Abstract
Inflammatory bowel disease (IBD) is caused by an uncontrolled immune response in the intestinal lumen, leading to inflammation in genetically predisposed individuals. Immunotherapy may be a promising approach to the treatment of IBD. Here, we show that transforming growth factor-β1 (TGF-β1) gene-modified immature dendritic cells (imDCs) could enhance the inhibitory function of imDCs and delay the progress of IBD induced by dextran sodium sulfate in mice. The results of fluorescence-activated cell sorter (FACS) demonstrated that this protective effect is mediated partially by inducing CD4+Foxp3+ regulatory T cells (Tregs) in mesentery lymph nodes to control inflammation. In vitro experiments also supported this hypothesis. In conclusion, we provide evidence that TGF-β1-modified bone marrow-derived imDCs may have a therapeutic effect to IBD.
Keywords: DCs, IBD, TGF-β1, Treg
Introduction
Inflammatory bowel disease (IBD), represented mainly by ulcerative colitis and Crohn's disease, is characterized as chronic, uncontrolled inflammation in the intestinal mucosa.1 The geographic distribution of IBD is not uniform, and higher incidence of IBD has been observed in industrialized countries.2 The etiology of IBD focuses on environmental triggers, genetic factors, immunoregulatory defects and microbial exposure.3 Current clinical therapy depends mainly on allopathic medicines, which can cause many systemic side effects.4 Therefore, development of novel treatments with improved target selectivity against IBD is required.
Transforming growth factor-β1 (TGF-β1) is a potent regulatory cytokine, which plays a well-established role in immunoregulation and tolerance. TGF-β1 has multiple inhibitory effects on the proliferation, differentiation and survival of T cells, B cells, macrophages and other immune cells.5 Furthermore, the defects either in TGF-β1 expression or in its signaling in T cells are correlated with several autoimmune diseases.6 TGF-β1 is also involved in modulation of intestinal inflammation.7
Dendritic cells (DCs) are antigen-presenting cells with the unique ability to induce primary immune responses.8 Mature DCs are potent antigen-presenting cells, but immature DCs (imDCs) have been shown to have no or lower expression of costimulatory molecules and exhibit tolerogenicity.9 ImDCs are not simply ignored but can lead to immunological tolerance, allowing possible application to the treatment of autoimmune diseases. However, the fact that imDCs tend to differentiate into mature DCs under certain inflammatory conditions or on encountering an immunological stimulus limits the clinical use of imDCs.
In our study, we investigated whether TGF-β1-modified bone marrow-derived immature DCs (TGF-β1-DCs) could show protective effects during the development of dextran sodium sulfate (DSS)-induced IBD in a mouse model and explored possible underlying mechanisms.
Materials and methods
Mice
Female C57BL/6 mice, 6–8 weeks of age, were purchased from Joint Ventures Sipper BK Experimental Animal (Shanghai, China) and kept in a specific pathogen-free environment (Hangzhou, China). The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Zhejiang University and animals were cared for according to Zhejiang University and National Institutes of Health guidelines.
Antibodies and reagents
FITC-conjugated antimouse I antibody (M5/114.15.2; Rat IgG2b), CD86 (GL1; Rat IgG2a) and CD11c (N418; Armenian Hamster IgG) antibodies, PE-conjugated antimouse CD80 (16-10A1; Armenian Hamster IgG), CD40 (1C10; Rat IgG2a) and CD4 (GK1.5; Rat IgG2b) antibodies, PE-Cy5-conjugated antimouse Foxp3 (FJK16s; Rat IgG2a) antibody and Foxp3 staining buffer set were from eBioscience (San Diego, CA, USA); recombinant murine granulocyte/macrophage colony-stimulating factor was from PeproTech (Rocky Hill, NJ, USA); ELISA kit for TGF-β1 was from R&D Systems (Minneapolis, MN, USA); DSS (molecular weight: 36 000–50 000) was from MP Biomedicals (Aurora, OH, USA).
DC culture and infection
Murine bone marrow-derived DCs were prepared as described previously with minor modification.11 Briefly, bone marrow mononuclear cells were prepared from mouse (6–8 weeks old) tibia and femur suspensions by depletion of red cells and cultured at a density of 2×106 cells/ml in six-well plates in RPMI 1640 medium supplemented with 10% fetal calf serum and 10 ng/ml recombinant murine granulocyte/macrophage colony-stimulating factor. Non-adherent cells were gently washed out after 48 h of culture; the remaining loosely adherent clusters were cultured for another 48 h and harvested for adenoviral (Ad) transduction. For Ad infection, 1×106 DCs were mixed with 5×107 plaque-forming unit of the viruses in a total volume of 1 ml of serum-free medium. After incubation for 24 h, DCs were washed five times before being collected for the next experiments.
Measurement of cytokines
The production of TGF-β1 and IL-12p70 from imDCs infected with Ad-TGF-β1, Ad-LacZ or day-6 DCs in vitro were detected by ELISA. In some experiments, DCs were stimulated with lipopolysaccharide (LPS) at final concentration of 1 µg/ml for 18 h.
Fluorescence-activated cell sorter analysis
DCs were harvested and washed twice with phosphate-buffered saline (PBS) containing 0.1% sodium azide plus 2% heat-inactivated fetal calf serum (wash buffer). Cells were then incubated with Fc receptor-blocking antibodies (PharMingen, San Diego, CA, USA) for 5 min. Fluorescent antibodies were then added at a concentration of 1 mg per 1×106 cells per 100 µl and cells were incubated at 4 °C for 30 min. The cells were washed twice with ice-cold PBS and then analyzed by flow cytometry using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA, USA).
