Abstract
The novel interleukin (IL)-1 family cytokine IL-33 has been shown to activate T helper 2 (Th2) lymphocytes, mast cells and basophils to produce an array of proinflammatory cytokines, as well as to mediate blood eosinophilia, IgE secretion and hypertrophy of airway epithelium in mice. In the present study, we characterized the activation of human eosinophils by IL-33, and investigated the underlying intracellular signaling mechanisms. IL-33 markedly enhanced eosinophil survival and upregulated cell surface expression of the adhesion molecule intercellular adhesion molecule (ICAM)-1 on eosinophils, but it suppressed that of ICAM-3 and L-selectin. In addition, IL-33 mediates significant release of the proinflammatory cytokine IL-6 and the chemokines CXCL8 and CCL2. We found that IL-33-mediated enhancement of survival, induction of adhesion molecules, and release of cytokines and chemokines were differentially regulated by activation of the nuclear factor (NF)-κB, p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) pathways. Furthermore, we compared the above IL-33 activities with two structurally and functionally related cytokines, IL-1β and IL-18. IL-1β, but not IL-18, markedly upregulated cell surface expression of ICAM-1. IL-1β and IL-18 also significantly enhanced eosinophil survival, and induced the release of IL-6 and chemokines CXCL8 and CCL2 via the activation of the NF-κB, p38 MAPK and ERK pathways. Synergistic effects on the release of IL-6 were also observed in combined treatment with IL-1β, IL-18 and IL-33. Taken together, our findings provide insight into IL-33-mediated activation of eosinophils via differential intracellular signaling cascades in the immunopathogenesis of allergic inflammation.
Keywords: allergy, cytokines, eosinophils, signal transduction
Introduction
Interleukin (IL)-33 was discovered as the eleventh member of the IL-1 family, which also includes IL-1β and IL-18.1, 2 It is a novel T helper 2 (Th2) proinflammatory cytokine abundantly expressed in human smooth muscle cells and bronchial epithelial cells.1 Administration of IL-33 in mice not only initiates the production of IgE and the Th2 cytokines IL-5 and IL-13, but also induces pathological changes including blood and bronchoalveolar lavage fluid eosinophilia, airway hyper-responsiveness, and epithelial cell hyperplasia and hypertrophy.1, 3, 4
Significantly higher serum IL-33 concentration has been reported in patients with Japanese cedar pollinosis than in healthy control subjects.5 In addition, IL-33 has been shown to promote the maturation and survival of human mast cells and to induce their secretion of cytokines IL-5 and IL-13, which promote the survival and effector functions of eosinophils.6, 7 Together with the results from mouse models,1, 3, 4 these results indicate that IL-33 might play a pivotal role in the exacerbation of allergic inflammation in allergic diseases mediated by the activation of eosinophils.
It was shown that IL-33 can signal via its receptor ST2 and activate downstream signaling molecules including inhibitor kappa B (IκB)-α and mitogen-activated protein kinases (MAPK), and abrogate IL-33-induced IL-5 and IL-13 production from CD4+ T lymphocytes, possibly by blocking nuclear factor (NF)-κB and MAPK pathways.1, 2, 8 In addition to the evidence of enhanced NF-κB activity in peripheral blood mononuclear cells of asthmatic patients,9 our previous studies also demonstrated that NF-κB signal transduction, p38 MAPK and extracellular signal-regulated kinase (ERK) pathways played pivotal roles in regulating IL-3, IL-5 and granulocyte macrophage colony-stimulating factor-induced intercellular adhesion molecule (ICAM)-1 expression and Th17 cytokine-elicited cytokine production from human eosinophils.10, 11
In an attempt to further investigate the activation of eosinophils induced by IL-33, we elucidated the intracellular signaling mechanisms regulating the survival, adhesion, and cytokine and chemokine release from eosinophils activated by IL-33, and we compared the effects of two other structurally and functionally related IL-1 family cytokines, IL-1β and IL-18.
Materials and methods
Reagents
Recombinant human IL-1β, IL-18 and IL-33 were purchased from PeproTec Inc. (Rocky Hill, NJ, USA), MBL (Naka-ku Nagoya, Japan), and R&D Systems Inc. (Minneapolis, MN, USA), respectively. IκB-α phosphorylation inhibitor BAY11-7082, ERK inhibitor U0126, c-Jun N-terminal kinase (JNK) inhibitor SP600125, p38 MAPK inhibitor SB203580, phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 and Janus kinase (JAK) inhibitor AG490 were purchased from Calbiochem Corp. (San Diego, CA, USA). SB203580 was dissolved in water whereas U0126, LY294002, SP600125, AG490 and BAY11-7082 were dissolved in dimethyl sulfoxide (DMSO). In all studies, the concentration of DMSO was 0.1% (v/v).
Endotoxin-free solutions
Cell culture medium was purchased from Gibco Invitrogen Corp. (Carlsbad, CA, USA), free of detectable lipopolysaccharide (LPS, <0.1 EU/ml). All other solutions were prepared using pyrogen-free water and sterile polypropylene plasticware. No solution contained detectable LPS, as determined by the Limulus amebocyte lyase assay (sensitivity limit 12 pg/ml; Biowhittaker Inc., Walkersville, MD, USA).
Isolation of human blood eosinophils from buffy coat and eosinophil culture
Fresh human buffy coat obtained from healthy volunteers of the Hong Kong Red Cross Blood Transfusion Service was diluted 1:2 with phosphate-buffered saline (PBS) at 4 °C and centrifuged using an isotonic Percoll solution (density, 1.082 g/ml; Amersham Pharmacia Biotech, Uppsala, Sweden) for 30 min at 1000g. The eosinophil-rich granulocyte fraction was collected and washed twice with cold PBS containing 2% fetal bovine serum (FBS) (Gibco Invitrogen Corp.). The cells were then incubated with anti-CD16 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) at 4 °C for 45 min and CD16-positive cells were depleted by passing through an LS+column (Miltenyi Biotec) within a magnetic field. With this preparation, the flow-through fraction contained eosinophils with a purity of at least 99% as assessed by Hemacolor rapid blood smear stain (E Merck Diagnostica, Darmstadt, Germany). The isolated eosinophils were cultured in RPMI 1640 medium (Gibco Invitrogen Corp.) supplemented with 10% FBS and 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Gibco Invitrogen Corp.).
Apoptosis and viability assay
Apoptosis and viability of eosinophils were assessed by the TACS Annexin V-fluorescein isothiocyanate (FITC) assay (Trevigen Inc., Gaithersburg, MD, USA) using flow cytometry (FACSCalibur, BD Biosciences Corp., San Jose, CA, USA) of eosinophils gated on the basis of their forward and side light scatter with any cell debris excluded from analysis. The population of viable cells was characterized by low mean fluorescence intensity (MFI) of both Annexin V-FITC and propidium iodide (PI).
Quantitative analysis of IL-6, CXCL8 and CCL2
Concentrations of the proinflammatory cytokine IL-6 and the chemokines CXCL8 and CCL2 in culture supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) kits from BD Pharmingen (San Diego, CA, USA).
