Abstract
Hypoxia is a major characteristic of the tumor microenvironment, and its effects on immune cells are proposed to be important factors for the process of tumor immune escape. It has been reported that hypoxia affects the function of dendritic cells and the antitumor function of T cells. Here we discuss the effects of hypoxia on T-cell survival. Our results showed that hypoxia induced apoptosis of T cells. Adenosine and adenosine receptors (AR) are important to the hypoxia-related signaling pathway. Using AR agonists and antagonists, we demonstrated that hypoxia-induced apoptosis of T cells was mediated by A2a and A2b receptors. Furthermore, we are the first, to our knowledge, to report that hypoxia significantly inhibited the expression of chemokine C receptor 7 (CCR7) of T cells via the A2R signal pathway, perhaps representing a mechanism of hypoxia-induced apoptosis of T cells. Collectively, our research demonstrated that hypoxia induces T-cell apoptosis by the A2R signaling pathway partly by suppressing CCR7. Blocking the A2R signaling pathway and/or activation of CCR7 can increase the anti-apoptosis function of T cells and may become a new strategy to improve antitumor potential.
Keywords: adenosine receptor, apoptosis, CCR7, T cells
Introduction
Tumor-associated immune escape indicates that tumor microenvironment plays immunosuppressive roles in innate and adaptive immune responses.1 Hypoxia is one of the common characteristics of solid tumor environments. It can affect the function and survival of immune cells that promote the escape of tumor cells.2, 3 We and others have previously demonstrated that hypoxia inhibited the maturation and migration of dendritic cells and indirectly affected T-cell activation and functioning.4, 5, 6 Acting primarily as effecter cells in tumor immunity, T cells are likely to encounter significant differences in oxygen tension, and the direct responses of T cells to hypoxia, therefore, may be an issue of concern.7 Various in vitro studies have indicated that hypoxia can affect the functions of T cells. Hypoxia significantly inhibited lymphocyte expression of interleukin 2 and proliferation8, 9, 10 and altered T-lymphocyte development and effector functions.11 Recently, Kiang et al. have shown that hypoxia triggered a series of biochemical alterations leading to apoptosis of Jurcat T cells.12 Whether hypoxia induces apoptosis of peripherally derived T cells is unclear.
Adenosine and adenosine receptor (AR) are essential components of the hypoxia-associated signaling pathway. Adenosine is an endogenous purine nucleoside and hypoxia can induce adenosine accumulation in the extracellular environment mainly through regulation of cellular metabolism.13 By interacting with G-protein-coupled receptors, adenosine can exert substantial immunosuppressive effects.14, 15 There are currently four clearly defined AR subtypes: Gi-protein-coupled A1 and A3 receptors, and Gs-protein-coupled A2a and A2b receptors.16, 17 T cells predominantly express A2aR and A2bR,17, 18, 19 depending on which adenosine inhibits T-cell-receptor-triggered activation and proliferation of T cells.20, 21 Deficiency of adenosine deaminase, a ubiquitous enzyme in the purine catabolic pathway, causes apoptosis of CD8 low-transitional and CD4+CD8+ double-positive thymocytes.22 Furthermore, T-cell apoptosis was abundant in the thymi of adenosine deaminase (−/−) mice.23 These results indicate that the adenosine signaling pathway is associated with T-cell survival. However, the role of adenosine and AR in the regulation of T-cell survival during hypoxia is unknown.
Chemokine C receptor 7 (CCR7) is an important mediator for lymphocyte homing.24 It has also been reported that CCR7 plays an essential role in T-cell differentiation and two subsets of memory T cells were distinguished depending on the expression of CCR7.25 Recently, a novel anti-apoptotic role for CCR7 was described in T cells,26, 27 and it was shown that the expression of CCR7 is regulated by hypoxia.6, 28 Therefore, we hypothesize that CCR7 may play an essential role in hypoxia-induced apoptosis of T cells. The primary goals of the present study were to elucidate the effect of hypoxia on T-cell apoptosis and to clarify the roles of the AR signal and CCR7 in this process.
