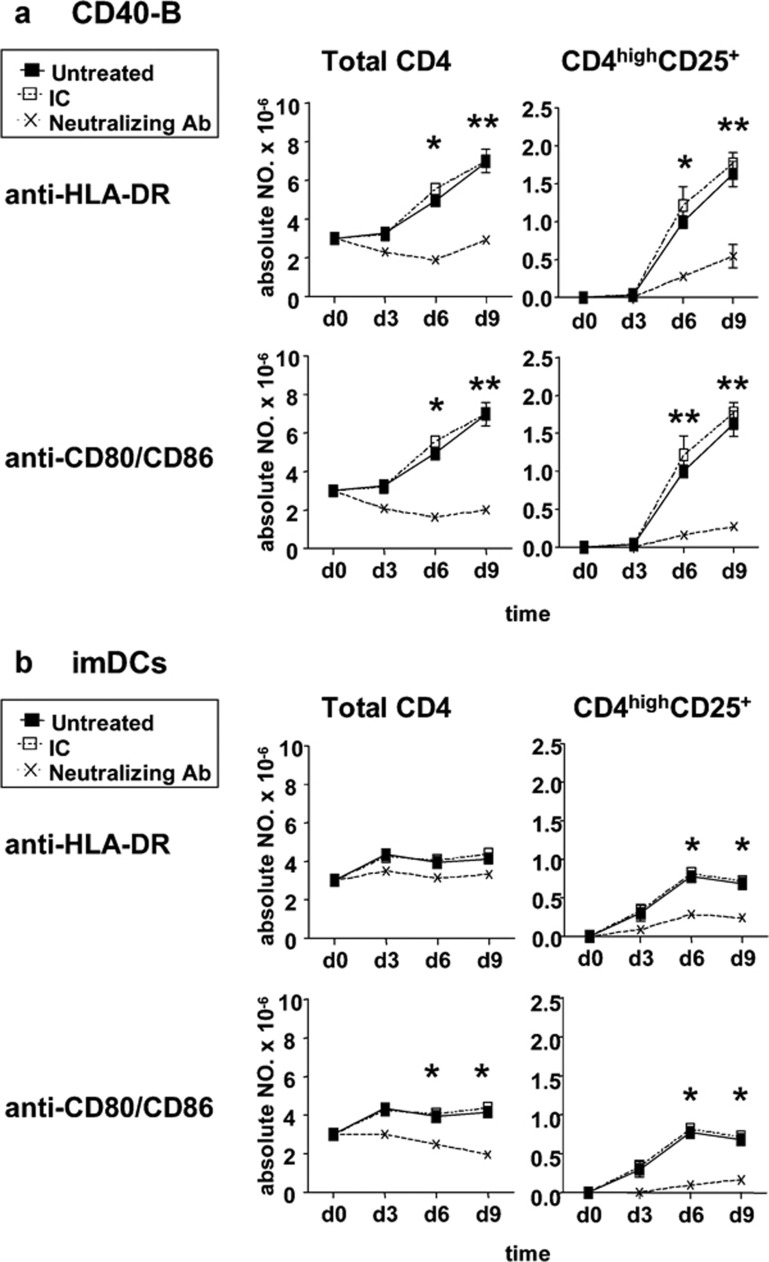
Generation of CD4highCD25+ Tregs is partially dependent on the expression of HLA-DR and CD80/86
To determine which molecules contributed to the dependence of cell–cell contact for the generation of CD4highCD25+ Tregs, neutralizing mAbs against HLA-DR or CD80/CD86 were added into cocultures. As shown in Figure 5, blockade of HLA-DR or CD80/CD86 significantly impaired the generation of CD4highCD25+ Tregs induced by either CD40-B or imDCs during 9 days of coculture. These data indicated that the generation of CD4highCD25+ Tregs partially relied on HLA-DR and CD80/CD86 expression on CD40-B or imDCs.
Figure 5.
Generation of CD4highCD25+ Tregs is partially dependent on HLA-DR and CD80/86 expression. Naive CD4+CD25− T cells isolated from normal PBMCs were cocultured with allogeneic CD40-B (a) or imDCs (b) at a T/B or T/DC ratio of 10/1 in the presence of neutralizing mAbs against HLA-DR, CD80/CD86 or relevant isotype control for 9 days. Absolute numbers of total CD4+ T cells and CD4highCD25+ Tregs were determined at the indicated times. Data are representative of four independent experiments. Two-tailed unpaired Student's t-tests were used for comparison (*P<0.05, **P<0.01). DC, dendritic cell; HLA-DR, human leukocyte antigen-DR; imDCs, immature DCs; mAbs, monoclonal antibodies; Tregs, regulatory T cells.