Western blot analysis
Eosinophils were washed with ice-cold PBS and lysed in 0.2 ml lysis buffer (20 mM Tris-HCl, pH 8.0, 120 mM NaCl, 1% Triton X-100, 10 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 0.05% 2-mercaptoethanol, 1X protease inhibitors). Cell debris was removed by centrifugation at 14 000g for 15 min, and the supernatant was boiled in Laemmli sample buffer (Bio-Rad Laboratory, Hercules, CA, USA) for 5 min. Equal amounts of protein were subjected to SDS–polyacrylamide gel electrophoresis before blotting onto a polyvinylidene difluoride membrane (GE Healthcare, Piscataway, NJ, USA). The membrane was blocked with 5% skim milk in Trisbuffered saline with 0.05% Tween 20, pH 7.6 for 1 h at room temperature and probed with primary rabbit antihuman glyceraldehyde-3-phosphate dehydrogenase or mouse antihuman ST2 antibodies (R&D Systems) at 4 °C overnight. After washing, the membrane was incubated with secondary goat antirabbit or sheep antimouse antibodies coupled to horseradish peroxidase (GE Healthcare) for 1 h at room temperature. Antibody–antigen complexes were then detected using an enhanced chemiluminescence detection system (GE Healthcare).
Immunofluorescence staining and flow cytometry
To determine the expression of adhesion molecules on the cell surface, the cells were washed and resuspended with cold PBS after the preceding treatments. After blocking with 2% human pooled serum for 20 min at 4 °C and washing with cold PBS, cells were incubated with FITC-conjugated mouse antihuman ICAM-1, mouse antihuman ICAM-3, mouse antihuman L-selectin antibodies or mouse IgG1κ isotype (R&D Systems) for 30 min at 4 °C in the dark. After washing, the cells were resuspended in 1% paraformaldehyde as a fixative and subjected to analysis.
To determine the intracellular expression of phosphorylated signaling molecules, cells were fixed with 4% paraformaldehyde for 10 min at 37 °C after the preceding treatments. After centrifugation, cells were permeabilized in ice-cold methanol for 30 min and then stained with FITC-conjugated mouse antihuman phosphorylated ERK1/2, phosphorylated p38 MAPK, phosphorylated IκB-α or mouse IgG1 antibodies (BD Pharmingen) for 30 min at 4 °C in the dark. Cells were then washed, resuspended and subjected to analysis. Expression of surface adhesion molecules and intracellular phosphorylated signaling molecules of 10 000 viable cells was analyzed by flow cytometry (FACSCalibur, BD Biosciences) as MFI, which includes both the changes of target molecule expression in individual cells and the percentage of cells expressing the target molecules.
Statistical analysis
All data are expressed as the means±SEM. Differences between groups were assessed by one-way ANOVA. A probability (P) <0.05 was considered significantly different. When ANOVA indicated a significant difference, the Bonferroni post hoc test was then used to assess the difference between groups. All analyses were performed using the Statistical Package for the Social Sciences statistical software for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Protein expression of IL-33 receptor
We examined the protein expression of the IL-33 receptor, ST2, in human eosinophils, as well as in several other control cell types: human neutrophils, human mast cell line (HMC)-1, human keratinocyte cell line HaCaT and human T lymphocytes. As shown in Figure 1, ST2 was constitutively expressed at the protein level in human eosinophils and T lymphocytes.
Figure 1.
Representative western blot of the protein expression of the IL-33 receptor ST2 in human eosinophils, neutrophils, HaCaT cells, HMC-1 cells and T lymphocytes. Total proteins were extracted from eosinophils, neutrophils, HaCaT cells, HMC-1 cells and T lymphocytes (1×107 cells). Equal amounts of protein (15 μg) were analyzed by western blot. Experiments were performed in triplicate with essentially identical results, and a representative blot is shown. HMC, human mast cell line; IL, interleukin.
Effects of IL-1β, IL-18 and IL-33 on the survival of human eosinophils
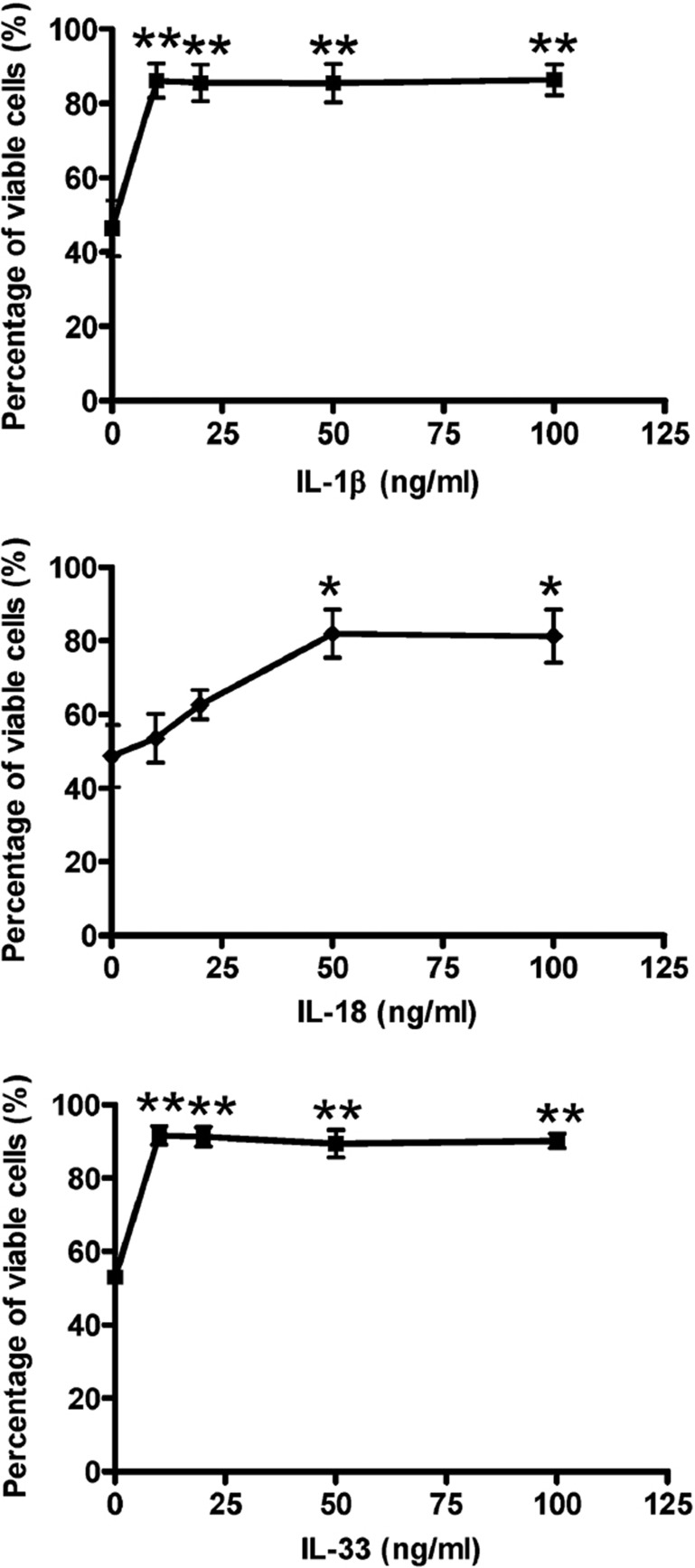
After incubation with IL-1β, IL-18 or IL-33 (10–100 ng/ml) for 48 h, the percentage of viable eosinophils increased significantly from 53 to 89% (Figure 2a), 46 to 86% (Figure 2b), and 49 to 82% (Figure 2c), respectively (all P<0.05). Among these cytokines, IL-18 enhanced the survival of eosinophils in a dose-dependent manner.