Materials and methods
Reagents
5′-N-ethyl-carboxamidoadenosine (NECA), a nonspecific agonist for all ARs, N6-cyclopentyladenosine (CPA), an agonist specific for A1 receptor, 2-[p-2-(carboxy-ethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine (CGS21680), an agonist specific for A2a receptor, N6-(3-iodobenzyl)adenosine-5'-N-methylluronamide (IB-MECA), an agonist specific for A3 receptor, 5-amino-2-(2-furyl)-7-phenylethyl-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidine (SCH58261), an antagonist specific for A2a receptor, phytohaemagglutinin, lipopolysaccharide (LPS), Ficoll and nylon were all obtained from Sigma (St. Louis, MO, USA). N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy] acetamide (MRS1706), an antagonist specific for A2b receptor was from Tocris (St. Louis, MO, USA). RPMI 1640 medium and fetal bovine serum was from Bio International (Auckland, New Zealand). Phycoerythrin (PE)-conjugated anti-CCR7 monoclonal antibody (mAb) and PE-Cy5-conjugated anti-CD3 mAb were purchased from BD PharMingen (San Diego, CA, USA). Annexin V/FITC Kit was from Bender Medsystems (Vienna, Austria).
Cell isolation and culture
Human peripheral blood mononuclear cells were isolated from heparinized blood of healthy donors (n=14) by Ficoll density gradient centrifugation, and suspended in serum-free RPMI 1640 medium. Next, peripheral blood mononuclear cells were cultured on six-well plates for 1 h at 37 °C to remove adherent monocytes, and then T lymphocytes were harvested using a nylon column. T cells were then resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum and 5 µg/ml phytohemagglutinin at a density of 1×106 cells/ml. One milliliter of the cell suspension was seeded per well into 12-well plates, with or without AR agonists/antagonists at normoxic (21% O2, 5% CO2, 74% N2) or hypoxic (1% O2, 5% CO2, 95% N2) conditions as indicated.
Normoxic and hypoxic culture conditions
To acquire normoxic conditions, the isolated T cells were incubated in a humidified cell culture incubator (HERA Cell 150, Kendro Laboratory Products GmbH, Langenselbold, Germany; 21% O2, 5% CO2 and 74% N2). Hypoxic incubation was performed in a sealed, anaerobic work station (Concept 400, Ruskin Technologies, Pencoed, Wales, UK), in which the hypoxic environment (1% O2, 94% N2 and 5% CO2), temperature (37 °C), and humidity (90%) were kept constant.
Apoptosis assay
T cells were cultured under the indicated conditions. After 24 h, cells were harvested and stained with 5 µl Annexin V-fluoresceine isothiocyanate (FITC) and 10 µl propidium iodide in binding buffer for 15 min according to the protocol provided by the manufacturer. The percentage of apoptotic cells was determined by flow cytometry.
Flow cytometric analysis
After stimulation, T cells were collected and washed with cold phosphate-buffered saline three times, then labeled with mouse antihuman PE-conjugated anti-CCR7 mAb and PE-Cy5-conjugated anti-CD3 mAb for 20 min in the dark. The cells were also stained with the corresponding mouse antihuman isotype-matched control antibodies. Then cells were washed with cold phosphate-buffered saline three times. The expression of CCR7 on CD3+ T cells was assayed by flow cytometer (FACSCalibur; Becton Dickinson, Mountain View, CA, USA). A minimum of 10 000 events per sample were collected for phenotypic analysis.
RNA extraction and reverse transcriptase polymerase chain reaction
Total RNA was isolated with Trizol according to the manufacturer's instruction. The RNA concentration was quantified by UV spectrophotometry at 260 nm and the purity and integrity was determined using the A260/A280 ratio and lab-on-chip assay (Agilent bioanalyzer, Santa Clara, CA, USA). Total RNA was treated with DNase (Fermentas, Burlington, Ontario, Canada), and then reverse-transcribed using RevertAid M-MuLV Reverse Transcriptase (Fermentas) with Oligo dT primers (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. One microliter of single-strand cDNA was used as a template for the polymerase chain reaction reaction with DNA polymerase (Invitrogen). Polymerase chain reaction amplification was performed after a hot start at 95 °C for 5 min followed by 30 cycles (94 °C for 30 s, 57 °C for 30 s and 72 °C for 45 s), with a final extension at 72 °C for 7 min. The housekeeping gene β-actin was used as an internal control for the normalization of RNA quantity and quality differences among the samples. Primer pairs for amplification are shown in Table 1.
