Abstract
Immunoglobulin superfamily (IgSF) members account for a large proportion of cell adhesion molecules that perform important immunological functions, including recognizing a variety of counterpart molecules on the cell surface or extracellular matrix. The findings that CD155/poliovirus receptor (PVR) and CD112/nectin-2 are the ligands for CD226/platelet and T-cell activation antigen 1 (PTA1)/DNAX accessory molecular-1 (DNAM-1), CD96/tactile and Washington University cell adhesion molecule (WUCAM) and that CD226 is physically and functionally associated with lymphocyte function-associated antigen-1 (LFA-1) on natural killer (NK) and activated T cells have largely expanded our knowledge about the functions of CD226, CD96, WUCAM and LFA-1 and their respective ligands, CD155, CD112, intercellular adhesion molecule (ICAM)-1 and junctional adhesion molecule (JAM)-1. The interactions of these receptors and their ligands are involved in many key functions of immune cells including naive T cells, cytotoxic T cells, NK cells, NK T cells, monocytes, dendritic cells, mast cells and platelets/megakaryocytes.
Keywords: CD112, CD155, CD226, IgSF, LFA-1
Introduction
The concept of the immunoglobulin superfamily (IgSF) was proposed in the 1980s.1 IgSF molecules are generally transmembrane glycoproteins; a large proportion of these are cell-adhesion molecules. IgSF members recognize counterpart molecules by three mechanisms: the interaction of IgSF members with each other, the interaction of IgSF members with integrin family members (e.g., intercellular adhesion molecule (ICAM)-1/lymphocyte function-associated antigen (LFA)-1, ICAM-1/Mac-1, vascular cell adhesion molecule -1/very late antigen-4 and vascular cell adhesion molecule-1/α4β7) and the interaction of IgSF members with other molecules (such as mucosal addressin cell adhesion molecule-1/L-selectin, mucosal addressin cell adhesion molecule-1/CD44, CD166/CD6, B- and T-lymphocyte attenuator (CD272)/herpesvirus entry mediator and CD47/thrombospondin). Interactions between IgSF members can be homophilic (CD31/CD31, CD56/CD56, neural cell adhesion molecule-1/neural cell adhesion molecule-L1 and CD66/CD66), between members with high homology (e.g., CD2/CD48, CD2/CD58 and CD244/CD48 within the CD2 subfamily), or heterophilic (CD4/MHC II, CD8/MHC I, CD28/B-7 and ICOS/ICOSL). In this review, we propose a novel cell interface consisting of homologous IgSF members, including two receptors, CD226 and CD96, two ligands, CD112 (nectin-2) and CD155 (poliovirus receptor, PVR), and their membrane-associated integrins, CD11a/CD18 (LFA-1) and αvβ3, with multiple immunological functions.
Ligands for CD226 and CD96
Nectin-2/CD112 has been identified as a ligand for CD226, whereas PVR/CD155 serves as a ligand for both CD226 and CD96 receptors. In addition, ICAM-1 and JAM-1 are the ligands for LFA-1, which is physically and functionally associated with CD226 on NK and activated T cells.
The human nectin family
Human nectin family members are Ca2+-independent immunoglobulin (Ig)-like adhesion molecules that homophilically or heterophilically trans-interact and cause cell–cell adhesion. The extracellular region of nectins is composed of three Ig-like domains: the V, C1-like and C2 types. Each nectin member can form homo-cis-dimers, followed by the formation of trans-dimers.2 The nectin family consists of nectin-1, -2, -3 and -4, among which nectin-1, -2 and -3 were named poliovirus receptor-related protein-1 (PRR-1), PRR-2 and PRR-3 and assigned as CD111, CD112 and CD113, respectively.3 Thus far, heterophilic interactions among nection-3/nectin-1, nectin-3/nectin-2, nectin-1/nectin-4 and nectin-3/PVR have been found.4
PVR/CD155
PVR/CD155 was named nectin-like molecule-5 (necl-5) because its domain structure is similar to that of nectins.2 It can be heterophilic, trans-interacting with nectin-3.4 The PVR/CD155 molecule contains a V domain, a C1-like domain and a C2 domain in its extracellular region.2, 5 CD155 and CD112 are constitutively expressed at low levels on epithelial and endothelial cells. Recently, PVR/necl-5/CD155 was found to be overexpressed in tumor-cell lines and primary tumors showing tumor-cell invasion and migration.6, 7
The human JAM family
The human junctional adhesion molecule (JAM) family is composed of JAM-1, JAM-2 and JAM-3, which show similar extracellular structures and high homology with respect to the V and C2 domains. They also participate in common homotypic interactions8 and were recently termed CD321, CD322 and CD323, respectively, at the Eighth International Conference on Human Leukocyte Differentiation Antigen.3 The contribution of JAM-1/CD321 to epithelial tight junctions and its expression by endothelial cells have been well described. It has also been confirmed that JAM-1/CD321 is a ligand for LFA-1 (αLβ2) and participates in LFA-1-dependent trans-endothelial migration of leukocytes,9 while JAM-2/CD322 and JAM-3/CD323 on endothelial cells can bind integrins very late antigen-4 (α4β1) and Mac-1 (αMβ2) on leukocytes, respectively.10 Thus, JAM-1/CD321-mediated junctional integrity and endothelial permeability are attributed to its ability to form homophilic interactions, while its heterophilic interaction with LFA-1 plays a key role in leukocyte transmigration.9, 11 In addition, like CD155, JAM-1/CD321 also forms a complex with the αvβ3 integrin on the surface of endothelial cells.12
Novel receptors for PVR/CD155 and nectin-2/CD112
Platelet and T-cell activation antigen 1 (PTA1)/DNAX accessory molecule-1 (DNAM-1)/CD226 is the receptor for both PVR/CD155 and nectin-2/CD112 ligands, while tactile/CD96 is the only receptor for PVR/CD155. LFA-1 is physically and functionally associated with CD226 on natural killer (NK) and T cells. In this regard, we would also like to consider this integrin molecule as a receptor upon the interaction of NK cells or T cells with target cells or endothelial cells.