Figure 2.
Effects of IL-1β, IL-18 and IL-33 on the viability of human eosinophils. Eosinophils (5×105 per well) were cultured with or without (a) IL-1β, (b) IL-18 or (c) IL-33 (10–100 ng/ml) for 48 h in a 24-well plate. Percentage of viability of eosinophils was assessed by the TACS Annexin V-FITC assay using flow cytometry. *P<0.05, **P<0.01 when compared with medium control. FITC, fluorescein isothiocyanate; IL, interleukin.
Effects of IL-1β, IL-18 and IL-33 on the expression of adhesion molecules on the surface of human eosinophils
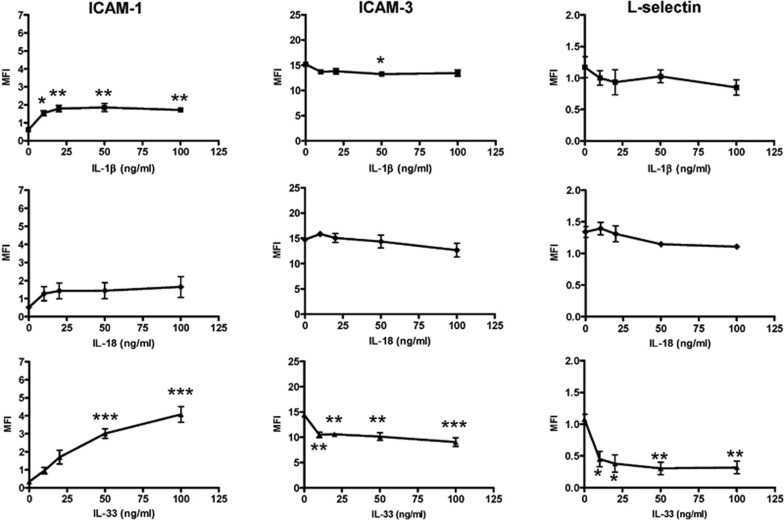
As shown in Figure 3, IL-33 markedly upregulated the expression of ICAM-1 on human eosinophils in a dose-dependent manner, but it significantly suppressed ICAM-3 and L-selectin expression (all P<0.05). IL-1β stimulated a significant increase in the expression of ICAM-1 on eosinophils, but not of ICAM-3 or L-selectin; IL-18 did not elicit any significant modulation of ICAM-1, ICAM-3 or L-selectin expression on eosinophils.
Figure 3.
Effects of IL-1β, IL-18 and IL-33 on surface expression of ICAM-1, ICAM-3 and L-selectin on human eosinophils. Eosinophils (5×105 cells) were cultured with or without IL-1β, IL-18 or IL-33 (10–100 ng/ml) for 16 h. The surface expression of (a) ICAM-1, (b) ICAM-3 and (c) L-selectin on 10 000 cells was analyzed by flow cytometry as MFI, which was normalized by subtracting the appropriate isotypic control and shown as the arithmetic mean±SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 when compared with medium control. ICAM, intercellular adhesion molecule; IL, interleukin; MFI, mean fluorescence intensity.
Effects of IL-1β, IL-18 and IL-33 on chemokine and cytokine release from human eosinophils
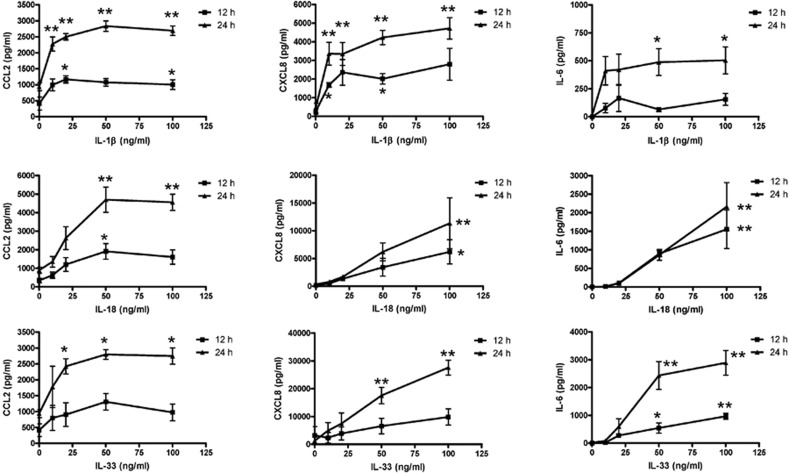
The cytokine expression profile in culture supernatants of eosinophils upon activation by IL-1β, IL-18 and IL-33 was first assessed semiquantitatively using the antibody-based RayBio human cytokine array V (RayBiotech Inc., Norcross, GA, USA). IL-1β, IL-18 and IL-33 (50 ng/ml) activated eosinophils (1.5×106 cells) to prominently induce the release of the chemokines CCL2 and CXCL8 and the proinflammatory cytokine IL-6 from among the 79 different cytokines that were screened after 48 h of incubation (data not shown). As shown in Figure 4, IL-1β, IL-18 and IL-33 activated eosinophils to release CCL2, CXCL8 and IL-6 in a dose-dependent manner, with higher release at 48 h than that at 24 h. Although IL-1β, IL-18 and IL-33 mediated comparable induction of CCL2 release, IL-33 elicited the strongest stimulation of CXCL8 and IL-6 release among the three IL-1 family cytokines. These induced cytokine and chemokine releases could be significantly suppressed by the transcriptional inhibitor actinomycin D and the translational inhibitor cycloheximide (data not shown), suggesting that IL-1β, IL-18 and IL-33 induce the release of newly synthesized CCL2, CXCL8 and IL-6 rather than preformed chemokines and cytokines from eosinophils.
Figure 4.
Effects of IL-1β, IL-18 and IL-33 on CCL2, CXCL8 and IL-6 release from human eosinophils. Eosinophils (1×106 cells) were cultured with or without IL-1β, IL-18 or IL-33 (50 ng/ml) for 12 and 24 h. Cell-free culture supernatant was collected and (a) CCL2, (b) CXCL8 and (c) IL-6 released into the supernatant were quantified using ELISA. Results are expressed as the arithmetic mean±SEM of three independent experiments. *P<0.05, **P<0.01 when compared with medium control. ELISA, enzyme-linked immunosorbent assay; IL, interleukin.