Table 1. Primer pairs for RT-PCR.
| Gene | Primers | Product (bp) |
|---|---|---|
| β-actin | For 5'-TTGCCGACAGGATGCAGAA-3' | 101 |
| Rev 5'-GCCGATCCACACGGAGTACT-3' | ||
| Adenosine A1 receptor | For 5'-GTTCACAGTTTTTTATTAGTCAC-3' | 109 |
| Rev 5'-AACATGAGTGTCAACTCC-3' | ||
| Adenosine A2a receptor | For 5'-CTTGGGTTCTGAGGAAGCAG-3' | 253 |
| Rev 5'-CAGCAGCTCCTGAACCCTAG-3' | ||
| Adenosine A2b receptor | For 5'-ATCTCCAGGTATCTTCTC-3' | 322 |
| Rev 5'-GTTGGCATAATCCACACAG-3' | ||
| Adenosine A3 receptor | For 5'-CTTGATTACTTCCACTGAGGTGG-3' | 334 |
| Rev 5'-CAACATCTTCTAGGCATCCTCC-3' |
Abbreviation: RT-PCR, reverse transcription polymerase chain reaction.
Statistical analysis
A paired t-test was used in the analysis of CCR7 expression and the T-cell apoptosis. For each test, P-values less than 0.05 (P<0.05) were considered statistically significant. All the statistical analyses were performed using SPSS version 11.5 for Windows (SPSS, Chicago, IL, USA).
Results
Hypoxia induced apoptosis of T lymphocytes
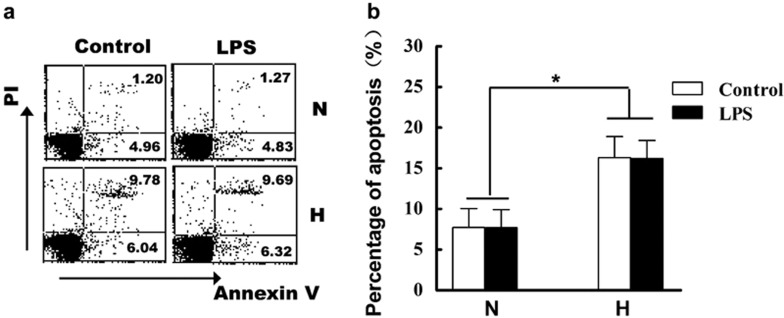
To investigate whether hypoxia affects T-cell apoptosis, we cultured freshly isolated blood T cells under normoxic or hypoxic conditions for 24 h. Next, apoptosis of T cells was assayed. The results demonstrated that hypoxia significantly induced the apoptosis of T cells (Figure 1). As shown in Figure 1b, the percentage of apoptosis in normoxia is 7.72±2.35%, and hypoxia increased the percentage of apoptosis to 16.30±2.60% (P<0.05). The response of T cells to an inflammatory stimulus, LPS, was also evaluated in parallel experiments as a control. The results indicated that LPS did not affect apoptosis of T cells regardless if stimulated under normoxia or hypoxia conditions.
Figure 1.
Hypoxia induces apoptosis of peripheral T cells. T cells were freshly isolated from heparinized blood of healthy donors as described in the section on ‘Materials and Methods'. Next, the isolated T cells were cultured with RPMI 1640 medium (supplemented with 10% fetal bovine serum and 5 µg/ml phytohemagglutinin) under normoxic (N) or hypoxic (H) conditions with or without LPS for 24 h. The percentage of apoptosis was assayed by flow cytometry and one representative result is shown (a). (b) Statistical analysis of apoptosis percentage (n=14). Data were the mean±SD of 14 independent experiments. *P<0.05, compared to the normoxic condition. LPS, lipopolysaccharide; PI, propidium iodide.