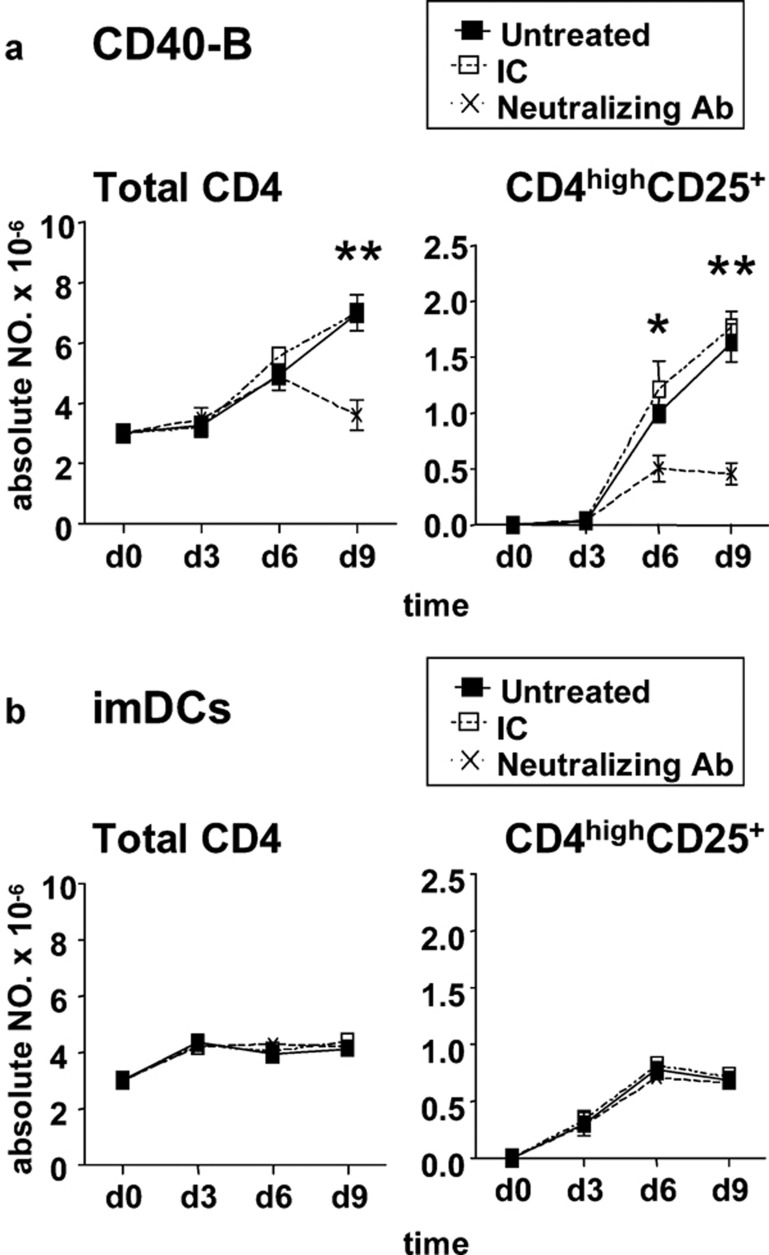
Endogenous IL-2 is primarily responsible for the difference in CD4highCD25+ Treg generation efficiency
To further determine the underlying mechanism involved in the difference in CD4highCD25+ Treg generation efficiency, a blocking assay using a neutralizing mAb against IL-2 was used. Neutralization of IL-2 significantly impaired the generation of CD4highCD25+ Tregs induced by CD40-B, but did not affect the generation of these cells induced by imDCs during 9 days of coculture (Figure 6). Furthermore, the generation efficiency of CD4highCD25+ Tregs by CD40-B became similar to that induced by imDCs under treatment with IL-2 neutralizing mAb (Figure 6). These results suggest that endogenous IL-2 was mainly responsible for the difference in CD4highCD25+ Treg generation efficiency.
Figure 6.