Synergistic effects of IL-1β, IL-18 and IL-33 on IL-6 release from human eosinophils
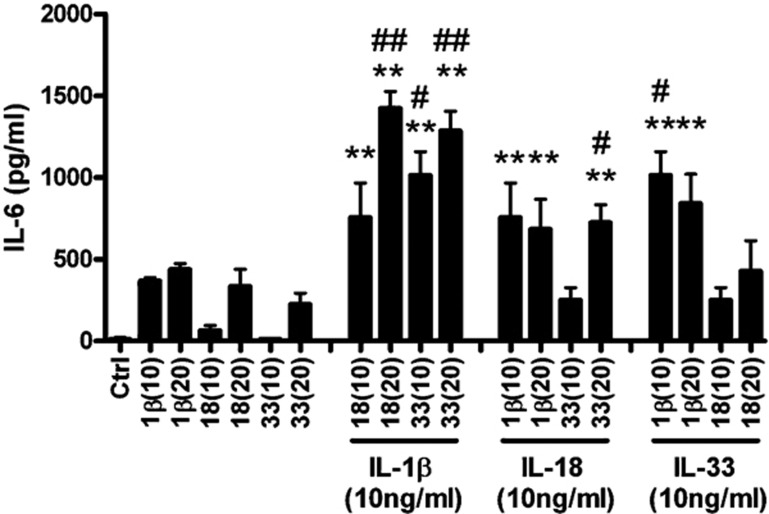
Figure 5 illustrates that combined treatment with IL-1β (10 ng/ml) and IL-33 (10 ng/ml) resulted in a significant synergistic increase in proinflammatory cytokine IL-6 release, whereas only additive effects were obtained for combined treatment with IL-1β (10 ng/ml) and IL-18 (10 ng/ml), or IL-18 (10 ng/ml) and IL-33 (10 ng/ml). Synergistic effects on IL-6 release could be further enhanced when the concentration of IL-33 was doubled (20 ng/ml) in combination with a fixed IL-1β concentration (10 ng/ml). In addition, synergistic increases in IL-6 production from human eosinophils resulted from combined treatment with IL-1β (10 ng/ml) and IL-18 (20 ng/ml), as well as with IL-18 (10 ng/ml) and IL-33 (20 ng/ml).
Figure 5.
Synergistic effects of IL-1β, IL-18 and IL-33 on IL-6 release from human eosinophils. Eosinophils (1×106 cells) were cultured with different concentrations of IL-1β, IL-18 and IL-33 in combination for 24 h. Cell-free culture supernatant was collected and IL-6 released into the supernatant was quantified using ELISA. Results are expressed as the arithmetic mean±SEM of three independent experiments. **P<0.01 when compared with medium control. #P<0.05, # #P<0.01 when compared with the sum of IL-6 production in treatment with individual cytokines (IL-33, IL-1β or IL-18) added alone. Ctrl, medium control; IL, interleukin; 1β(10), IL-1β (10 ng/ml); 1β(20), IL-1β (20 ng/ml); 18(10), IL-18 (10 ng/ml); 18(20), IL-18 (20 ng/ml); 33(10), IL-33 (10 ng/ml); 33(20), IL-33 (20 ng/ml).
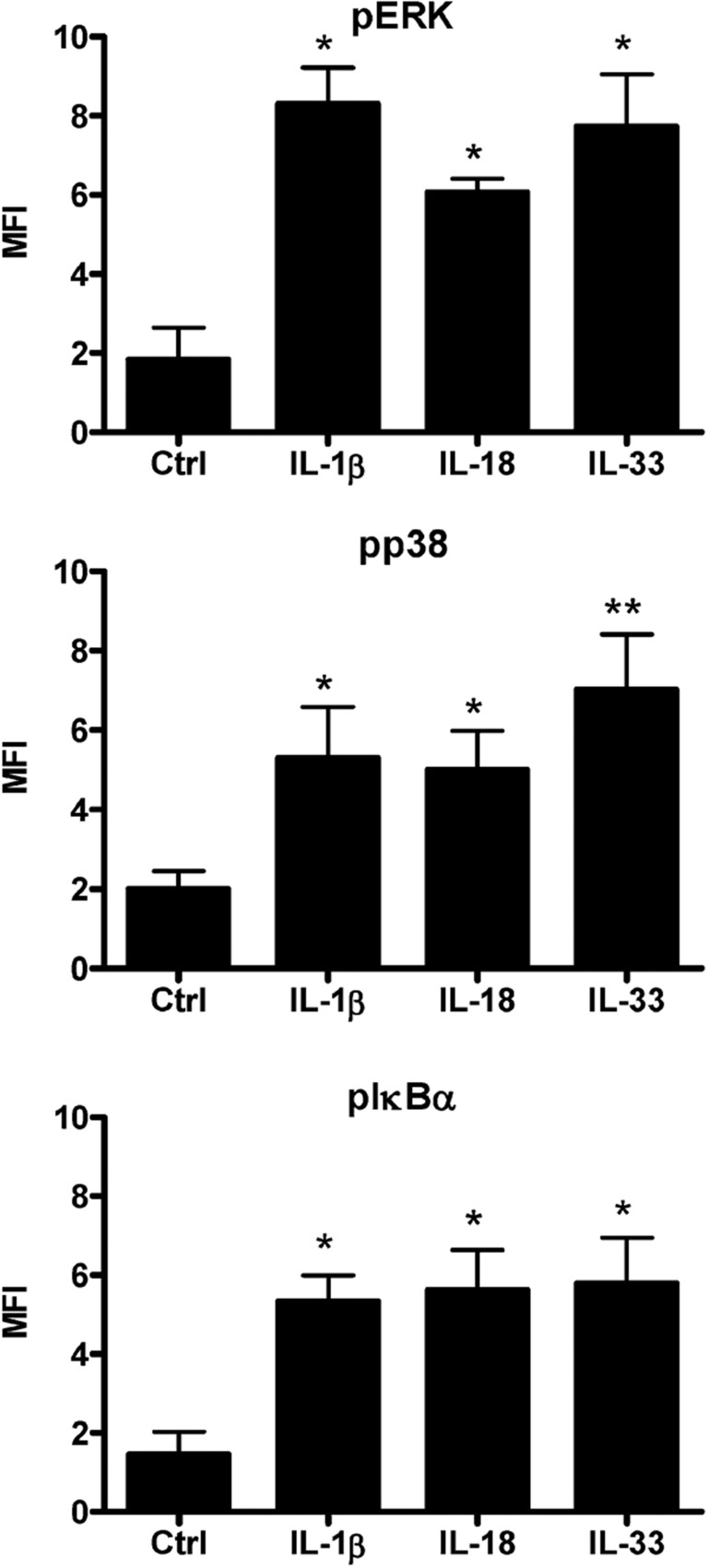
Intracellular staining of phospho-ERK, phospho-p38 MAPK and phospho-IκB-α in human eosinophils
As shown in Figure 6, ERK, p38 MAPK and IκB-α were rapidly activated in eosinophils upon stimulation with IL-1β, IL-18 or IL-33 for 15 min. Phosphorylation of ERK, p38 MAPK and IκB-α in eosinophils was differentially induced upon IL-1β, IL-18 and IL-33 stimulation.