Mixed lymphocyte reaction
T cells were purified from the spleens of BALB/c mice for in vitro culture in round-bottom 96-well plates. In each well, 2×105 splenic T cells were seeded with either control C57BL/6-derived DCs or genetically modified C57BL/6-derived DCs (TGF-β1 or Lac Z). On day 5 of culture, 1 µCi of [3H]-thymidine was added to each well 18 h before harvest. Cultured cells were harvested and 3H incorporation was measured in a microplate beta counter (Wallac, Gaithersburg, MD, USA).
Induction and treatment of DSS colitis
Mice were randomized into groups with identical average body weight. Acute colitis was induced by giving DSS in acidified drinking water, 1.5% (w/v), for 9 days. The day that mice started to drink DSS was regarded as day 0. For the treatment group, mice were injected intraperitoneally (i.p.) with TGF-β1-imDCs (2×106/mouse/injection) on days 2, 3 and 5. Mice that drank DSS only or normal water were used as controls.
Assessment of DSS colitis
For all animals, body weight, rectal bleeding and stool consistency were monitored daily. Intestinal bleeding was measured by using the one-step fecal occult blood test (W.H.P.M. Bioresearch & Technology Co., Ltd, El Monte, CA, USA), as well as by observation of bleeding signs on the anus or gross bleeding. The daily disease activity index (DAI) was calculated by grading the following parameters on a scale of 0–4: change in weight (0, ≤1% 1, 1–5% 2, 5–10% 3, 10–15% 4, >15%), intestinal bleeding (0, negative; 4, positive) and stool consistency (0, normal; 2, loose stools; 4, diarrhea). The combined scores were then divided by 3 to obtain the final DAI (maximum score: 4). Mice were killed 9 days following disease induction, and the colon was collected and evaluated for microscopic damage.
Microscopic scoring
Proximal, medial and distal portions of colon were fixed in 10% phosphate-buffered formalin. Paraffin-embedded sections were stained with hematoxylin and eosin. The evaluation system was used as described previously.12 The degree of histological damage and inflammation was graded in a blinded fashion. The following parameters scored were used: (i) amount of inflammation (0, none; 1, mild; 2, moderate; 3, severe; 4, accumulation of inflammatory cells in the gut lumen); (ii) distribution of lesions (0, none; 1, focal; 2, multifocal; 3, nearly diffuse; 4, diffuse) and (iii) depth of inflammation and layers involved (0, none; 1, mucosa only; 2, mucosa and submucosa; 3, limited transmural involvement; 4, transmural). The overall histological score was the sum of the three parameters (maximum score: 12).
Analysis of CD4+Foxp3+ regulatory T cell in vivo
Lymphocytes were isolated from murine spleens or mesentery lymph nodes (mLNs). Cells were first stained with PE-conjugated antimouse CD4 antibodies and then the Foxp3 intracellular staining was carried out according to the manufacturer's protocol. The relative Foxp3+ regulatory T cell (Treg) numbers were detected by fluorescence-activated cell sorter (FACS).
Induction of CD4+Foxp3+ Treg in vitro
Splenocytes were isolated from the spleens of C57 mice and cultured in vitro in 24-well plates. In each well, 1×106 splenocytes were cocultured with 1×105 TGF-β1-DCs, LacZ-DCs or control-DCs in the presence of 0.5 µg/ml of anti-CD3 for 3 days. The percentage of CD4+Foxp3+ Treg in splenocytes was detected by FACS.
Trafficking of DCs in vivo
DCs were labeled with PKH26 probe according to the manufacturer's instructions. Briefly, 1×107 cells in 1 ml of diluent C were mixed with 12 µl PKH26 (red fluorescent cell linker; Sigma-Aldrich, St Louis, MO, USA) dissolved in 3 ml of diluent C and stained for 4 min at room temperature. Labeling was terminated by incubation with cold PBS (10% fetal bovine serum) for 1 min and finally the cells were washed twice in RPMI 1640. IBD mice or normal control mice were injected i.p. with PKH26-labeled DCs or unlabeled DCs. The mice were killed 24 h after injection, and the splenocytes and lymphocytes from mLNs were collected and subjected to FACS analysis.
Immunohistochemistry
Murine colon tissues were fixed in 10% formalin, dehydrated in ethanol and embedded in paraffin. Tissues were then cut into 4-µm sections, mounted on slides and dried at 60 °C for 4 h. Then, deparaffinization and rehydration of the sections were performed. Sections were pretreated by boiling in citrate buffer (pH 6.0) for 20 min to retrieve antigen. After cooling, sections were treated in 3% hydrogen peroxide solution for 10 min and washed in PBS twice for 3 min each. Then, the sections were incubated with primary antibodies. All antibodies were diluted 1:50 in PBS containing 5% skim milk and incubated at 4 °C overnight. After washing in PBS, the slides were incubated in the corresponding secondary antibodies for 30 min. After being washed in PBS, the slides were immersed in diaminobenzidine solution for 2 min and then washed with water. The slides were placed in a hematoxylin bath for 30 s, washed in tap water for 10 min and dehydrated through gradient alcohol and xylene three times for 1 min each. The slides were then coverslipped and mounted with permanent mounting media. Immunohistochemical images of the sections were captured under standard conditions. For each section, we captured five visual fields randomly. The photos were analyzed by Image-Pro Plus 5.0. We analyzed the positive area by using the mean density, which was defined by the integrated optical density divided by the actual area that represented the quantity of cytokine.
