Abstract
In several mammalian species, including humans, coronavirus infection can modulate the host immune response. We show a potential role of dendritic cells (DC) in murine coronavirus-induced immune modulation and pathogenesis by demonstrating that the JAW SII DC line and primary DC from BALB/c mice and p/p mice with reduced expression of the murine coronavirus receptor, murine CEACAM1a, are susceptible to murine coronavirus infection by a receptor-dependent pathway.
Coronavirus infections can cause severe alterations of the host immune response in several species by mechanisms that are not completely understood (7, 9, 33). Severe acute respiratory syndrome (SARS) in humans is caused by a new coronavirus (12, 17, 28, 37) and is associated with a decrease in peripheral T cells, B cells, and NK cells (10). In this paper we investigate the possible role of dendritic cells (DC) in immune modulation and pathogenesis of the murine coronavirus, mouse hepatitis virus strain A59 (MHV-A59). MHV causes a variety of diseases in mice, including hepatitis, enteritis, respiratory infection, acute encephalitis, and chronic demyelination in the central nervous system (7). Outbreaks of MHV infection in laboratory mouse colonies are expensive to remedy and significantly compromise research.
MHV infection modulates the immune system and alters host responses to other pathogens and noninfectious agents. For example, allogeneic skin transplants grafted onto mice 3 weeks after recovery from acute MHV infection survive longer than grafts onto uninfected mice (9). MHV infection can cause impaired function and/or loss of B-cell and T-cell populations, and immunomodulation has been attributed to infection of macrophages; however, the precise mechanisms of immunomodulation are not well understood (7). Here we describe receptor-dependent MHV infection of murine DC and discuss the possible role of DC in MHV-induced immune modulation.
DC are the most potent antigen-presenting cells of the immune system and the keystone of the adaptive immune response. Many different viruses have been shown to interact with DC, but the outcomes differ significantly. For example, vaccinia virus inoculation of DC results in an abortive infection that blocks DC maturation (16), while Venezuelan equine encephalitis (VEE) virus infection of DC is a mechanism for delivery of virus from the periphery to the lymph nodes and spleen (27). A single amino acid substitution in the VEE E2 glycoprotein prevents infection of DC in vivo and blocks virus spread (27). Although human immunodeficiency virus type 1 does not infect DC, it binds to DC-SIGN, a lectin on the plasma membrane of DC, and is carried on migrating DC to susceptible T cells (20). Similarly, Ebola virus binds to DC-SIGN and DC-SIGNR but does not use them for entry into DC, although binding of virions to DC enhances infection of macrophages and endothelial cells (40). DC-SIGN mediates entry into DC for human cytomegalovirus and dengue virus (22, 41), but the means of entry are not yet known for other viruses, including VEE virus, measles virus, influenza virus, herpes simplex virus type 1, varicella-zoster virus, lymphocytic choriomeningitis virus, vesicular stomatitis virus, pseudorabies virus, parainfluenza virus type 3, and Sindbis virus (1, 3, 19, 30, 34-36, 38, 39).
In the present study, we used a murine DC cell line (JAWS II; ATCC catalog no. CRL-1194) and cultures of primary murine bone marrow-derived DC (BMDC) to test whether murine coronavirus MHV-A59 can bind to and infect DC via its receptor, murine CEACAM1a, a cell surface glycoprotein in the carcinoembryonic antigen (CEA) family within the immunoglobulin superfamily (2). Several other CEA-related murine glycoproteins, including CEACAM1b, CEACAM2, and bCEA, also have MHV receptor activity (6, 13, 14, 32, 45, 46). MHV infection of murine cells that express CEACAM1a can be blocked with an anti-murine CEACAM1a monoclonal antibody (MAb) called CC1 (15). CEACAM1a is expressed on apical membranes of epithelial cells in the gastrointestinal and respiratory tracts, kidneys, B cells, neutrophils, macrophages, activated T cells, thymic stromal cells, and small vascular endothelial cells (8, 21, 23, 29). Kammerer et al. showed that isoforms of CEACAM1a expressed on BALB/c and C57BL/6 BMDC are signal-transducing molecules that regulate early maturation and activation of DC (25).