Figure 6.
Activation of ERK, p38 MAPK, NF-κB in human eosinophils upon IL-1β, IL-18 and IL-33 stimulation. Eosinophils (5×105 cells) were cultured with or without IL-1β, IL-18 or IL-33 (50 ng/ml) for 15 min. After fixation and permeabilization, the intracellular contents of phosphorylated (a) ERK, (b) p38 MAPK and (c) IκB-α in 10 000 permeabilized eosinophils were measured by intracellular staining and flow cytometry. Results were normalized by subtracting the appropriate isotypic control and shown as the arithmetic mean±SEM of three independent experiments. *P<0.05, **P<0.01 when compared with medium control. Ctrl, medium control; ERK, extracellular signal-regulated kinase; IL, interleukin; MAPK, mitogen-activated protein kinase; NF, nuclear factor.
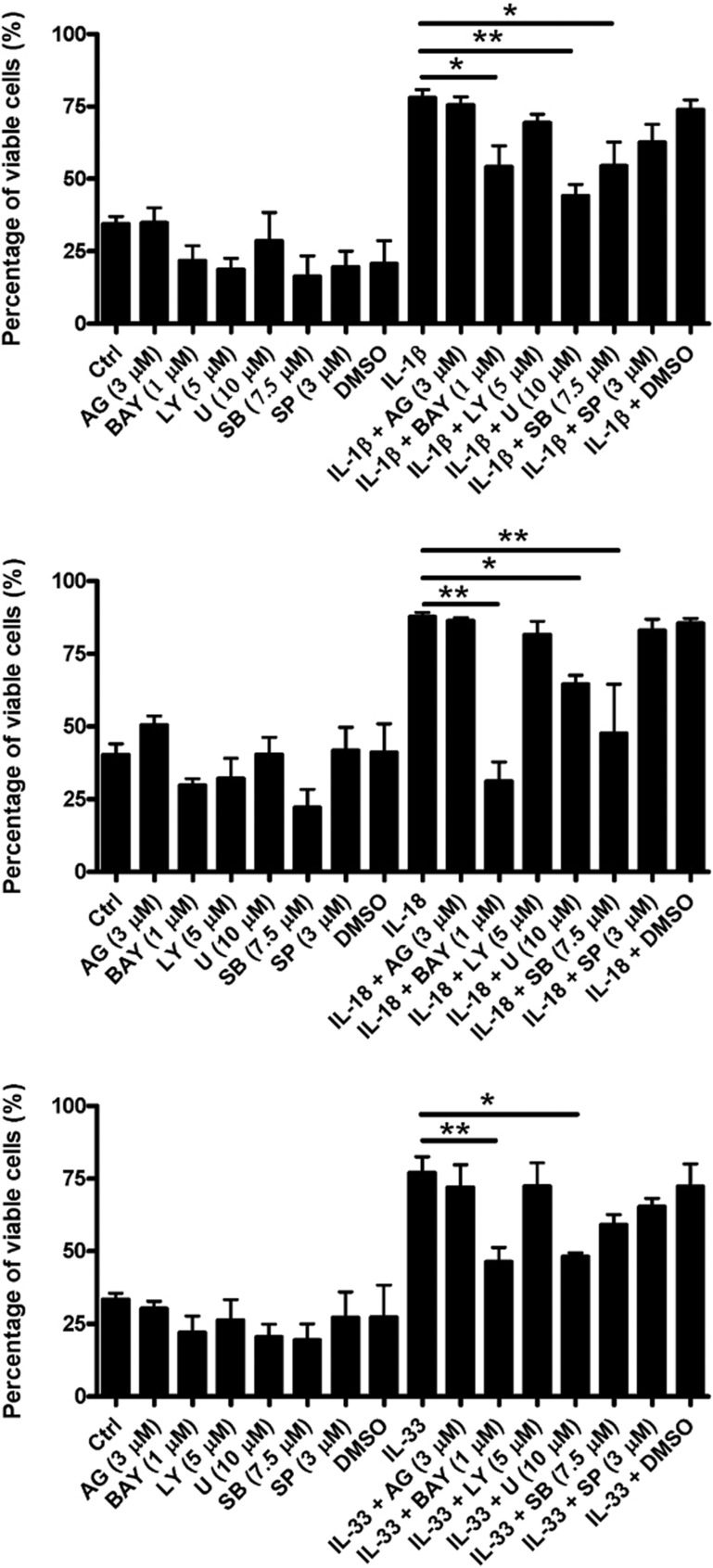
Effects of different inhibitors on IL-1β-, IL-18- and IL-33-induced survival enhancement of human eosinophils
Figure 7 shows that pre-treatment with the IκB kinase inhibitor BAY11-7082, the ERK inhibitor U0126 or the p38 MAPK inhibitor SB203580 for 30 min significantly abolished both IL-1β- and IL-18-induced survival enhancement of human eosinophils, while pre-treatment with BAY11-7082 and U0126 for 30 min also markedly abolished IL-33-induced survival enhancement of human eosinophils (all P<0.05). No significant difference of cell viability was found between the medium control (Ctrl) group and the groups treated with different signaling inhibitors, namely, AG490 (3 μM), BAY11-7082 (1 μM), LY294002 (5 μM), U0126 (10 μM), SB203580 (7.5 μM) and SP600125 (3 μM) alone.
Figure 7.
Effects of different inhibitors on IL-1β-, IL-18- and IL-33-induced survival enhancement of human eosinophils. Eosinophils (5×105 cells) were pre-treated with inhibitors for 30 min followed by incubation with or without (a) IL-1β, (b) IL-18 and (c) IL-33 (50 ng/ml) in the presence of inhibitors for a further 48 h. Percent viability of eosinophils was assessed by the TACS Annexin V-FITC assay using flow cytometry and are expressed as the arithmetic mean±SEM of three independent experiments. *P<0.05, **P<0.01 when compared between groups denoted by the horizontal lines. AG, AG490; BAY, BAY11-7082; Ctrl, medium control; FITC, fluorescein isothiocyanate; IL, interleukin; LY, LY294002; U, U0126; SB, SB203580; SP, SP600125.