Statistical analysis
Results were compared using the Student's t-test and ANOVA. Values of P<0.05 were considered to be statistically significant.
Results
The production of TGF-β1 in TGF-β1-imDCs
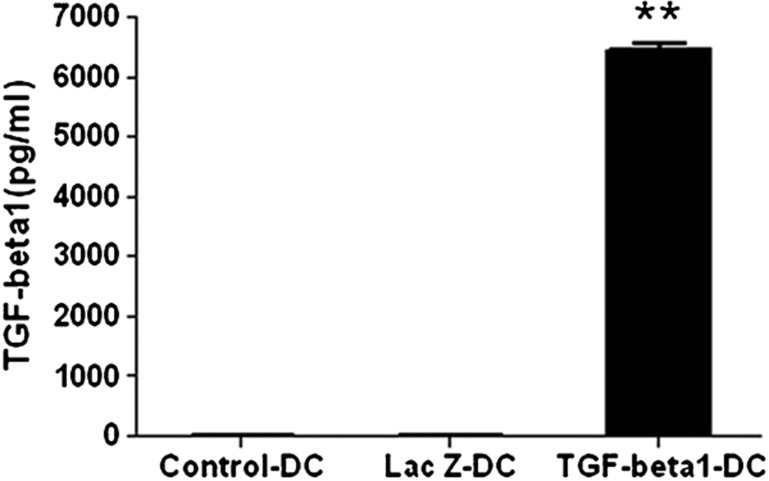
To test whether imDCs are successfully transferred by Ad-TGF-β1, we first detected the production of TGF-β1. Culture supernatants of TGF-β1-DCs or LacZ-DCs were harvested 48 h after transfection of Ad-TGF-β1 or Ad-LacZ,13 respectively; culture supernatants of day-6 DCs were used as a control. The level of TGF-β1 was detected by ELISA. The secretion of TGF-β1 in the TGF-β1-DCs was much stronger than in the LacZ-DCs or control-DCs (Figure 1). This result demonstrated that the TGF-β1 gene could be efficiently transferred into imDCs using Ad-TGF-β1.
Figure 1.
Level of TGF-β1 in the culture supernatant of TGF-β1-DCs. Six-day-old DCs were infected with Ad-TGF-β1 or Ad-LacZ at an MOI of 50 for 24 h in serum-free medium. After infection of adenovirus for 24 h, culture supernatants of the infected DCs were collected. The level of TGF-β1 in the culture supernatants was measured by ELISA. **P<0.01; data are representative of three independent experiments (n=3). Ad, adenoviral; DC, dendritic cell; MOI, multiplicity of infection; TGF-β1, transforming growth factor-β1.
Characteristics of TGF-β1-imDCs
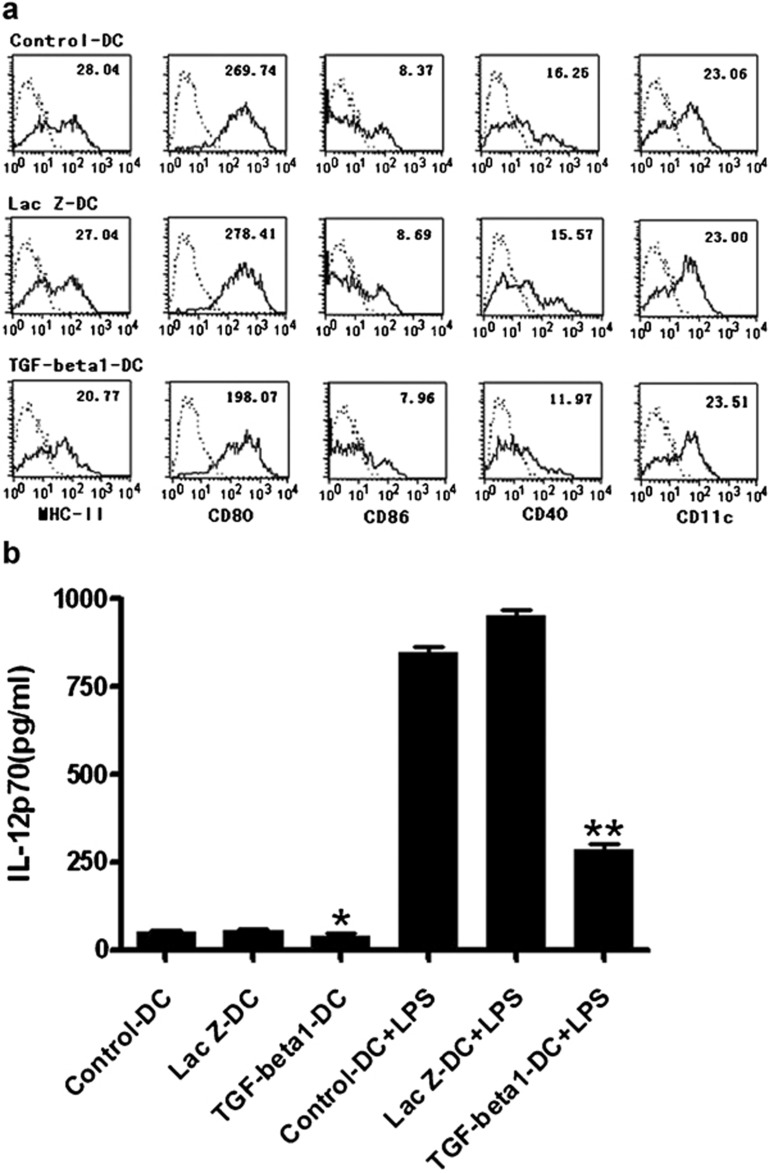
Genetic modification of DCs by immunosuppressive molecules can enhance their potential tolerogenicity.14 To determine whether TGF-β1 gene-modified bone morrow-derived imDCs have a phenotype characteristic of imDCs, we detected the surface markers of TGF-β1-DCs by FACS. As shown in Figure 2a, TGF-β1-DCs expressed the mouse DC marker CD11c and exhibited the immature phenotype with low expression of MHC-II, CD80, CD86 and CD40 when compared with day-6 control DCs and LacZ-DCs. These results implied that the TGF-β1 gene downregulated antigen presentation of bone morrow-derived imDCs.