PTA1/DNAM-1/CD226
CD226 was initially described as T-lineage-specific activation antigen 1, involved in the differentiation of human cytotoxic T cells from their precursors.13 Subsequently, this molecule was identified on platelets and was shown to be involved in platelet activation and aggregation; hence, it was renamed as PTA1.14 In the 1990s, PTA1 was cloned by two independent groups and the DNAX group called it DNAM-1.15, 16 The PTA1/CD226 gene is located on chromosome 18q22–23 and is a 65-kDa transmembrane glycoprotein consisting of an extracellular region with two Ig-V-like domains, a transmembrane region and a cytoplasmic region containing tyrosine- and serine-phosphorylated sites.15, 16 The CD226 expression on T cells and cytotoxicity of cytotoxic lymphocyte are upregulated by IL-2, IL-15 and tumor-necrosis factor-α and downregulated by transforming growth factor-β.17 Transcription factors such as activator protein-1 and Ets might participate in the regulation of CD226 expression.18 Importantly, CD226 is physically and functionally associated with LFA-1 on NK cells and activated T cells, and crosslinking of LFA-1 induces tyrosine phosphorylation of CD226, suggesting that CD226 is important for costimulatory signals initiated by LFA-1 ligation.19 This finding was further supported by the fact that the dynamic assembly of the LFA-1–PTA1 complex is formed via intracellular interaction following T-cell activation.20 CD226 is highly conserved among humans, apes, monkeys and mice, suggesting that it has important evolutionary biological functions (Figure 1).21 In 2003, PVR/CD155 and PRR-2/nectin-2/CD112 were identified as ligands for CD226.22 This finding dramatically highlighted the molecular mechanism of CD226-mediated functions. Recently the interaction of murine CD226 with the PVR homolog was also identified in the murine system.23
Figure 1.

Comparison of the protein sequences among human, gibbon, monkey and mouse CD226. The predicated signal peptide (Met−18 to Cys−1) and transmembrane sequences (Leu251 to Leu265 for human and gibbon CD226, Arg251 to Leu265 for monkey CD226 and Leu253 to Tyr267 for mouse CD226) are underlined. The conserved cysteines (Cys37, Cys108, Cys152 and Cys222 for human, gibbon and monkey CD226, and Cys37, Cys109, Cys153 and Cys224 for mouse CD226) predicated to form the disulfide bonds that stabilize the two V-like domains are boxed. The key phosphorylation sites (Tyr322 and Ser329 for human, gibbon and monkey CD226, and Tyr319 and Ser326 for mouse CD226) are indicated by ♦ and *, respectively. The murine CD226 ORF encodes a protein of 333 amino acids, which is 3 amino acid shorter than that of human CD226. Compared with human CD226, mouse CD226 has 53% identity at the amino acid level, while human, gibbon and monkey CD226 protein have the same length of 336 amino acid residues and their CD226 sequences have 93–95% amino acid identity.