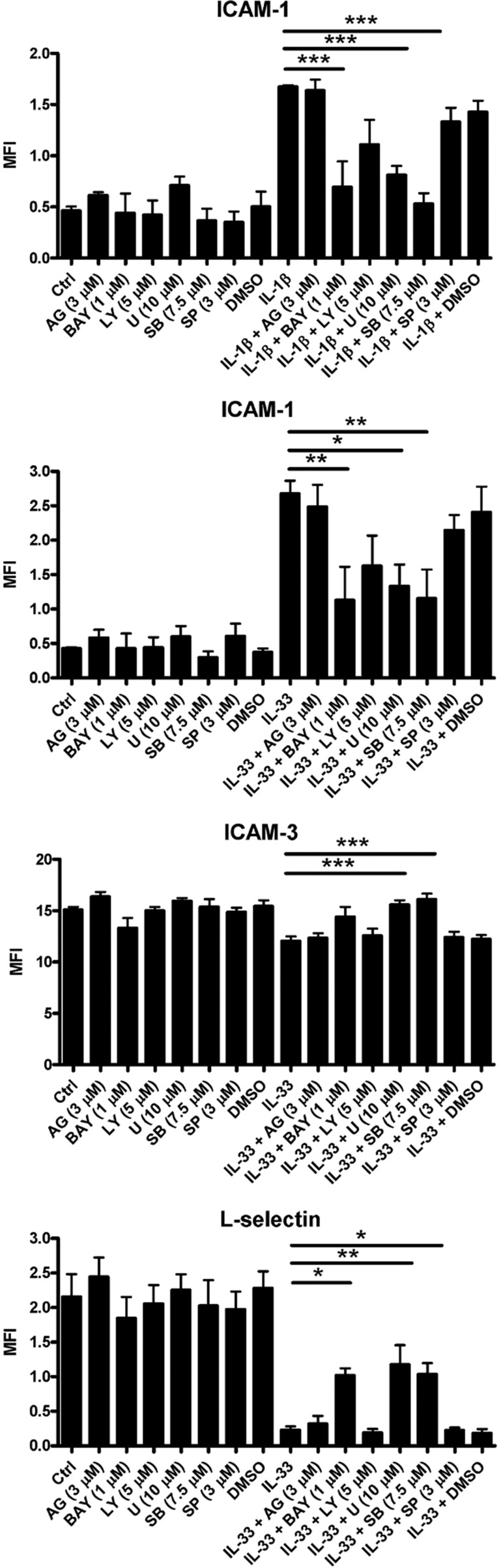
Effects of different inhibitors on IL-1β- and IL-33-mediated surface expression of adhesion molecules on human eosinophils
As shown in Figure 8, pre-treatment with the IκB kinase inhibitor BAY11-7082, the ERK inhibitor U0126 and the p38 MAPK inhibitor SB203580 for 30 min significantly abolished IL-1β- and IL-33-induced ICAM-1 expression on human eosinophils (all P<0.05). Marked suppression of IL-33-induced ICAM-3 expression on human eosinophils was found after pre-treatment with U0126 and SB203580 for 30 min, as was significant inhibition of L-selectin expression on human eosinophils after pre-treatment with BAY11-7082, U0126 and SB203580 for 30 min (all P<0.05).
Figure 8.
Effects of different inhibitors on IL-1β- and IL-33-induced adhesion molecule expression on human eosinophils. Eosinophils (5×105 cells) were pre-treated with inhibitors for 30 min followed by incubation with or without IL-1β or IL-33 (50 ng/ml) in the presence of inhibitors for a further 16 h. (a, b) ICAM-1, (c) ICAM-3, (d) L-selectin expression on 10 000 cells was analyzed by flow cytometry as MFI, which was normalized by subtracting the appropriate isotypic control and shown as the arithmetic mean±SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 when compared between groups denoted by the horizontal lines. AG, AG490; BAY, BAY11-7082; Ctrl, medium control; LY, LY294002; U, U0126; SB, SB203580; SP, SP600125.
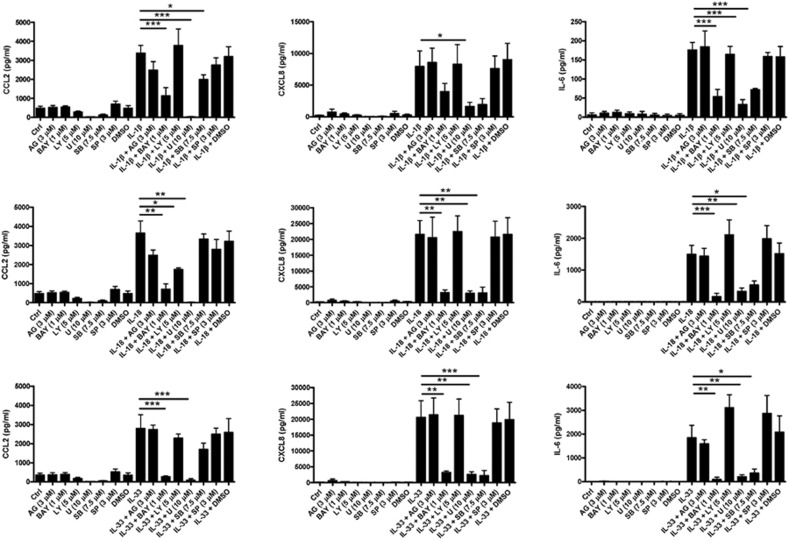
Effects of different inhibitors on IL-33-, IL-1β- and IL-18-induced release of CCL2, CXCL8 and IL-6 from human eosinophils
Figure 9a shows that pre-treatment with the IκB kinase inhibitor BAY11-7082, the ERK inhibitor U0126 and the p38 MAPK inhibitor SB203580 for 30 min significantly abolished IL-1β-induced CCL2 release, while pre-treatment with BAY11-7082, the PI3K inhibitor LY294002 and U0126 for 30 min markedly suppressed IL-18-induced CCL2 release. Pre-treatment with BAY11-7082 and U0126 for 30 min significantly inhibited IL-33-induced CCL2 release from human eosinophils (all P<0.05). As shown in Figure 9b, pre-treatment with BAY11-7082, U0126 and SB203580 for 30 min significantly abolished IL-18- and IL-33-induced CXCL8 release, while pre-treatment with U0126 for 30 min also markedly suppressed IL-1β-induced CXCL8 release from human eosinophils (all P<0.05). Furthermore, Figure 9c shows that pre-treatment with BAY11-7082, U0126 and SB203580 for 30 min significantly abolished IL-1β-, IL-18- and IL-33-induced IL-6 release from human eosinophils (all P<0.05).
Figure 9.
Effects of different inhibitors on IL-1β-, IL-18- and IL-33-induced CCL2, CXCL8 and IL-6 release from human eosinophils. Eosinophils (5×105 cells) were pre-treated with inhibitors for 45 min followed by incubation with or without (a) IL-1β, (b) IL-18 and (c) IL-33 (50 ng/ml) in the presence of inhibitors for a further 24 h. Cell-free culture supernatant was collected and CCL2, CXCL8 and IL-6 released into the supernatant were quantified using ELISA. Results are expressed as the arithmetic mean±SEM of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 when compared between groups denoted by the horizontal lines. AG, AG490; BAY, BAY11-7082; Ctrl, medium control; ELISA, enzyme-linked immunosorbent assay;LY, LY294002; U, U0126; SB, SB203580; SP, SP600125.