Figure 2.
Characteristics of TGF-β1-DCs. (a) Flow cytometric analysis of murine DCs. After infection of adenovirus for 48 h, each group of DCs was stained with FITC-coupled antibodies specific for MHC-II, CD11c, CD80, CD86, or the corresponding isotype controls. (b) Levels of IL-12p70 in the culture supernatant of TGF-β1 gene-modified immature DCs. Six-day-old DCs were infected with Ad-TGF-β1 or Ad-LacZ at an MOI of 50 for 24 h in serum-free medium. After washing five times, the cells were stimulated with LPS (100 ng/ml) for 20 h. Levels of IL-12p70 in the culture supernatants were determined by ELISA. *P<0.05, **P<0.01; data are representative of three independent experiments (n=3). Ad, adenoviral; DC, dendritic cell; LPS, lipopolysaccharide; MOI, multiplicity of infection; TGF-β1, transforming growth factor-β1.
IL-12, which is produced mainly by activated myeloid DCs, plays a pivotal role in the differentiation and expansion of Th1 cells.15, 16 LPS can induce the maturation of the imDCs. Thus, we detected IL-12p70, a member of the IL-12 family, in the culture supernatants from TGF-β1-DCs and LacZ-DCs 24 h after transfection of Ad-TGF-β1, Ad-LacZ and day-6 DCs with or without LPS stimulation. As shown in Figure 2b, there was no difference inIL-12 secretion between TGF-β1-DCs, LacZ-DCs and control-DCs. However, after stimulation with 1 µg/ml of LPS for 18 h, the level of IL-12 was significantly lower in the supernatant from TGF-β1-DCs than from LacZ-DCs or control-DCs.
The in vitro inhibitory effect of TGF-β1-DCs
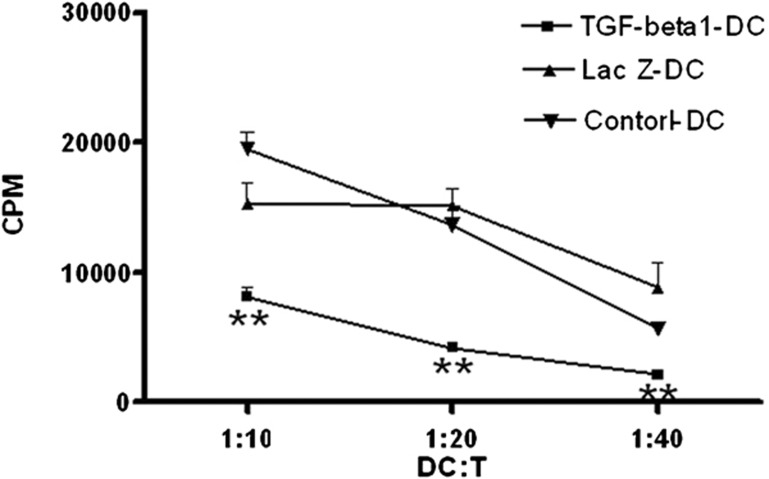
To determine the ability of TGF-β1-DCs to inhibit the proliferation of T cells, TGF-β1-DCs, LacZ-DCs and control-DCs were cocultured with T cells derived from BALB/c mice, as described above. The allostimulatory ability of TGF-β1-DCs was much weaker than that of LacZ-DCs or control-DCs (Figure 3). TGF-β1-DCs could significantly inhibit the proliferation of T cells in a dose-dependent manner. These results indicated that the modification of TGF-β1 endowed imDCs with enhanced immunosuppressive capacity.
Figure 3.
In vitro function of TGF-β1-DCs. T cells (1×106 cells/ml) from BALB/c (H-2kd) spleen were stimulated with C57BL/6 (H-2kb) bone-marrow DCs (non-infected or infected either Ad-TGF-β1 or Ad-Lac Z) at serial ratios. Eighteen hours before the fifth day in culture, 1 µCi of [3H]-thymidine was added to each well. **P<0.01; data are representative of three independent experiments (n=3). DC, dendritic cell; TGF-β1, transforming growth factor-β1.