Tactile/CD96
In 2004, CD96, T-cell activation increased late expression (tactile), was also found to be another receptor for PVR/CD155.24 Notably, CD226 and CD96 share a very high homology with a common structural feature; all extracellular domains are V-like (three V domains for CD96 and two V domains for CD226). Although CD96 was cloned in 1992 and belongs to the T-cell activation antigen family,25 its function was unknown until PVR/CD155 was identified as a ligand for CD96 and the interaction of CD96 with CD155 was shown to be involved in NK cell adhesion to target cells and cytotoxicity. Thus, the existence of a dual-receptor system (CD226 and CD96) that could recognize PVR/CD155 further underlies the crucial role of this ligand in NK-mediated recognition of target cells.24
Washington University cell adhesion molecule
Washington University cell adhesion molecule (WUCAM) is another recently identified novel receptor for PVR/CD155.26 WUCAM sequences aligned with nectin-1–4, PVR/CD155, CD226 and CD96 in the distal Ig-V-type domain, according to the National Center Biotechnology Information cDNA database. WUCAM has a single Ig-V-type domain, a transmembrane-spanning sequence and a short cytoplasmic tail. Similar to nectins and nectin-like molecules, the cytoplasmic domain of WUCAM also exhibits a putative type-I PSD-95/discs large/zona occludens-1-binding motif. Furthermore, the interaction between WUCAM on human follicular B helper T cells and PVR/CD155 on follicular dendritic cell (DC) within the germinal center mediates adhesion of T follicular helper cells to follicular DCs and might facilitate the generation of T-cell-dependent antibody responses.26
CD226 and LFA-1 form a cell interface guarding the regulation of signal junctions
LFA-1 is recruited into lipid rafts during T-cell activation and is involved in the formation of peripheral supramolecular activation clusters that surround central supramolecular activation clusters at the immunological synapse.27, 28, 29 Stimulation of T cells with anti-CD3 induces physical association of CD226 with LFA-1, for which the serine phosphorylation of human CD226 at residue 329 is responsible. Protein kinase C phosphorylates Ser329 of CD226 and plays a critical role for both CD226 adhesion and signaling.30 Once LFA-1 and CD226 associate with each other, crosslinking with LFA-1 induces tyrosine phosphorylation of human CD226 at residue 322, for which the Fyn protein tyrosine kinase is responsible, mediating costimulatory signals for triggering T-cell differentiation and proliferation. Serine 329 of human CD226 is required for lipid raft recruitment of CD226.30, 31 More recently, results from mouse models also demonstrated that LFA-1 is necessary for recruitment of CD226 into lipid rafts. Furthermore, serine phosphorylation of murine CD226 is mainly required for the association of CD226 with LFA-1, which allows CD226 entry into lipid-raft compartments.32
Strikingly, CD226, nectin-2 and JAM all contain PDZ-binding domains at their C-termini, and all of them have been shown to bind different putative type-I PSD-95/discs large/zona occludens-1-containing proteins.20, 33, 34 In resting T cells, CD226 binds the carboxyl-terminal domain of isoforms of the actin-binding protein 4.1G, which is known to associate with the membrane-associated guanylate-kinase homolog, human discs large. Upon T-cell stimulation with phorbol ester, a ternary complex among CD226, human discs large, 4.1G and the cytoskeleton is formed. These dynamic associations provide the structural basis for a regulated molecular adhesive complex that serves to cluster and transport LFA-1 and associated molecules.20
Interaction of CD226 and CD96 with their ligand mediates multiple immunoregulation mechanisms
Based on the accumulating evidence provided in this review, we propose a concept of a novel cell interface or platform consisting of CD226 and CD96 receptors, their ligands, PVR/necl-5/CD155 and PRR-2/nectin-2/CD112, and membrane-associated molecules LFA-1 and αvβ3. The interaction of these receptors with their ligands is involved in many important immunological functions. The features of the structure, the interaction and functions of these receptors and ligands are described below.
There is a high homology between PVR/necl-5/CD155 and PRR-2/nectin-2/CD112 and their receptors. Both human CD155 and CD112 genes are located on chromosome 19q13.2–13.4.2 Dendrogram analysis of V domains from IgSF members indicates that CD226, CD96, CD155 and CD112 are closely related (Figure 2). Therefore, the interaction of these ligands and receptors is very similar to that within the CD2 subfamily; members of this family also share a high homology and interact with each other.35 Interestingly, some integrin family members interact with CD226, PVR/necl-5/CD155 and JAM-1/CD321. In addition to the interactions between LFA-1 with CD226 and LFA-1 and JAM-1, CD155 and JAM-1 associate with αvβ3 on endothelial cells, mediating the adhesion of endothelial cells to extracellular matrix vitronectin and fibronectin (Figure 3). Cross-recognition exists between these ligands and receptors. As mentioned before, CD226 can bind PVR/necl-5/CD155 and PRR-2/nectin-2/CD112, while CD155 can be recognized by two receptors, CD226 and CD96. LFA-1 (αLβ2) constitutively associates with CD226 on NK cells and on activated T cells and could bind ICAM-1/CD54 and JAM-1/CD231 expressed on endothelia cells (Figure 3).
Figure 2.