Discussion
IL-33 has been proposed to be a novel regulator of the immune system that initiates maturation, migration and activation of various types of leukocytes, including human Th2 lymphocytes,12, 13 mast cells,6 basophils,8, 13, 14 natural killer cells13 and eosinophils.8, 15, 16 In addition, IL-1β was identified as a potent mediator that initiates differentiation of Th17 lymphocytes to drive autoimmune responses,17 while IL-18 was widely known as an inducer that stimulates IFN-γ production from T lymphocytes and natural killer cells to promote Th1 responses.18 Intriguingly, it has been reported that both IL-1β and IL-18 could also activate human eosinophils.19, 20 Indeed, how IL-33 and the two other IL-1 family cytokines IL-1β and IL-18 orchestrate and promote allergic inflammatory responses is still poorly understood. Noting the fact that IL-1β, IL-18 and IL-33 share protein sequence homology,1 a receptor unit, and Toll-like/IL-1-receptor structure in the intracellular receptor domain,21 we investigated the intracellular signaling pathways involved in the activation of the biological functions of human eosinophils by IL-1β, IL-18 and IL-33, paying special attention to the modulation of adhesion molecule expression, survival enhancement, and stimulation of cytokine and chemokine release.
Activation of eosinophils could first be regulated at the ligand/receptor-binding level. We found that human eosinophils constitutively express the IL-33 receptor ST2, consistent with previous studies.15, 16 As soluble ST2 was shown to attenuate eosinophil infiltration and Th2 cytokine production in an asthmatic murine model,22, 23 the significant increase in the release of soluble ST2 in viral infection24 and asthmatic exacerbation25 may help amplify the negative regulatory effects of soluble ST2 in these disease conditions. Utilizing antagonistic effects of anti-ST2 and recombinant ST2 proteins in clinical applications may facilitate the development of new therapeutic strategies for allergic diseases.
Delayed eosinophil apoptosis is a central mechanism of eosinophilia in allergic inflammatory responses.26, 27 Our results demonstrated that IL-33, as well as IL-1β and IL-18, was able to markedly enhance the survival of eosinophils (Figure 2), suggesting that these molecules may promote eosinophilia at the site of allergic inflammation. Noting the fact that enhanced ICAM-1 expression was found in bronchial asthma attacks28 and the discovery of the fibronectin and hyaluronan-binding properties of ICAM-1,29, 30 our finding that IL-33- and IL-1β-induced upregulation of cell surface expression of ICAM-1 on human eosinophils (Figure 3) may provide an additional biochemical basis for IL-33- and IL-1β-enhanced eosinophil adhesiveness to extracellular matrix components at inflamed tissues such as bronchial epithelium. In addition, an ICAM-1/CD18 interaction was found to initiate firm eosinophil adhesion to bronchial epithelial cells.31 Despite the high expression of ICAM-3 on resting stage human eosinophils, an anti-inflammatory role of ICAM-3 in inducing apoptosis of eosinophils has been suggested.32 The switch to enhanced ICAM-1 and reduced ICAM-3 expression on activated eosinophils upon IL-33 stimulation may favor the initiation of proinflammatory responses. L-selectin initiates tethering and rolling of eosinophils on endothelial cells prior to their firm adhesion and transendothelial migration.33 Our results are in agreement with previous evidence revealing downregulation of the surface expression of L-selectin on activated eosinophils after allergen challenge in asthmatic conditions.34 It has been hypothesized that the potent effects of IL-33 in modulating ICAM-1, ICAM-3 and L-selectin expression on eosinophils may help promote their adhesion and transmigration into the inflamed tissues.
Aberrant increases in proinflammatory cytokine and chemokine secretion in allergic asthmatic patients were reported in clinical studies.35, 36 We found that allergic rhinitis patients showed higher plasma IL-33 median concentration than normal controls (33.6 versus 9.8 pg/ml). It has been reported that CCL2 concentration in bronchoalveolar lavage fluid was found to be elevated in allergic asthmatic patients.37 In addition, CCL2 can initiate leukocyte infiltration and inflammatory responses by inducing chemotaxis of monocytes, T lymphocytes, natural killer cells, eosinophils and basophils, and by degranulation and histamine release from both basophils and mast cells.38, 39, 40 Taken together with the evidence demonstrating IL-33 as a chemoattractant for Th2 lymphocytes,12 the significant CCL2 release from eosinophils upon IL-1β, IL-18 and IL-33 activation may facilitate the locomotion of both eosinophils and other leukocytes to promote the development of allergic inflammation. A prior study has revealed that enhanced IL-6 and CXCL8 release in body fluid resulted from allergen challenge in atopic patients.41 The neutrophil chemoattractant CXCL8 was shown not only to enhance surface adhesion molecule expression on neutrophils to facilitate their migration and accumulation in the tissue of inflammation, but also to stimulate neutrophils to secrete granular proteins and produce superoxide.42 Although IL-33 could not activate neutrophils,15 our results suggest that it may collaborate with IL-1β and IL-18 to recruit neutrophils into the inflamed tissues.
The central roles of IL-6 in host defenses and acute inflammation have been well delineated.43 Lipopolysaccharide also activates dendritic cells and macrophages to release IL-33,1 and the enhanced IL-6 production induced by IL-33, as well as IL-1β and IL-18, may amplify the inflammatory responses in microbial infection in addition to exacerbating allergic inflammation. Furthermore, both IL-1β and IL-6 were reported to promote differentiation of Th17 lymphocytes,17 which are characterized by their distinct IL-17A and IL-17F production, possibly promoting leukocyte recruitment and inflammatory processes in allergies.44 The synergistic effects of IL-6 production mediated by the combined effects of IL-1β and IL-18, IL-1β and IL-33, as well as IL-18 and IL-33, could amplify Th17 polarization with a paracrine loop mechanism and thereby elicit more profound allergic inflammation effects.
Eosinophil activation is first initiated by cell surface interaction of receptors and soluble ligands, followed by activation of various signaling pathways regulating transcription of proinflammatory genes.45 The receptors for IL-1β, IL-18 and IL-33 all belong to the IL-1 receptor/Toll-like receptor superfamily and share the Toll-IL-1 receptor unit in their intracellular domain for signaling.46 The significant role of the Toll-IL-1 receptor domain in inflammation and host defenses has been elucidated.47 Since IL-1β, IL-18 and IL-33 all activate eosinophils and induce the same array of cytokines and chemokines, we postulated that these IL-1 family cytokines may share and transmit overlapping signal transduction pathways. Our study revealed the involvement of the NF-κB, ERK and p38 MAPK pathways in regulating IL-1 family cytokine (IL-1β, IL-18 and IL-33)-mediated survival of eosinophils, surface adhesion molecule expression, and allergic inflammation-related cytokine and chemokine release from eosinophils. Since NF-κB regulates the transcription of proinflammatory genes encoding cytokines and adhesion molecules for initiating chronic inflammation, the critical role of NF-κB in the pathogenesis of asthma has been suggested in previous studies.48, 49 In addition, mounting evidence has revealed that the ERK and p38 MAPK cascades could regulate proliferation, differentiation, cytokine production, chemotaxis and apoptosis of various inflammatory cells, including Th2 lymphocytes, mast cells and eosinophils.49, 50 Therefore, the activation of the NF-κB, ERK and p38 MAPK pathways in IL-1β-, IL-18- and IL-33-induced eosinophil activation further confirms the importance of these signal transduction pathways in the pathogenesis of allergic inflammation.