TGF-β1-DCs can delay the progress of murine IBD induced by DSS
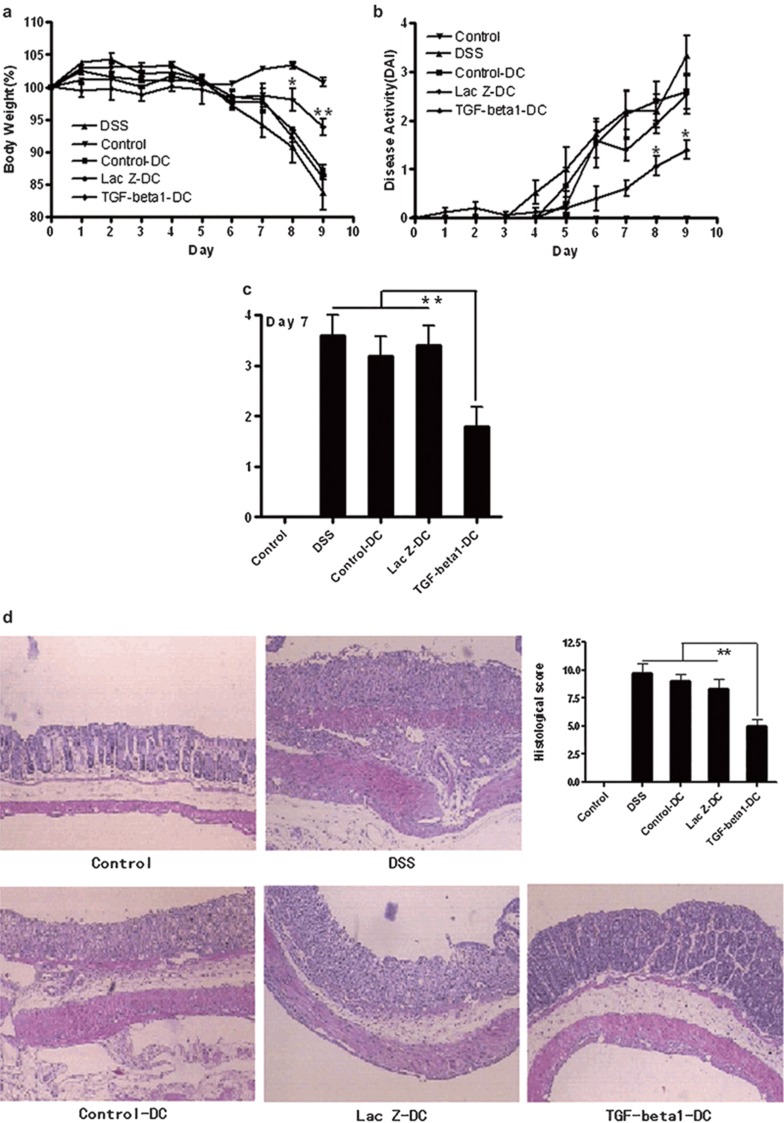
To assess the effect of TGF-β1-DCs in the development of IBD, a murine model of acute DSS-induced IBD was used. Mice with established DSS-induced IBD were injected with TGF-β1-DCs, LacZ-DCs or control-DCs on days 2, 3 and 5, respectively. Compared to the water-control group, the weight loss of DSS-induced mice (without treatment) was significantly enhanced from day 5 after induction, and reached 19% by day 9. Similarly, the DSS-induced mice treated with control-DCs or LacZ-DCs also experienced weight loss, which reached 10% by day 9. However, the weight loss in mice treated with TGF- β1-DCs was significantly alleviated compared to the DSS-control group, which decreased only 7% compared to their original weight by day 9 (Figure 4a). The daily DAI and the intestinal bleeding were calculated as described in the section on ‘MATERIALS AND METHODS'. The scores of the group with the treatment of TGF-β1-DCs were less than groups with control-DCs or LacZ-DCs (Figure 4b and c). Histological assessment of colonic damage in control-DCs, LacZ-DCs or untreated mice was performed 9 days after DSS induction. The results revealed extensive, severe, nearly diffused inflammation of the mucosa, submucosa and, in some cases, all of the intestinal layers (transmural inflammation). This was associated with pronounced disruption of the normal architecture and crypt loss. Less damage with greater conservation of the glandular structure was revealed in colons from DSS-induced mice treated with TGF-β1-DCs. Only limited leukocyte infiltration was found in the mucosa and submucosa (Figure 4d). Taken together, these results strongly supported the idea that TGF-β1-DCs could significantly delay the development of murine IBD induced by DSS.
Figure 4.
TGF-β1-DCs delayed the progress of murine IBD induced by DSS. Acute colitis was induced by giving 1.5% DSS in acidified drinking water for 9 days. Groups of mice were injected i.p. with TGF-β1-DCs, LacZ-DCs or control-DCs (2×106/mouse/injection) on days 2, 3 and 5. (a) Body weight loss. Each point represents average weight data pooled from five mice±SE. (b) Disease activity index (DAI, combined score of body weight, bleeding and stool consistency). Each point represents average DAI data pooled from five mice. (c) Rectal bleeding was detected on days 7 and 9 by the one-step fecal occult blood test. Each column represents average data scores pooled from five mice. (d) Histological appearance 9 days after DSS induction. Representative colonic sections are stained with hematoxylin and eosin. Magnification: ×40. *P<0.05, **P<0.01; data are representative of three independent experiments (n=5). DAI, disease activity index; DC, dendritic cell; DSS, dextran sodium sulfate; IBD, inflammatory bowel disease; TGF-β1, transforming growth factor-β1.