Dendrogram analysis of IgSF member V domains. The dendrogram of immunoglobulin superfamily member V domains was analyzed by ClustalX software. Analysis results showed that the first V domains of CD226 and CD96 have the closest relationship with respect to sequence similarity comparison. The V domains of nectin-2/CD112 and PVR/CD155 have the highest homology. C1L, C1-like; CNS, central nervous system; DC, dendritic cell; ICAM-1, intercellular adhesion molecule-1; IgSF, immunoglobulin superfamily; JAM-1, junctional adhesion molecule-1; LFA-1, lymphocyte function-associated antigen-1; NK, natural killer; NKT, natural killer T cell; PTA1, platelet and T-cell activation antigen 1; PVR, poliovirus receptor; WUCAM, Washington University cell adhesion molecule.
Figure 3.

Cross-recognition between ligands CD155, CD112, CD54, CD321 and their receptors CD226, CD96, LFA-1 and WUCAM. Tactile/CD96, PTA1/DNAM-1/CD226, LFA-1 and WUCAM are expressed on T cells, NK cells, NK T cells, monocytes, megakaryocytes or platelets, while their ligands PVR/CD155, nectin-2/CD112, ICAM-1/CD54 and JAM-1/CD321 are expressed on endothelial cells and target cells such as tumor cells. Both PVR/CD155 and JAM-1/CD321 are associated with the αvβ3 integrin. PTA1/DNAM-1/CD226 recognizes ligands PVR/CD155 and nectin-2/CD112, while tactile/CD96 recognizes PVR/CD155 but not nectin-2/CD112. LFA-1/αLβ2 physically and functionally associated with CD226 bound to ICAM-1/CD54 and JAM-1/CD321. C1L, C1-like; DNAM-1, DNAX accessory molecular-1; HSV, herpes simplex virus; ICAM-1, intercellular adhesion molecule-1; JAM-1, junctional adhesion molecule-1; LFA-1, lymphocyte function-associated antigen-1; NK, natural killer; PTA1, platelet and T-cell activation antigen 1; PVR, poliovirus receptor; WUCAM, Washington University cell adhesion molecule.
Surprisingly, the CD226 and CD96 ligands (CD155, CD112, CD231 and CD54, which is the ligand for LFA-1) can form homophilic dimers and serve as receptors for Poliovirus,36 some strains of the herpes simplex virus,2, 37 Rhinovirus38 and Reovirus,39 respectively. Interestingly, these cells are known to allow endocytosis of the corresponding virus via interaction with their V domains (Figure 4). More recently, it was found that the human cytomegalovirus viral product UL141 can block CD155, impeding signaling through the activating receptor, CD226, on NK cells and contributing to the protection of virus-infected cells from NK-mediated attack.40
Figure 4.

CD155, CD112, CD321 and CD54 serve as virus receptors. CD155 and CD112, the ligands for receptors CD226 and CD96, and CD54 and CD321, the ligands for LFA-1, which is closely associated with CD226, serve as receptors for Poliovirus, herpes simplex virus (HSV) and Rhinovirus and Reovirus, respectively. In addition, human cytomegalovirus (HCMV) viral product UL141 can block CD155, which may contribute to the protection of virus-infected cells from NK-mediated attack. CTL, cytotoxic lymphocyte; DC, dendritic cell; iDC, immature DC; Ig, immunoglobulin; JAM-1, junctional adhesion molecule-1; LFA-1, lymphocyte function-associated antigen-1; NK, natural killer; TCR, T-cell receptor.
Interactions of CD226 and CD96 with their ligands contribute to many of the immunological functions that are characteristic of various immunocytes, including lymphoid cells (Th, cytotoxic lymphocyte, NK and NK T) and myeloid cells (monocytes, dendritic cells, platelets/megakaryocytes lineage and mast cells) (Figure 5 and Table 1).
Figure 5.
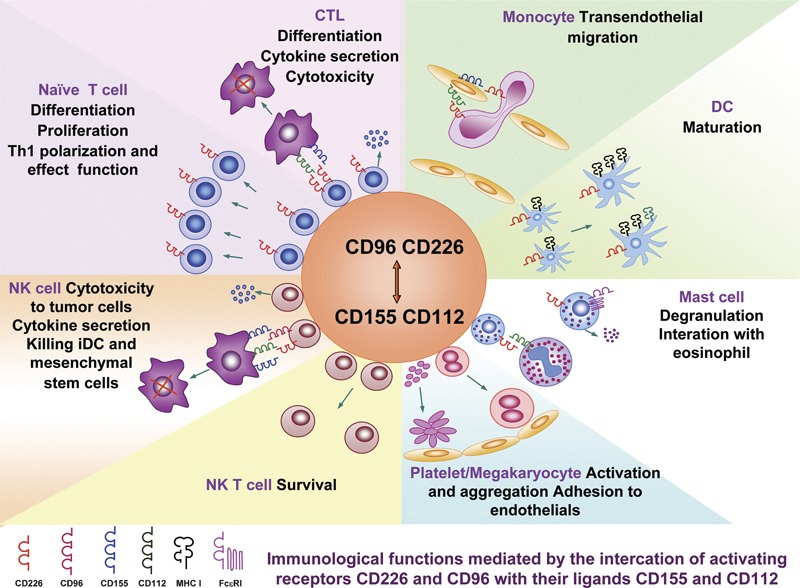
The interactions of CD226 and CD96 with their ligands participate in multiple immunological functions. Detailed explanations are described in Table 1. CTL, cytotoxic lymphocyte; DC, dendritic cell; NK, natural killer.