Effects of hypoxia on the expression of AR subtypes
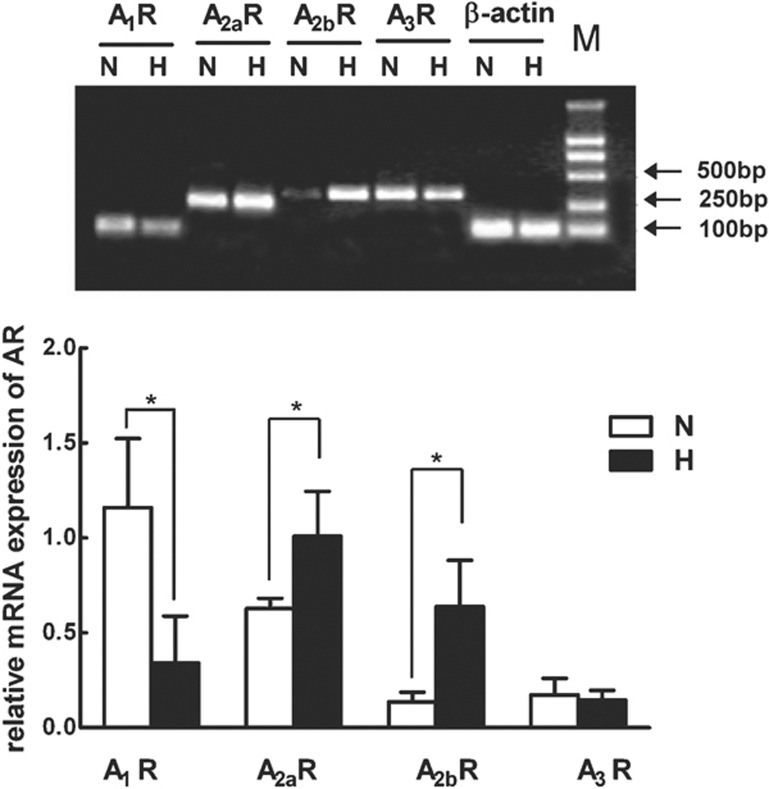
Because hypoxia is a potent stimulus for the release of adenosine, we presume the adenosine–AR signaling pathway may play essential roles in hypoxia-induced apoptosis of T cells. Therefore, we assayed the expression pattern of genes encoding AR subtypes in T cells and the effect of hypoxia on the AR subtypes by using reverse transcription polymerase chain reaction. The experimental data demonstrated that all four AR subtypes were expressed in T cells during hypoxia. A1R was downregulated and A2R (including A2aR and A2bR) was upregulated compared with their levels during normoxia (P<0.05). In addition, we found hypoxia had no effect on A3 receptor (P>0.05) (Figure 2).
Figure 2.
Effects of hypoxia on the expression of AR subtypes in T cells. Total RNA was isolated from human peripheral blood T cells cultured under normoxic (N) or hypoxic (H) conditions. RNA samples isolated from four different donor-derived T cells were reverse transcribed and tested for adenosine receptors A1, A2a, A2b, A3 and β-actin mRNA expression by RT-PCR analysis. Relative levels of adenosine receptors mRNA transcripts were calculated in relation to the values obtained in parallel for the reference gene (β-actin). Data represent mean±SD of four independent experiments. *P<0.05, compared to the normoxic condition. AR, adenosine receptor; RT-PCR, reverse transcription polymerase chain reaction.
Hypoxia induced apoptosis of T cells via A2 receptor
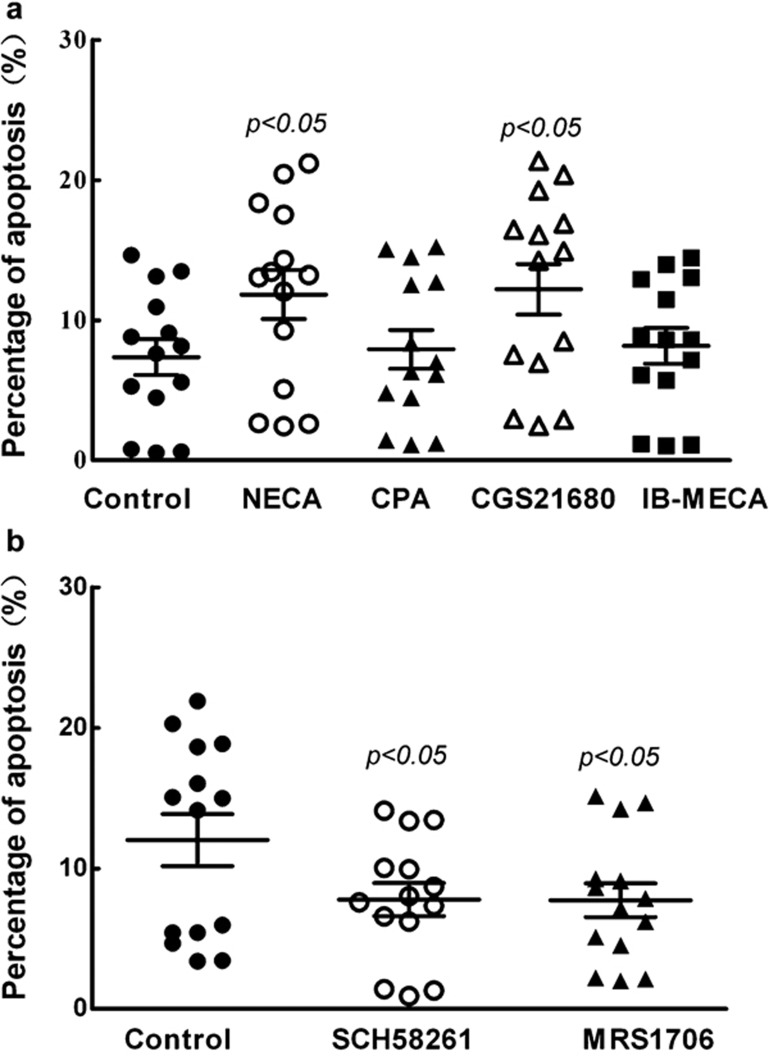
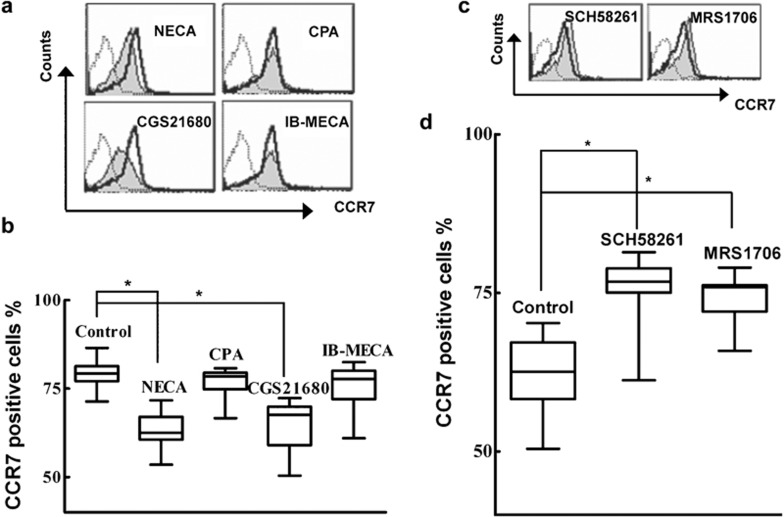