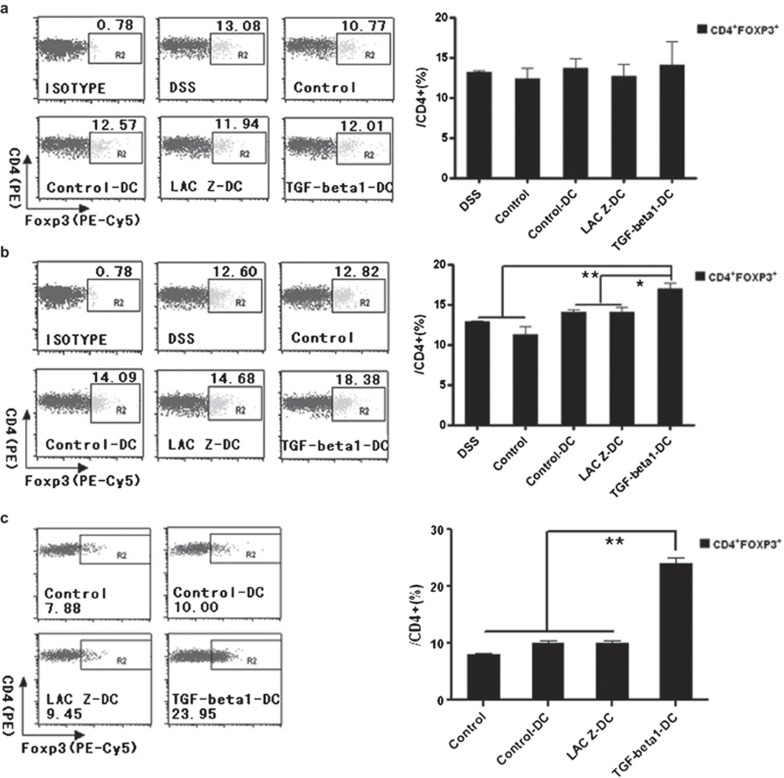
TGF-β1-DCs increased CD4+Foxp3+ Tregs in mLNs from the inflammatory site in vivo and in vitro
To explore the mechanism of the protective effect of TGF-β1-DCs in murine IBD, day-9 mice were killed, and the spleens and the mLNs were isolated. CD4+Foxp3+ Tregs were detected by FACS. As shown in Figure 5a, the percentage of CD4+Foxp3+ Tregs in splenocytes of mice treated with TGF-β1-DCs was similar to those in other groups. In contrast, a higher percentage of CD4+Foxp3+ Tregs were observed in the lymphocytes from mLNs from the inflammatory site (Figure 5b), indicating that the induction of CD4+Foxp3+ Tregs might contribute to the protective effect of TGF-β1-DCs.
Figure 5.
TGF-β1-DCs can increase the relative numbers of CD4+Foxp3+ Tregs in vivo and in vitro. (a) The relative CD4+Foxp3+ Treg numbers from spleens of each group mice. (b) The relative CD4+Foxp3+ Treg numbers from mLNs of each group mice. CD4+ cells were gated and analyzed for Foxp3+ Tregs. (c) TGF-β1-DCs can increase the relative numbers of CD4+Foxp3+ Tregs in vitro. *P<0.05, **P<0.01; data are representative of three independent experiments (n=3). DC, dendritic cell; TGF-β1, transforming growth factor-β1.
To further confirm the ability of TGF-β1-DCs to induce Tregs, splenocytes of normal mice were isolated and cocultured with TGF-β1-DCs. After 3 days, the number of CD4+Foxp3+ Tregs was analyzed by FACS. As shown in Figure 5c, compared to control-DCs and LacZ-DCs, TGF-β1-DCs could induce higher percentages of CD4+Foxp3+ Tregs. These results indicated that TGF-β1-DCs could strongly induce CD4+Foxp3+ Tregs.
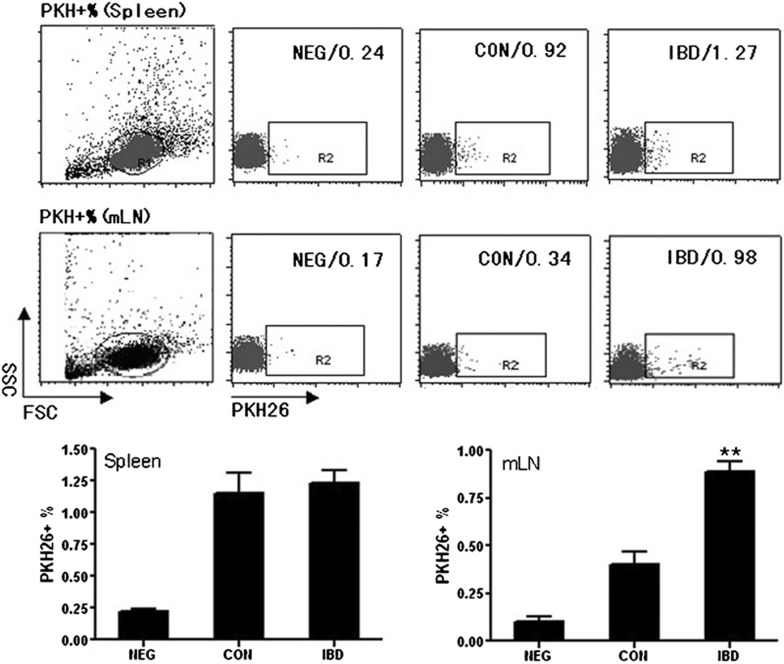
Lymphocytes from mLNs of IBD mice recruited more TGF-β1-DCs than those from normal mice
To determine whether DCs could target inflammatory sites in vivo, we injected mice i.p. with PKH-26-labeled DCs (1×106/mouse) or non-labeled DCs (unlabeled DCs served as an autofluorescent background control). DC transport to mLNs and spleen was identified by FACS analysis 24 h later. There were few differences in the percentages of PKH26+ DCs in the spleens between IBD mice and control mice. However, the PKH26+ DCs in mLNs from IBD mice statistically increased when compared with those from control mLNs (Figure 6). These results suggested that DCs could be effectively targeted to the inflammatory sites.