Table 1. The interaction of receptors CD226 and CD96 with their ligands CD112 and CD155 involving multiple immunological functions.
| Receptor-expressing cells | Ligand-expressing cells | Immunological functions | Reference |
|---|---|---|---|
| NK cells | Tumor cells | ||
| NCR (NKp30, NKp40 and NKp46), NKG2D and CD226 are the major activating receptors on NK cells. CD96 is also expressed on NK cells | NCR ligands are still unknown. The ligands for NKG2D and CD226 are MICA/B and ULBPs as well as CD112 and CD155 respectively which are expressed on a variety of tumor cells. CD155 can also serve as a ligand for CD96 | Killing tumor cells is mediated by the interaction of activating receptors with their ligands. The susceptibility to lysis by NK cells directly correlates with surface expression of CD155 on some neuroblasts, while the downregulation of CD226 in some leukemia may be involved in the mechanisms of tumor escape. Consistent expression of CD112 and CD155 exists in freshly isolated myeloid leukemias in which MICA/B and ULBPs are either absent or weakly expressed. Accordingly, NK-mediated lysis of these leukemias is dependent on CD226 expression. CD96 promotes NK cell adhesion to target cells and stimulates cytotoxicity of activated NK cells. Together with CD226 expression, NK cells have evolved a dual receptor system that mediates NK cell adhesion and triggering of effector functions against tumors | 24, 42, 43, 44 |
| Dendritic cells (DC) | |||
| Murine CD8α+ DC express CD112 and CD155. In particular, CD112 expression is upregulated on immature dendritic cells (iDC) as compared with monocytes | In autologous model, iDCs are efficiently killed by NK cells to which the interaction of CD226 with its ligands contributes. CD226 may play a role in NK-mediated ‘quality control' of DC maturation by selecting those DCs maturation by the high expression of MHC class I molecules and costimulatory ligands to optimize the ability to prime T cells | 54 | |
| Mesenchymal stem cells (MSCs) | |||
| Human MSCs express ULBPs, CD112 and CD155 | Activated NK cells are capable of killing MSC for which the interaction of NKp30, NKG2D and CD226 with their ligands is responsible | 56 | |
| Cytotoxic T lymphocytes (CTL) | Tumor cells | ||
| CD226 is expressed on CTL and its expression is upregulated by cytokines (IL-2 and TNF-α) and alloantigen stimulation | Tumor cells express CD112 and CD155 | The interaction of CD226 with its ligands promotes CTL-mediated cytotoxicity against tumor cells and allograft. In vivo, murine CD8+ T cells are responsible for the rejection of CD226 ligands-expressing RMA tumors | 13, 15, 17, 23, 45 |
| Naive T cells | CD226 is involved in LFA-1-mediated costimulatory signal of triggering naive T-cell differentiation and proliferation. LFA-1 ligation induces aggregation and activation of focal adhesion kinase (FAK) at sites of LFA-1–ICAM interaction and activated FAK then bind to Fyn. The Fyn is responsible for CD226 tyrosine phosphorylation induced by LFA-1 signal. Fyn kinase may also play an important role for Th1 polarization from CD4+ naïve T cells | 47 | |
| Helper T cells (Th1) | Antimurine CD226 treatment in vivo reduces significantly Th1-cell expansion, and delays onset and reduces the severity of Th1-mediated autoimmune disease, EAE, suggesting that CD226 may play an important role in activation and effect function of Th1 cells | 49 | |
| NK T cells | CD226 is significantly downregulated on NK T cells from active SLE patients and surviving is selectively deficient in NK T cells from the patients which may cause anti-CD95-induced apoptosis of these NKT cells. Preactivation of CD226 could rescue NK T cells from anti-CD95-induced apoptosis | 50 | |
| Monocytes | Endothelial cells | ||
| Monocytes express CD226 | CD155 is expressed at junctions on primary vascular endothelial cells | The interaction of CD226 with CD155 regulates monocyte migration through endothelial junctions and occurs during diapedesis step. This binding is independent of LFA-1 | 52 |
| Dendritic cells | |||
| CD8α+ dendritic cells express CD226 | Crosslinking CD226 induces maturation of CD8+α DC which can prime Th1 cells by antigen presentation | 45 | |
| Mast cells | Eosinophils | ||