To confirm whether upregulation of A2R plays a role in the regulation of T-cell apoptosis, AR agonists and antagonists were used. Under normoxia, agonists specific for A2a receptor (CGS21680) and nonspecific agonists for A2b receptor (NECA) both increased T-cell apoptosis (P<0.05), whereas A1 receptor-specific agonist (CPA) and A3 receptor-specific agonist (IB-MECA) had no effect on T-cell survival (Figure 3a). These results indicated that A2a, and perhaps A2b, are involved in the regulation of T-cell apoptosis. However, under hypoxic condition, A2b antagonists (MRS1706) prevented hypoxia-induced apoptosis of T cells (P<0.05); the function of A2b in the process of T-cell apoptosis was thus confirmed (Figure 3b). Additionally, A2a receptor antagonists (SCH58261) had similar functioning to A2b receptor antagonists (Figure 3b), and furthermore, demonstrated the function of A2a in the induction of T-cell apoptosis. These results suggest that the A2R signaling pathway is involved in the regulation of T-cell apoptosis.
Figure 3.
Hypoxia induces apoptosis of T cells through adenosine receptors A2a and A2b. The freshly isolated T cells were cultured under normoxic conditions with adenosine receptor agonists and hypoxic conditions with A2R antagonists for 24 h and the apoptosis was determined by flow cytometry. (a) T cells were treated with adenosine receptor agonists under normoxia. Agonists used were specific for A1 receptor (CPA), A2a receptor (CGS21680), A3 receptor (IB-MECA), or nonspecific for A2b receptor (NECA). (b) T cells were treated with adenosine receptor antagonists under hypoxia. The antagonists used were specific for A2a receptor (SCH58261) or A2b receptor (MRS1706). CGS21680, 2-[p-2-(carboxy-ethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine; CPA, N6-cyclopentyladenosine; IB-MECA, N6-(3-iodobenzyl)adenosine-5'-N-methylluronamide; MRS1706, N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy] acetamide; NECA, 5′-N-ethyl-carboxamidoadenosine; SCH58261, 5-amino-2-(2-furyl)-7-phenylethyl-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidine.