Endogenous IL-2 is primarily responsible for the difference in CD4highCD25+ Treg generation efficiency induced by CD40-B and imDCs. Naive CD4+CD25− T cells isolated from normal PBMCs were cocultured with allogeneic CD40-B (a) or imDCs (b) at a T/B or T/DC ratio of 10/1 in the presence of the neutralizing mAbs against IL-2 or relevant isotype control for 9 days. Absolute numbers of total CD4+ T cells and CD4highCD25+ Tregs were determined at the indicated times. Data are representative of four independent experiments. Two-tailed unpaired Student's t-tests were used for comparison (*P<0.05, **P<0.01). DC, dendritic cell; imDCs, immature DCs; mAbs, monoclonal antibodies; PBMCs, peripheral blood mononuclear cells; Tregs, regulatory T cells.
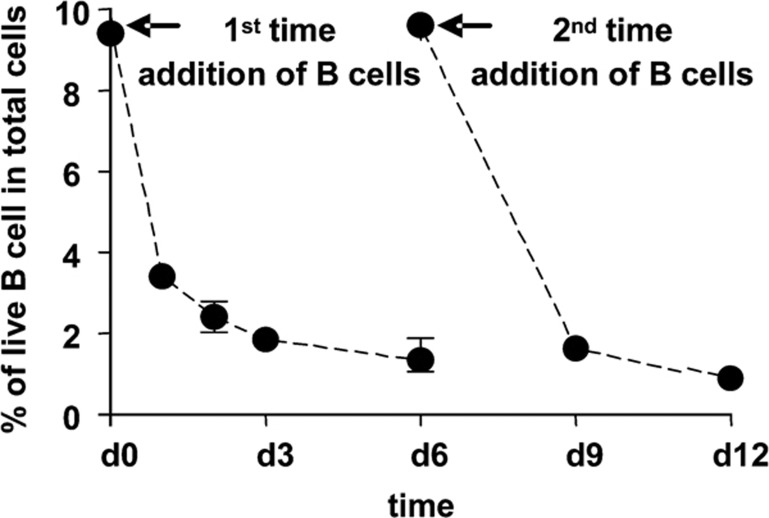
Almost all CD40-B are dead after 6 days of coculture
To examine the viability of CD40-B in coculture with allogeneic naive CD4+CD25− T cells, the percentage of live B cells in coculture was determined. As shown in Figure 7, the percentage of live CD40-B in the coculture gradually decreased during 6 days of coculture, and similar results were also found following the addition of CD40-B from day 6 to day 12 of coculture. After 12 days of coculture, fewer than 1% of the harvested cells were B cells (data not shown).
Figure 7.
Almost all CD40-B are dead within 6 days of coculture. Naive CD4+CD25− T cells isolated from normal PBMCs were cocultured with allogeneic CD40-B at a T/B ratio of 10/1 for 12 days and CD40-B were added repeatedly on day 6 of coculture. The percentage of live B cells was determined as described in the text. Data are representative of more than 10 independent experiments. PBMCs, peripheral blood mononuclear cells.
Discussion
The induction of antigen-specific Tregs is important for the prevention of T-cell-mediated inflammatory diseases and maintenance of immune homeostasis. The generation of large numbers of antigen-specific Tregs ex vivo is critical for the development of clinical immunotherapies based on the adoptive transfer of Tregs. Although both CD40-B and imDCs, as professional APCs,22, 24, 25, 26 can be used for the generation of antigen-specific Tregs,20, 21, 22, 24 we demonstrated for the first time that CD40-B are more potent than imDCs to induce and expand alloantigen-specific CD4highCD25+ Tregs. Additionally, the cells induced by CD40-B have greater suppressive capacity than those induced by imDCs.
Similar to previous reports that cell–cell contact is required for the induction of CD4+ Tregs by imDCs or plasmacytoid DCs,27, 28 we found that cell–cell contact was essential for the generation of both imDCs- and CD40-B-induced CD4highCD25+ Tregs (Figure 4). Activation of T cells by APCs require cell–cell contact through two signals, i.e., the interaction between TCR and MHC molecules loaded with antigen and the cross-talk between CD28 and CD80/CD86.29 Here our data showed that the generation of CD4highCD25+ Tregs by either CD40-B or imDCs was partially dependent on the expression of HLA-DR and CD80/86 (Figure 5), indicating that these two signals are required for the induction of CD4+ Tregs. In addition, we also found that CD40-B expressed higher levels of MHC-II and CD80 than imDCs (Figure 1) and that blockade of HLA-DR and CD80/CD86 reduced the generation of CD4+ Tregs by CD40-B to levels comparable to those induced by imDCs (Figure 5). These results suggest that the difference in HLA-DR and CD80 expression may account, at least in part, for the difference in CD4highCD25+ Treg generation efficiency between CD40-B and imDCs.