| Mast cells express a high level of CD226 | Eosinophils express CD112 | CD226 synergizes with FcεR I on mast cells to augment degranulation through a pathway involving Fyn, LAT, PLCγ2 and CD18 which causes IgE-dependent hyperactivity. CD226 on human mast cells is engaged by CD112 on eosinophils to elicit its costimulatory effect which may function as a costimulatory signaling switch | 55 |
| Megakaryocytes/platelets | Vascular endothelial cells | ||
| CD226 is expressed on platelets and primary megakaryocytes and megakaryocytic cell lines UT7/TPO, HEL, CMK, Dami and Mo7e cells | Endothelial cells express CD112 and CD155 | The interaction of CD226 with its ligands mediates megakaryocytic cell adhesion to vascular endothelial cells. When CD34+ cells from human bone marrow are incubated in the presence of CD226 mAb plus LFA-1 mAb, the ploidy of generated megakeryocytes is significantly shifted to high classes. Crosslinking of CD226 mediates platelet activation and aggregation during which CD226 is phosphorylated and PKC is involved in. thrombin-activated platelets can bind to HUVEC during which CD226 is tyrosine-phosphorylated and the tyrosine at residue 304 plays an important role in its adhesion function | 14, 51, 52 |
CTL, cytotoxic lymphocyte; EAE, experimental autoimmune encephalomyelitis; HUVEC, human umbilical vein endothelial cells; IgE, immunoglobulin E; LAT, linker of activation of T cells; ICAM, intercellular adhesion molecule; LFA-1, lymphocyte function-associated antigen-1; mAb, monoclonal antibody; MICA/B, MHC class I-related chain molecule A/B; NCR, natural cytotoxicity receptors; NK, natural killer; NKG2D, natural killer group 2D; PKC, protein kinase C; PLCγ2, phospholipase C γ2; TNF-α, tumor-necrosis factor-α TPO, thrombopoietin; ULBPs, UL1b-binging protein.
(i) The balance of the expression of activating and inhibitory receptors on the NK cell surface and their ligand expression on target cells determine the cytotoxicity of NK cells.41 In addition to the expression of NK receptors and ligands, reduced or lack of MHC class I molecule expression, which serves the major ligands of NK inhibitory receptors, is also required for NK cytotoxicity.41 CD226 is a major activating receptor on NK and activated T cells and mediates the cytotoxicity via its binding to CD155 or/and CD112 on target cells (e.g., tumor cells).41 The susceptibility to lysis by NK cells directly correlates with the surface expression of CD155 on some tumor cells. The downregulation of PVR/CD226 in some forms of leukemia could be involved in tumor-escape mechanisms. This property has recently received a great deal of attention.42, 43, 44 In vivo experiments using syngeneic mice also indicated that CD112- or CD155-transduced RMA tumor cells could be rejected by CD226-expressing CD8+ T and NK cells.45 It should be noted that CD226 could not induce cytotoxicity by resting NK cells. However, using crosslinked monoclonal antibodies, CD226 synergized with CD16, 2B4/CD244 or NK p46/CD335 on resting NK cells, increasing the levels of Ca2+ flux and cytokine secretion (tumor-necrosis factor-α and interferon-γ) and enhancing the cytotoxicity of resting NK cells, as determined by the redirected lysis assay.46 Similar to CD226, CD96 promoted NK killing activity by the interaction with CD155 on targets.24 An advance in the role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance was recently reviewed.47
(ii) CD226 is involved in LFA-1-mediated costimulatory signaling that triggers naive T-cell differentiation and proliferation.48 Importantly, in vivo anti-CD226 treatment significantly reduces Th1 cell expression. It also delays onset and reduces the severity of the Th1-mediated autoimmune disease, experimental autoimmune encephalomyelitis, suggesting that CD226 is a costimulatory molecule that may play an important role in activation and effect functions of Th1 cells.49 It should be pointed out that CD226 could participate in early-stage T-cell development, since the murine CD226/Ig fusion protein could inhibit the production of thymocytes, particularly decreasing the proportion of CD4+CD8+ and CD4−CD8+ subpopulations during fetal thymus organ culture (unpublished data).