CCR7 mediated the apoptosis of T cells and hypoxia inhibited the expression of CCR7 via A2R
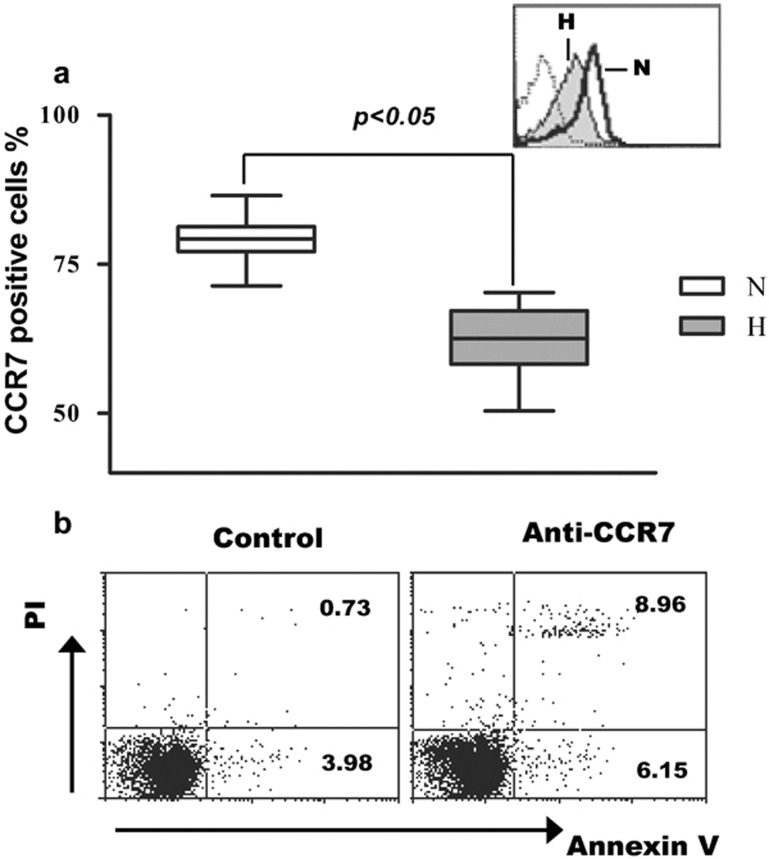
CCR7 expression is important for lymphocyte homing to tissues, and CCR7 also plays a role in CD8+ T-cell protection from apoptosis. To determine whether CCR7 regulates hypoxia-induced apoptosis of T cells, we assayed the effects of hypoxia on the expression of CCR7. The results demonstrated that hypoxia significantly suppressed CCR7 expression in T cells (P<0.05) (Figure 4a), and blocking the CCR7 signaling pathway with anti-CCR7 antibody was associated with a significant increase in the apoptosis of T cells (P<0.05) (Figure 4b). These results indicate that CCR7 may play an essential role in hypoxia-induced T-cell apoptosis.
Figure 4.
Hypoxia inhibits the expression of CCR7, and CCR7 mediates the apoptosis of T cells. T cells (n=14) were cultured under normoxic and hypoxic conditions for 24 h and the CCR7 expressions were determined by flow cytometry. (a) The thin line represents staining with a control isotype-matched antibody. The bold line and grey solid histograms represent normoxia and hypoxia, respectively. (b) T cells were cultured under normoxic condition with anti-CCR7 antibody or isotype control for 24 h and the apoptosis of T cells was assayed by flow cytometry. CCR7, chemokine C receptor 7; H, hypoxia; N, normoxia.
To evaluate whether A2R-regulated apoptosis of T cells under hypoxia is mediated by CCR7, a pharmacological approach using AR agonists and antagonists was chosen. T cells were treated with the AR agonist for 24 h under normoxic condition and the expression of CCR7 was assayed. As shown in Figure 5a and b, nonspecific agonist, NECA and A2a receptor-specific agonist, CGS21680, can significantly inhibit CCR7 expression (P<0.05), whereas agonists for A1 and A3 receptors had no effects on the expression of CCR7. To further determine whether A2b regulates CCR7 expression while accounting for the finding that hypoxia upregulated A2R, we cultured T cells under hypoxic conditions with A2R antagonists and assayed CCR7 expression. The results demonstrated that inactivation of both A2a and A2b by antagonists induced the upregulation of CCR7 (P<0.05) (Figure 5c and d). Taken together, we confirmed that A2R-mediated induction of T-cell apoptosis under hypoxia partially acts via A2R suppression of CCR7.
Figure 5.