Figure 6.
Lymphocytes from mLNs of IBD mice recruit more TGF-β1-DCs than those from normal mice. DCs were labeled with PKH26 and injected into normal mice (CON) and IBD model mice (IBD). NEG was a group of normal mice injected with unlabeled DCs as negative control. The mice were killed 24 h after injection, and the splenocytes and lymphocytes from mLNs were collected and subjected to FACS analysis. **P<0.01; data are representative of three independent experiments (n=3). DC, dendritic cell; IBD, inflammatory bowel disease; mLN, mesentery lymph node; FACS, fluorescence-activated cell sorter.
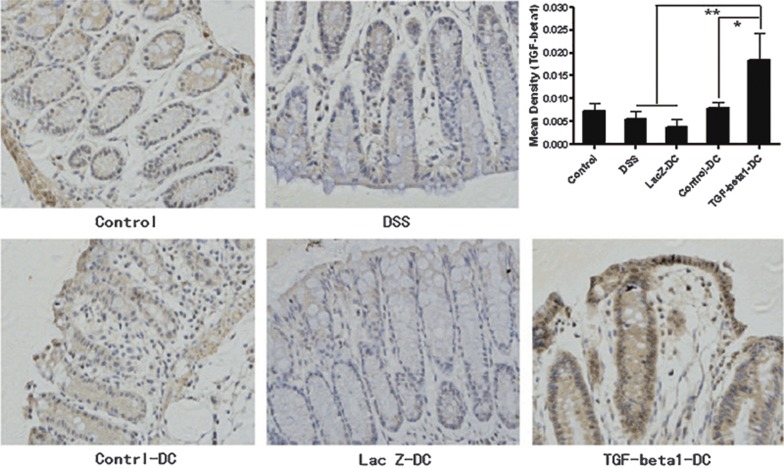
TGF-β1-DCs increased the level of TGF-β1 in colon tissues
As TGF-β1 has a crucial role in the differentiation of Th0 cells into Tregs, we examined the level of TGF-β1 in mouse colon tissues by immunohistochemistry (Figure 7). The results showed that the TGF-β1-DC group had enhanced levels of TGF-β1 versus other groups. These results provided a possible explanation for the induction of Tregs.
Figure 7.
TGF-β1-DCs can increase the level of TGF-β1 in colon tissues. The figures show the appearance of TGF-β1 via immunohistochemistry at the inflammatory site. The yellow and brown parts in the figures are positive sections, representing the localization of TGF-β1. The immunohistochemical images of the sections were captured under standard conditions. For each section, we captured five visual fields randomly. The photos were analyzed by Image-Pro Plus 5.0. The quantity of cytokine was indicated by mean density. Mean density=integrated optical density/actual area. *P<0.05, **P<0.01; data are representative of three independent experiments (n=5). DC, dendritic cell; TGF-β1, transforming growth factor-β1.
Discussion
Although the pathogenesis of IBD is not yet understood completely, it seems that the excessive, cytokine-driven intestinal inflammation caused by a disordered immune response is considered to be a critical pathogenic factor.17 The studies on the immunological pathogenesis in IBD might contribute to the development of novel therapies in the future.
DCs are the most potent antigen-presenting cells, which can promote the activation and proliferation of primary T cells. However, despite the fact that mature DCs are potent antigen-presenting cells, imDCs have been shown to have a lower expression of co-stimulatory molecules and can be tolerogenic.18 Recently, an increasing number of studies on DCs focused on their ability to induce immunological tolerance. ImDCs have limited capability to induce proliferation of T lymphocytes and subsequent incompetence or anergy of T cells.19 Furthermore, imDCs can also induce allograft tolerance, suggesting a solution to allograft rejection.20 TGF-β1 is a key factor during the differentiation of Th cells, which can upregulate Foxp3 expression in Th0 cells, driving them to differentiate into CD4+Foxp3+ Tregs.16 Here, we successfully transferred the TGF-β1 gene into bone marrow-derived imDCs by using adenovirus, and detected a high level of TGF-β1 in the supernatant of these DCs. Meanwhile, the DCs could induce higher percentages of CD4+Foxp3+ Tregs in vitro. Tregs play a crucial role in immune tolerance, which regulate the responses to either pathogen or self-antigen and inhibit the proliferation of autoreactive T lymphocytes.15, 16 Our study showed that TGF-β1-DCs had more immature characteristics, with lower expressions of MHC-II, CD80, CD86 and CD40. The results of the mixed lymphocyte reaction demonstrated that TGF-β1-DCs could effectively inhibit the proliferation of allogenic lymphocytes in vitro. Also, TGF-β1-DCs could significantly decrease the expression of IL-12, which is a necessary cytokine in the differentiation of Th1 cells,21 after LPS stimulation. Taken together, these results demonstrated that TGF-β1-DCs had potential immunosuppressive properties.