(iii) CD226-expression deficiency may cause death receptor-induced apoptosis of NK T cells from systemic lupus erythematosus patients.50
(iv) CD226 mediates platelet activation and aggregation, as well as platelet and megakaryocyte adhesion to vascular endothelial cells.14, 51 Moreover, CD226 was expressed on Lin− cells derived from human adult bone marrow; when CD34+ cells from adult bone marrow were incubated in the presence of CD226 and LFA-1 monoclonal antibodies, the ploidy of generated megakaryocytes was significantly shifted to high classes.52
(v) The interaction of CD226 and CD155 regulates monocyte migration through endothelial junctions.53 In addition, JAM-1 molecules also contribute to LFA-1 dependent trans-endothelial migration of T cells and polymorphonuclear neutrophils, as well as LFA-1-mediated arrest of T cells.9
(vi) In the mouse, CD8 α+ DCs also express CD226, and crosslinking CD226 induces maturation of CD8 α+ DCs.45 On the other hand, the interaction of CD226 with CD155 and CD112 contributes to NK-mediated lysis of immature DCs and mature DCs. Analogously, mature DCs are protected from NK-mediated lysis by high surface expression of HLA class I molecules on mature DCs; these act as ligands for inhibitory receptors on NK cells. Immature DCs are efficiently killed by NK cells due to CD226 function. Therefore, CD226 might play a role in NK-mediated ‘quality control' of DC maturation by selecting those DCs that show a high expression of HLA class I molecules and costimulatory ligands, optimizing their ability to prime T cells.54
(vii) Human mast cells express a high level of CD226 and eosinophils express CD112. CD226 synergized with FcεR I on mast cells and its engagement augments degranulation through a pathway involving Fyn, LAT, PLCγ2 and CD18. Blocking CD112 expression on eosinophils with neutralizing antibodies normalized the hyperactivity resulting from IgE-dependent activation of mast cells cocultured with eosinophils.55
(viii) Activated NK cells are capable of killing mesenchymal stem cells; this may be due to interactions of the activating receptors (NKp30/CD337, NKG2D/CD314 and CD226) on NK cells with their ligands (ULBPs, PVR/CD155 and nectin-2/CD112) on mesenchymal stem cells.56
Concluding remarks
Information about the structure and function of CD226 and CD96 and their ligands has significantly increased in past few years. However, the cell signaling pathways triggered by CD226 and CD96 are still poorly understood. Therefore, additional studies could focus on putative cellular signaling pathways that engage the cytoplasmic domains of CD226 and CD96. The relationship between membrane molecules and the soluble forms of their ligands and receptors is unknown, and the in vivo physiological and pathological significance of the receptor families discussed above requires further investigation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 30801003, 30972683 and 30901310).
References
- Williams AF, Barclay AN. The immunoglobulin superfamily – domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94:655–667. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Available from: http://www.hlda8.org
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276:43205–43215. doi: 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- Mueller S, Wimmer E. Recruitment of nectin-3 to cell–cell junctions through trans-heterophilic interaction with CD155, a vitronectin and poliovirus receptor that localizes to alphavbeta3 integrin-containing membrane microdomains. J Biol Chem. 2003;278:31251–31260. doi: 10.1074/jbc.M304166200. [DOI] [PubMed] [Google Scholar]
- Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49:236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Muller WA. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Naik UP, Eckfeld K. Junctional adhesion molecule 1 (JAM-1) J Biol Regul Homeost Agents. 2003;17:341–347. [PubMed] [Google Scholar]
- Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and alphavbeta3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and alphavbeta3 complex. Blood. 2003;102:2108–2114. doi: 10.1182/blood-2003-04-1114. [DOI] [PubMed] [Google Scholar]
- Burns GF, Triglia T, Werkmeister JA, Begley CG, Boyd AW. TLiSA1, a human T lineage-specific activation antigen involved in the differentiation of cytotoxic T lymphocytes and anomalous killer cells from their precursors. J Exp Med. 1985;161:1063–1078. doi: 10.1084/jem.161.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JL, Dunn SM, Jin B, Hillam AJ, Walton S, Berndt MC, et al. Characterization of a novel membrane glycoprotein involved in platelet activation. J Biol Chem. 1989;264:13475–13482. [PubMed] [Google Scholar]
- Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- Sherrington PD, Scott JL, Jin B, Simmons D, Dorahy DJ, Lloyd J, et al. TLiSA1 (PTA1) activation antigen implicated in T cell differentiation and platelet activation is a member of the immunoglobulin superfamily exhibiting distinctive regulation of expression. J Biol Chem. 1997;272:21735–21744. doi: 10.1074/jbc.272.35.21735. [DOI] [PubMed] [Google Scholar]
- Jin B, Scott JL, Vadas MA, Burns GF. TGF beta down-regulates TLiSA1 expression and inhibits the differentiation of precursor lymphocytes into CTL and LAK cells. Immunology. 1989;66:570–576. [PMC free article] [PubMed] [Google Scholar]