Hypoxia downregulates the expression of CCR7 through the adenosine receptor A2. The freshly isolated T cells (n=14) were cultured under normoxic conditions with adenosine receptor agonists and under hypoxic conditions with adenosine receptor antagonists for 24 h. The expression of CCR7 was assayed by flow cytometry. (a) T cells were treated with adenosine receptor agonists under normoxia. Agonists used were specific for the A1 receptor (CPA), the A2a receptor (CGS21680), the A3 receptor (IB-MECA), and nonspecific for A2b receptor (NECA). (b) Statistical analysis of effects of adenosine receptor agonists on the expression of CCR7 under normoxia. (c) T cells were treated with A2aR (SCH58261) and A2bR (MRS1706) antagonists under hypoxia. (d) Statistical analysis of effects of A2 agonists on the expression of CCR7 under hypoxia. CCR7, chemokine C receptor 7; CGS21680, 2-[p-2-(carboxy-ethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine; CPA, N6-cyclopentyladenosine; IB-MECA, N6-(3-iodobenzyl)adenosine-5'-N-methylluronamide; MRS1706, N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy] acetamide; NECA, 5′-N-ethyl-carboxamidoadenosine; SCH58261, 5-amino-2-(2-furyl)-7-phenylethyl-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidine.
Discussion
Physiologic adaptation and pathophysiological response of immune cells to hypoxia is an area of intense investigation. The effect of hypoxia on the function of immune cells is considered to be essential mechanism for tumor immune escape. Many in vitro studies have shown that hypoxia regulates cytokines and the proliferation of lymphocytes. In this study, we found that hypoxia can induce the apoptosis of peripheral T cells. Apoptosis of T cells induced by hypoxia may represent a mechanism for tumor immunosuppression. However, it has been reported that hypoxia protected activated T cells from activation-induced cell death via the HIF-1-adrenomedullin cascade.7 These contradictory conclusions indicate that the effects of hypoxia on T-cell survival are correlated with the activation status of T cells. Furthermore, our results showed that an inflammatory stimulus, LPS, had no effect on the apoptosis of T cells regardless if stimulated under normoxic or hypoxic conditions, suggesting that hypoxia, but not mediators such as LPS, is the potent stimulus inducing T-cell apoptosis.
Hypoxia can cause the marked accumulation of extracellular adenosine29 and change the cellular function mediated by different ARs. The hypoxic microenvironment of solid tumors induces accumulation of adenosine. This accumulation of adenosine plays an essential role in cell-mediated antitumor immune responses. It has been reported that adenosine inhibited T-cell adhesion to tumor cells and cytotoxic activity via signaling primarily through A2a and A3 adenosine receptors.1 Furthermore, it has been reported that the adenosine signaling pathway was associated with T-cell survival,22, 23 but whether hypoxia-induced apoptosis of T cells is mediated by the adenosine signal, in addition to knowing which subtypes of AR are involved in this process, is unclear. To our knowledge, we are the first group to report that hypoxia differentially regulated AR subtype expression in T cells. Our results showed that hypoxia upregulated A2aR and A2bR, and the upregulation of A2R indicated that A2R may play an essential role in hypoxia-induced apoptosis of T cells. To test the A2R function on the induction of T-cell apoptosis, AR agonists and antagonists were used. Agonists for A2aR (CGS21680) increased T-cell apoptosis under normoxic condition. Nonspecific agonists for A2bR (NECA) also induced apoptosis of T cells. Under hypoxic conditions, specific antagonists for A2aR and A2bR reduced T-cell apoptosis, which further supported the role of A2R in the regulation of T-cell apoptosis. Furthermore, hypoxia downregulated A1R and did not change the expression of A3R, and as expected, agonist and antagonist experiments proved A1R and A3R indeed had no effect on T-cell survival. In conclusion, we have shown that hypoxia regulated the apoptosis of T cells mainly through the A2R signal pathway. This may represent a crucial mechanism by which the tumor microenvironment inhibits the antitumor activity of T lymphocytes.
For T cells, CCR7 not only exerts functions for lymphocyte homing but also plays an essential role in T-cell protection from apoptosis.27 The CCR7+ subset of circulating CD8+ T cells is significantly less sensitive to apoptosis than CD8+ T cells lacking CCR7. CCR7 expression correlated with higher Bcl-2 levels and signaling mechanism studies demonstrated that CCR7 protects effector T cells from apoptosis through the phosphatidylinositol 3-kinase/Akt pathway.26 CCR7 also plays essential roles in regulating dendritic cell apoptosis via Akt-mediated phosphorylation/inhibition of glycogen synthase kinase-3beta.30, 31 In this study, using an anti-CCR7 antibody, we further verified the anti-apoptosis effect of CCR7 (as shown in Figure 4b). We found hypoxia significantly inhibited CCR7 expression on T cells which implied that CCR7 may play important roles in hypoxia-induced T-cell apoptosis. Interestingly, our results demonstrated that hypoxia-induced downregulation of CCR7 in T cells was regulated by the A2R signaling pathway. These results suggest that hypoxia-induced inhibition of CCR7 regulated by the A2R signaling pathway may weaken the protective role of CCR7 in T-cell apoptosis and supports essential roles of CCR7 in hypoxia-induced apoptosis of T cells. Taken together, our results indicate that hypoxia-induced apoptosis of T cells may be mediated by the A2R signaling pathway by partially suppressing CCR7 expression. Therefore, novel cancer immunotherapy protocols may include the application of antagonists of A2R and/or activation of CCR7 to prevent the apoptosis of T cells and lead to more efficient elimination of tumors by T lymphocytes.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30671902 and No. 30872321) and the Natural Science Foundation of Shandong Province (No. Y2008C02 and No. Y2006C122).