The maturation state of APCs is believed to be a control point for the induction of CD4+ Tregs through modifications in the activation state of T cells.30, 31 Our data also confirmed this, as CD4+ Tregs were able to be induced by imDCs (Figures 2 and 3). However, CD40-B, as mature APCs with high levels of MHC-I, MHC-II and CD80 expression (Figure 1), can also be used to generate Tregs (Figures 2 and 3),20 suggesting that the stimulatory or tolerogenic role of APCs may not only rely on the maturation state of APCs, at least for CD40-B. In fact, the T/B ratio seems critical for the induction of immunity or tolerance. CD40-B could induce CD4+ and CD8+ T-cell responses at a T/B ratio of 4/1,25, 26 whereas they could also efficiently induce both CD4+ and CD8+ Tregs when the T/B ratio was 10/1.20, 21 These data suggest that the stimulatory or tolerogenic role of CD40-B may depend on the strength of the activation signal received by T cells, but not on the maturation state of CD40-B.32
Without any exogenous cytokines, total CD4+ T cells did not expand significantly, although CD4highCD25+ Tregs were induced by imDCs during the first 6 days of coculture (Figure 2), indicating that CD4highCD25+ Tregs were directly converted from their naive precursors but did not undergo proliferation. However, CD40-B significantly induced and expanded both total CD4+ T cells and CD4highCD25+ Tregs under the same coculture conditions, suggesting that CD40-B not only induced Tregs but also promoted the proliferation of Tregs.
High doses of exogenous IL-2 are usually required for the generation of human polyclonal or antigen-specific CD4+ Tregs in vitro,10, 11, 12 although it has been reported that IL-2 from activated CD4+CD25− T cells could help the expansion of CD4+CD25+ Tregs.33 We did not detect IL-2 expression in CD4+ T cells cocultured with either imDCs or CD40-B in our system (data not shown). On the contrary, CD40-B were capable of secreting IL-2 to help the induction and expansion of induced CD4highCD25+ Tregs.20 In contrast, no IL-2 expression could be found in imDCs (data not shown). In addition, using a neutralizing mAb to block IL-2, we further demonstrated that endogenous IL-2 was mainly responsible for the observed difference in CD4highCD25+ Treg generation efficiency (Figure 6). Therefore, endogenously produced IL-2 is favorable for alloantigen-specific CD4highCD25+ Treg induction and expansion induced by CD40-B.
Our results also indicated that CD4highCD25+ Tregs induced by CD40-B have greater suppressive capacity than those induced by imDCs. The fact that CD40-B induced higher levels of CD25 and Foxp3 expression in CD4highCD25+ Tregs than did imDCs (Figure 2) may account for this. On the other hand, CD4highCD25+ Tregs induced by imDCs expressed higher levels of CD103 than those induced by CD40-B (Figure 2). CD103 has been regarded as an important molecule in the suppressive function of Tregs induced by imDCs,34, 35 but its role in Tregs induced by CD4-B is still unknown. In a previous study,20 we found that the inhibitory capacity of CD4highCD25+ Tregs induced by CD40-B was mainly dependent on CTLA-4. Taken together, these results suggested that the suppressive functions of Tregs induced by CD40-B and imDCs might be regulated by several molecules and pathways to different degrees, a possibility that will require further study.
Finally, we found that almost all CD40-B were dead within 6 days of coculture (Figure 7). This spontaneous clearance of CD40-B may further reduce the cost of purifying CD4highCD25+ Tregs and accelerate the use of Treg-based immunotherapy in clinical applications.
In conclusion, CD40-B cells were more able to induce and expand alloantigen specific CD4+ Tregs than imDCs, which may result from endogenous IL-2 production, along with the high expression of MHC-II and costimulatory molecule CD80, in CD40-B cells. Using CD40-B cells to generate large numbers of antigen-specific Tregs may facilitate the Treg-based immunotherapy in the treatment of allograft rejection, GVHD and autoimmune diseases in the future.