- Jian JL, Zhu CS, Xu ZW, Ouyang WM, Ma DC, Zhang Y, et al. Identification and characterization of the CD226 gene promoter. J Biol Chem. 2006;281:28731–28736. doi: 10.1074/jbc.M601786200. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, et al. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11:615–623. doi: 10.1016/s1074-7613(00)80136-3. [DOI] [PubMed] [Google Scholar]
- Ralston KJ, Hird SL, Zhang X, Scott JL, Jin BQ, Thorne RF, et al. The LFA-1-associated molecule PTA-1 (CD226) on T cells forms a dynamic molecular complex with protein 4.1G and human discs large. J Biol Chem. 2004;279:33816–33828. doi: 10.1074/jbc.M401040200. [DOI] [PubMed] [Google Scholar]
- Tian F, Li D, Xia H, Liu X, Jia W, Sun C, et al. Isolation of cDNAs encoding gibbon and monkey platelet and T cell activation antigen 1 (PTA1) DNA Seq. 1999;10:155–161. doi: 10.3109/10425179909033941. [DOI] [PubMed] [Google Scholar]
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Miyamoto A, Hara A, Honda S, Shibuya K, Shibuya A. Identification and characterization of murine DNAM-1 (CD226) and its poliovirus receptor family ligands. Biochem Biophys Res Commun. 2005;329:996–1000. doi: 10.1016/j.bbrc.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172:3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- Wang PL, O'Farrell S, Clayberger C, Krensky AM. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol. 1992;148:2600–2608. [PubMed] [Google Scholar]
- Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39:695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. The immunological synapse. Arthritis Res. 2002;4 Suppl 3:S119–S125. doi: 10.1186/ar559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci. 2002;115:963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- Marwali MR, MacLeod MA, Muzia DN, Takei F. Lipid rafts mediate association of LFA-1 and CD3 and formation of the immunological synapse of CTL. J Immunol. 2004;173:2960–2967. doi: 10.4049/jimmunol.173.5.2960. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Lanier LL, Phillips JH. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor. J Immunol. 1998;161:1671–1676. [PubMed] [Google Scholar]
- Shirakawa J, Shibuya K, Shibuya A. Requirement of the serine at residue 329 for lipid raft recruitment of DNAM-1 (CD226) Int Immunol. 2005;17:217–223. doi: 10.1093/intimm/dxh199. [DOI] [PubMed] [Google Scholar]
- Shirakawa J, Wang Y, Tahara-Hanaoka S, Honda S, Shibuya K, Shibuya A. LFA-1-dependent lipid raft recruitment of DNAM-1 (CD226) in CD4+ T cell. Int Immunol. 2006;18:951–957. doi: 10.1093/intimm/dxl031. [DOI] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, et al. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell–cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–249. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud F, Ponce de Leon M, Baribaud I, Whitbeck JC, Xu R, et al. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322:286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Staunton DE, Merluzzi VJ, Rothlein R, Barton R, Marlin SD, Springer TA. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- Tomasec P, Wang EC, Davison AJ, Vojtesek B, Armstrong M, Griffin C, et al. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol. 2005;6:181–188. doi: 10.1038/ni1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221–226. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42:463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, Moretta L, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Shibuya K, Kai H, Miyamoto A, Morikawa Y, Ohkochi N, et al. Tumor rejection by the poliovirus receptor family ligands of the DNAM-1 (CD226) receptor. Blood. 2006;107:1491–1496. doi: 10.1182/blood-2005-04-1684. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359–366. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Shirakawa J, Kameyama T, Honda S, Tahara-Hanaoka S, Miyamoto A, et al. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J Exp Med. 2003;198:1829–1839. doi: 10.1084/jem.20030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Schubart AS, Reddy J, Meyers JH, Monney L, Sabatos CA, et al. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. J Immunol. 2005;175:1558–1565. doi: 10.4049/jimmunol.175.3.1558. [DOI] [PubMed] [Google Scholar]
- Deng T, Liu SW, Wu Q, Liu Y, Ju W, Liu JY, et al. CD226 expression deficiency causes high sensitivity to apoptosis in NK T cells from patients with systemic lupus erythematosus. J Immunol. 2005;174:1281–1290. doi: 10.4049/jimmunol.174.3.1281. [DOI] [PubMed] [Google Scholar]
- Kojima H, Kanada H, Shimizu S, Kasama E, Shibuya K, Nakauchi H, et al. CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. J Biol Chem. 2003;278:36748–36753. doi: 10.1074/jbc.M300702200. [DOI] [PubMed] [Google Scholar]
- Ma D, Sun Y, Lin D, Wang H, Dai B, Zhang X, et al. CD226 is expressed on the megakaryocytic lineage from hematopoietic stem cells/progenitor cells and involved in its polyploidization. Eur J Haematol. 2005;74:228–240. doi: 10.1111/j.1600-0609.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, et al. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med. 2004;199:1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer–dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- Bachelet I, Munitz A, Mankutad D, Levi-Schaffer F. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): a novel interface in the allergic process. J Biol Chem. 2006;281:27190–27196. doi: 10.1074/jbc.M602359200. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]