References
- Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int J Oncol. 2008;32:527–535. [PubMed] [Google Scholar]
- Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- Zhao P, Li XG, Yang M, Shao Q, Wang D, Liu S, et al. Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol Immunol. 2008;45:2187–2195. doi: 10.1016/j.molimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Qu X, Yang MX, Kong BH, Qi L, Lam QL, Yan S, et al. Hypoxia inhibits the migratory capacity of human monocyte-derived dendritic cells. Immunol Cell Biol. 2005;83:668–673. doi: 10.1111/j.1440-1711.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Darmanin S, Fu Q, Chen J, Cui H, Wang J, et al. Hypoxia suppresses the production of matrix metalloproteinases and the migration of human monocyte-derived dendritic cells. Eur J Immunol. 2005;35:3468–3477. doi: 10.1002/eji.200526262. [DOI] [PubMed] [Google Scholar]
- Makino Y, Nakamura H, Ikeda E, Ohnuma K, Yamauchi K, Yabe Y, et al. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J Immunol. 2003;171:6534–6540. doi: 10.4049/jimmunol.171.12.6534. [DOI] [PubMed] [Google Scholar]
- Naldini A, Carraro F. Hypoxia modulates cyclin and cytokine expression and inhibits peripheral mononuclear cell proliferation. J Cell Physiol. 1999;181:448–454. doi: 10.1002/(SICI)1097-4652(199912)181:3<448::AID-JCP8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, Juneau PL, Masserant S. Influence of tumour physico-chemical conditions on interleukin-2-stimulated lymphocyte proliferation. Br J Cancer. 1992;66:619–622. doi: 10.1038/bjc.1992.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerberg AL, Goldberg LI, Lederman HM. Effects of hypoxia on interleukin-2 mRNA expression by T lymphocytes. Crit Care Med. 1994;22:197–203. doi: 10.1097/00003246-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Krishnan S, Lu X, Li Y. Inhibition of inducible nitric-oxide synthase protects human T cells from hypoxia-induced apoptosis. Mol Pharmacol. 2008;73:738–747. doi: 10.1124/mol.107.041079. [DOI] [PubMed] [Google Scholar]
- Gorlach A. Control of adenosine transport by hypoxia. Circ Res. 2005;97:1–3. doi: 10.1161/01.RES.0000174112.36064.77. [DOI] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- Sugiyama H, Chen P, Hunter M, Taffs R, Sitkovsky M. The dual role of the cAMP-dependent protein kinase C alpha subunit in T-cell receptor-triggered T-lymphocytes effector functions. J Biol Chem. 1992;267:25256–25263. [PubMed] [Google Scholar]
- Benveniste P, Cohen A. p53 expression is required for thymocyte apoptosis induced by adenosine deaminase deficiency. Proc Natl Acad Sci USA. 1995;92:8373–8377. doi: 10.1073/pnas.92.18.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apasov SG, Blackburn MR, Kellems RE, Smith PT, Sitkovsky MV. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling. J Clin Invest. 2001;108:131–141. doi: 10.1172/JCI10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–140. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Kim JW, Ferris RL, Whiteside TL. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res. 2005;11:7901–7910. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- Parmiani G, Castelli C, Rivoltini L. Chemokine receptor 7, a new player in regulating apoptosis of CD8+ T cells in cancer patients. Clin Cancer Res. 2005;11:7587–7588. doi: 10.1158/1078-0432.CCR-05-1965. [DOI] [PubMed] [Google Scholar]
- Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–3734. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL. CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J Immunol. 2009;183:6282–6295. doi: 10.4049/jimmunol.0804093. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sánchez N, Riol-Blanco L, de la Rosa G, Puig-Kröger A, García-Bordas J, Martín D, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104:619–625. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]