Acknowledgments
This work was supported in part by the Seed Funding for Basic Research, University Research Committee, the University of Hong Kong (HKU), Hong Kong, China (WT); General Research fund, Research Grants Council of Hong Kong, Hong Kong, China (WT); the Area of Excellence Scheme of the University Grants Committee, Hong Kong, China (Grant AoE/M-12/06; WT and YLL); Edward Sai-Kim Hotung Paediatric Education and Research Fund (YLL, WT) and HKU postgraduate studentships (JZ).
References
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Toubi E. The role of CD4+CD25+ T regulatory cells in autoimmune diseases. Clin Rev Allergy Immunol. 2008;34:338–344. doi: 10.1007/s12016-007-8043-0. [DOI] [PubMed] [Google Scholar]
- Lysaght J, Jarnicki AG, Mills KH. Reciprocal effects of Th1 and Treg cell inducing pathogen-associated immunomodulatory molecules on anti-tumor immunity. Cancer Immunol Immunother. 2007;56:1367–1379. doi: 10.1007/s00262-007-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S, Apetoh L, Chalmin F, Ladoire S, Mignot G, Puig PE, et al. CD4CD25 Tregs control the TRAIL-dependent cytotoxicity of tumor-infiltrating DCs in rodent models of colon cancer. J Clin Invest. 2008;118:3751–3761. doi: 10.1172/JCI35890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann H, Adams E, Fairchild P, Cobbold S. Regulation and privilege in transplantation tolerance. J Clin Immunol. 2008;28:716–725. doi: 10.1007/s10875-008-9249-5. [DOI] [PubMed] [Google Scholar]
- Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, et al. L-selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–3812. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- Gil-Guerrero L, Dotor J, Huibregtse IL, Casares N, Lopez-Vazquez AB, Rudilla F, et al. In vitro and in vivo down-regulation of regulatory T cell activity with a peptide inhibitor of TGF-β1. J Immunol. 2008;181:126–135. doi: 10.4049/jimmunol.181.1.126. [DOI] [PubMed] [Google Scholar]
- Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, et al. In vitro-expanded human CD4+CD25+ T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-β to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol. 2008;38:915–917. doi: 10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849–7858. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam KT, et al. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009;183:3742–3750. doi: 10.4049/jimmunol.0901329. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL, et al. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858–865. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad M, Kristensen NN, Kury E, Claesson MH. Characterization of T-regulatory cells, induced by immature dendritic cells, which inhibit enteroantigen-reactive colitis-inducing T-cell responses in vitro and in vivo. Immunology. 2004;113:499–508. doi: 10.1111/j.1365-2567.2004.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepmann K, Biester S, Plskova J, Muckersie E, Duncan L, Forrester JV. CD4+CD25+ T regulatory cells induced by LPS-activated bone marrow dendritic cells suppress experimental autoimmune uveoretinitis in vivo. Graefes Arch Clin Exp Ophthalmol. 2007;245:221–229. doi: 10.1007/s00417-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. 2009;5:e1000543. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K, Bedke T, Enk AH. Regulatory conversation between antigen presenting cells and regulatory T cells enhance immune suppression. Cell Immunol. 2007;250:1–13. doi: 10.1016/j.cellimm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Rutella S, Lemoli RM. Regulatory T cells and tolerogenic dendritic cells: from basic biology to clinical applications. Immunol Lett. 2004;94:11–26. doi: 10.1016/j.imlet.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Tu WW, Zheng J, Liu YP, Lau YL. Stimulatory or tolerogenic role of CD40-activated B cells depends on the strength of the activation to T cells response. Blood. 2009;114:747–748. [Google Scholar]
- Jung YJ, Seoh JY. Feedback loop of immune regulation by CD4+CD25+ Treg. Immunobiology. 2009;214:291–302. doi: 10.1016/j.imbio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Giroux M, Yurchenko E, St-Pierre J, Piccirillo CA, Perreault C. T regulatory cells control numbers of NK cells and CD8α+ immature dendritic cells in the lymph node paracortex. J Immunol. 2009;179:4492–4502. doi: 10.4049/jimmunol.179.7.4492. [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Fitzpatrick D, Boisvert A, Baba N, Bouguermouh S, Sarfati M, et al. Expression of CD103 identifies human regulatory T-cell subsets. J Allergy Clin Immunol. 2009;118:1342–1349. doi: 10.1016/j.jaci.2006.07.034. [DOI] [PubMed] [Google Scholar]