In conclusion, this is a comprehensive report on the activation of eosinophils by IL-1β, IL-18 and IL-33. This activation occurs via the NF-κB, ERK and p38 MAPK pathways and results in survival enhancement, modulation of surface adhesion molecule expression, and cytokine and chemokine release. Our study also demonstrated the synergistic effects of combining IL-1β and IL-18, IL-1β and IL-33, as well as IL-18 and IL-33 on induction of IL-6 release. Taken together with previous reports, the significance of this study inevitably lies in how IL-33 and its related IL-1 family cytokines IL-1β and IL-18 could be involved in the pathogenesis of allergic inflammation such as the paracrine activation of Th17 lymphocytes. Further investigation of the underlying mechanisms of eosinophil activation may provide more clues for the development of novel therapeutic strategies for eosinophil-associated allergic diseases.
Acknowledgments
This study was supported by Research Grant Committee General Research Fund, Hong Kong (Project code: CU08757; principal investigator: CK Wong; co-investigator: CWK Lam).
References
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan T, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Kondo Y, Yoshimoto T, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia C, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–1881. doi: 10.1111/j.1365-2222.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardo R, Chanez P, Mathieu M, Bruno A, Costanzo G, Gougat C, et al. Persistent activation of nuclear factor-κB signaling pathway in severe uncontrolled asthma. Am J Respir Crit Care Med. 2003;168:1190–1198. doi: 10.1164/rccm.200205-479OC. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ip WK, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor-induced adhesion molecule expression on eosinophils by p38 mitogen-activated protein kinase and nuclear factor-κB. Am J Respir Cell Mol Biol. 2003;29:133–147. doi: 10.1165/rcmb.2002-0289OC. [DOI] [PubMed] [Google Scholar]
- Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J. Immunol. 2008;180:5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- Whitcomb EA, Dinarello CA, Pincus SH. Differential effects of interleukin-1 alpha and interleukin-1 beta on human peripheral blood eosinophils. Blood. 1989;73:1904–1908. [PubMed] [Google Scholar]
- Wang W, Tanaka T, Okamura H, Sugita M, Higa S, Kishimoto T, et al. Interleukin-18 enhances the production of interleukin-8 by eosinophils. Eur J Immunol. 2001;31:1010–1016. doi: 10.1002/1521-4141(200104)31:4<1010::aid-immu1010>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- Becerra A, Warke RV, de Bosch N, Rothman AL, Bosch I. Elevated levels of soluble ST2 protein in dengue virus infected patients. Cytokine. 2008;41:114–120. doi: 10.1016/j.cyto.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–573. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- Kankaanranta H, Lindsay MA, Giembycz MA, Zhang X, Moilanen E, Barnes PJ. Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol. 2000;106:77–83. doi: 10.1067/mai.2000.107038. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hashimoto S, Imai K, Amemiya E, Yamaguchi M, Yachi A, et al. Elevation of serum soluble intercellular adhesion molecule-1 (sICAM-1) and sE-selectin levels in bronchial asthma. Clin Exp Immunol. 1994;96:110–1115. doi: 10.1111/j.1365-2249.1994.tb06239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser R, Fehr J, Olgiati L, Bruijnzeel PL. Migration of primed human eosinophils across cytokine-activated endothelial cell monolayers. Blood. 1992;79:2937–2945. [PubMed] [Google Scholar]
- McCourt PA, Ek B, Forsberg N, Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J Biol Chem. 1994;269:30081–30084. [PubMed] [Google Scholar]
- Burke-Gaffney A, Hellewell PG. A CD18/ICAM-1-dependent pathway mediates eosinophil adhesion to human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1998;19:408–418. doi: 10.1165/ajrcmb.19.3.3179. [DOI] [PubMed] [Google Scholar]
- Kessel JM, Sedgwick JB, Busse WW. Ligation of intercellular adhesion molecule 3 induces apoptosis of human blood eosinophils and neutrophils. J Allergy Clin Immunol. 2006;118:831–836. doi: 10.1016/j.jaci.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- Mengelers HJ, Maikoe T, Brinkman L, Hooibrink B, Lammers JW, Koenderman L. Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med. 1994;149:345–351. doi: 10.1164/ajrccm.149.2.8306028. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui D, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillie-Leblond I, Hammad H, Desurmont S, Pugin J, Wallaert B, Tonnel A, et al. CC chemokines and interleukin-5 in bronchial lavage fluid from patients with status asthmaticus. Potential implication in eosinophil recruitment. Am J Respir Crit Care Med. 2000;162:586–592. doi: 10.1164/ajrccm.162.2.9907014. [DOI] [PubMed] [Google Scholar]
- Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, et al. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153:1398–1404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- Conti P, Boucher W, Letourneau R, Feliciani C, Reale M, Barbacane R, et al. Monocyte chemotactic protein-1 provokes mast cell aggregation and [3H]5HT release. Immunology. 1995;86:434–440. [PMC free article] [PubMed] [Google Scholar]
- Dunzendorfer S, Kaneider NC, Kaser A, Woell E, Frade JM, Mellado M, et al. Functional expression of chemokine receptor 2 by normal human eosinophils. J Allergy Clin Immunol. 2001;108:581–587. doi: 10.1067/mai.2001.118518. [DOI] [PubMed] [Google Scholar]
- Rose CE Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10:273–288. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- Ferreira MA. Cytokine expression in allergic inflammation: systematic review of in vivo challenge studies. Mediators Inflamm. 2003;12:259–267. doi: 10.1080/09629350310001619717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Wong CK, Zhang J, Ip WK, Lam CW. Intracellular signal transduction in eosinophils and its clinical significance. Immunopharmacol Immunotoxicol. 2002;24:165–186. doi: 10.1081/iph-120003748. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- O'Neill L. The Toll/interleukin-1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans. 2000;28:557–563. doi: 10.1042/bst0280557. [DOI] [PubMed] [Google Scholar]
- Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ip WK, Lam CW. Biochemical assessment of intracellular signal transduction pathways in eosinophils: implications for pharmacotherapy. Crit Rev Clin Lab Sci. 2004;41:79–113. doi: 10.1080/10408360490427624. [DOI] [PubMed] [Google Scholar]
- Duan W, Wong WS. Targeting mitogen-activated protein kinases for asthma. Curr Drug Targets. 2006;7:691–698. doi: 10.2174/138945006777435353. [DOI] [PubMed] [Google Scholar]