In the acute DSS-induced IBD murine model, weight loss, intestinal bleeding and DAI were applied as measures of the degree of progression of IBD disease.22 The results indicated that TGF-β1-DCs could delay the weight loss, relieve the intestinal bleeding and reduce the DAI scores of IBD mice. All these results illustrated that TGF-β1-DCs could delay the development of IBD.
The critical protective function of Tregs involved in IBD and the marked upregulation of CD4+Foxp3+ Treg by TGF-β1-DCs in vitro suggest that TGF-β1-DCs could induce the production of TGF-β1 in vivo to offer a protective effect against IBD. The lymphocytes were initially separated from spleens and mLNs of IBD and control mice, where the percentages of CD4+Foxp3+ Treg in both groups were detected by FACS. In the group of TGF-β1-DCs mice, we found a significant enhancement of the percentage of CD4+Foxp3+ Tregs in mLNs; however, the percentage of CD4+Foxp3+ Tregs in spleens was not increased. It has been long noted that TGF-β1 plays important roles in inducing the differentiation of Tregs. The results presented here showed an increased level of TGF-β1 from colon tissues in TGF-β1-DC group mice relative to the other groups of mice. This result suggested that a high level of TGF-β1 at sites of immunity could induce the production of Tregs in mLNs. Furthermore, stronger DC chemotaxis was observed in lymphocytes from mLNs in IBD mice, relative to that of normal mice by cell tracing experiments in vivo. This illustrated that TGF-β1-DCs could accumulate at local immunity sites and release large amounts of TGF-β1, leading to a high level of TGF-β1. Moreover, selective migration of TGF-β1-DCs into the mLNs of inflammatory sites could somewhat confine systemic immunosuppression, allowing possible clinical application. Notably, some DCs could also migrate to spleens. TGF-β1 alone did not induce the increase in Tregs, probably due to the lack of antigen stimulation.23 Compared to the lymphocytes from mLNs, there was a much lower chance of spleen cells encountering an intestinal antigen, suggesting that they were probably not responsible for inducing Tregs. Tregs from mLNs might migrate to the spleen, leading to the increase of total number of Tregs there. The number of these cells could be ignored, with respect to the large number of Tregs in the spleen. This study is the first to report that DCs modified by the TGF-β1 gene could show a pronounced protective effect during the disease process of IBD in DSS-induced mice, and the protective function was linked to the induced percentage of CD4+Foxp3+ Tregs in mLNs. The local induction was due to increased recruitment of DCs to intestinal inflammatory sites in mice, which led to high levels of TGF-β1 locally.
In summary, DCs modified by the TGF-β1 gene in this study showed a therapeutic effect on IBD in mice. Therefore, our study highlights the potential of this technique as a new approach to IBD treatment in the clinic.
Acknowledgments
We thank Professor Lin-Rong Lu for his critical review of the manuscript. This work was supported by the National Key Basic Research Program of China (Grant 2007CB512400), the National High Technology Research and Development Program of China (Grants 2006AA02A239 and 2007AA021102), the National Natural Science Foundation of China (Grant 30671909 and 30972725) and Natural Science Foundation of Zhejiang Province (Z2090042).
References
- Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel diseases. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sitaraman S, Gewirtz AT. Intestinal epithelial cell regulation of mucosal inflammation. Immunol Res. 2004;29:55–68. doi: 10.1385/IR:29:1-3:055. [DOI] [PubMed] [Google Scholar]
- Kuhbacher T, Fölsch UR. Practical guidelines for the treatment of inflammatory bowel disease. World J Gastroenterol. 2007;13:1149–1155. doi: 10.3748/wjg.v13.i8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16–26. doi: 10.1097/00054725-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;192:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–175. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MH, Tang H, Guo ZH, An HZ, Zhu XJ, Song WG, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Aharonia R, Sonegoa H, Brennerb O, Eilamb R, Arnon R. The therapeutic effect of glatiramer acetate in a murine model of inflammatory bowel disease is mediated by anti-inflammatory T-cells. J Pharmacol Exp Ther. 2006;318:68–78. doi: 10.1124/jpet.106.103192. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Wang QX, Zhang LH, Pan JP, Zhang M, Lu GH, et al. TGF-β1 gene modified immature dendritic cells exhibit enhanced tolerogenicity but induce allograft fibrosis in vivo. J Mol Med. 2002;80:514–523. doi: 10.1007/s00109-002-0346-2. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Wing K, Fehervari Z, Sakaguchi S. Emerging possibilities in the development and function of regulatory T cells. Int Immunol. 2006;18:991–1000. doi: 10.1093/intimm/dxl044. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Leehler R, Ng WF, Steinman RM. Dendritic cells in transplantation: friend or foe. Immunity. 2001;14:357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placek K, Coffre M, Maiella S, Bianchi E, Rogge L. Genetic and epigenetic networks controlling T helper 1 cell differentiation. Immunology. 2009;127:155–162. doi: 10.1111/j.1365-2567.2009.03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]