To evaluate the possible role of CEACAM1a on murine DC in the pathogenesis and immune modulation of MHV infection, we used flow cytometry to analyze the expression of CEACAM1a and the DC marker CD11c on a murine DC line (JAWS II) and primary BMDC from BALB/c mice and from genetically manipulated p/p mice, which are partially resistant to MHV-A59 infection and express reduced levels of CEACAM1a in the liver, kidneys, and colon (4). A total of 106 JAWS II cells were incubated with mouse anti-CEACAM1a MAb CC1, hamster anti-CD11c MAb HLC, or isotype-matched control MAbs followed by a fluorescein isothiocyanate-conjugated anti-mouse or anti-hamster immunoglobulin. Figure 1 shows that JAWS II DC expressed both CEACAM1a and CD11c. For BALB/c mice, CD11c and CEACAM1a were also expressed on BMDC obtained from the bone marrow of femurs and tibiae after depletion of CD4+ and CD8+ T cells, B220+ B cells, and major histocompatibility complex class II-positive maturing myeloid cells by magnetic cell sorting following the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladback, Germany). A total of 106 BMDC, in 4 ml of medium (RPMI [Gibco]; 10% fetal bovine serum, 2% penicillin-streptomycin, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 0.06 mM HEPES, 0.02 mM 2-mercaptoethanol) supplemented with 3 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml and 5.5 ng of interleukin 4 (IL-4)/ml, were split and refed on day 2 and incubated for 5 days before use. With and without GM-CSF/IL-4, more than 95% of the negatively selected cells from murine bone marrow expressed CEACAM1a (data not shown). In addition, 61% of cells grown in GM-CSF and IL-4 expressed both CD11c and major histocompatibility complex class II, another commonly used marker for BMDC (data not shown; 24, 47).
FIG. 1.
Expression of CEACAM1a and CD11c on murine dendritic cells. The JAWS II line of murine DC and primary cultures of murine BMDC from BALB/c mice and p/p mice that have a genetically modified ceacam1a gene were analyzed by flow cytometry for surface expression of the MHV receptor, murine CEACAM1a (CD66a), and the DC marker CD11c.
It was previously shown that p/p mice are much more resistant to MHV-A59 infection than are wild-type BALB/c mice (4). We included the p/p mice in this study to determine if their partial resistance to MHV occurred at the level of DC. Surprisingly, BMDC from p/p mice expressed the same amount of CEACAM1a as did DC from BALB/c mice (Fig. 1). Thus, manipulation of the ceacam1a gene in p/p mice, which changed expression of CEACAM1a isoforms on other tissues (4), did not alter the expression of CEACAM1a protein on DC.
To test whether MHV-A59 causes productive infection of DC, JAWS II DC and BMDC from BALB/c or p/p mice were incubated with MHV-A59 at a multiplicity of infection of 10 PFU/cell for 1 h at 37°C. The inocula were removed, excess virus was washed away, and the cells were incubated at 37°C. Supernatant media were collected at intervals after virus inoculation and centrifuged to remove cell debris. The yield of infectious virus in the medium was titrated by plaque assay on murine 17 Clone 1 cells (18). Figure 2 shows that MHV-A59 produced infectious virus in the JAWS II DC line and BMDC from BALB/c and p/p mice. Since immunofluorescence showed that all cells were infected and 3 × 105 cells produced over 105 PFU/ml in 0.5-ml samples, each infected DC produced approximately 1 PFU. By 8 h after inoculation, small multinucleate syncytia had formed (Fig. 3), and fusion of DC was very extensive by 24 h after inoculation (data not shown). Trypan blue exclusion studies showed that more than 80% of DC were killed by the virus within 24 h after inoculation (data not shown). Immunolabeling and viral growth curves showed that DC of p/p mice were as susceptible to infection as DC from BALB/c mice. Therefore, the partial resistance of p/p mice to MHV is probably due to reduced levels of CEACAM1a expressed on other target cells for the virus such as hepatocytes or enterocytes (4).
FIG. 2.
Replication of murine coronavirus MHV-A59 in murine DC. JAWS II DC and primary cultures of BALB/c BMDC from wild-type or p/p mice were inoculated with MHV-A59, and the yield of released virus was determined by plaque titration on murine fibroblasts.
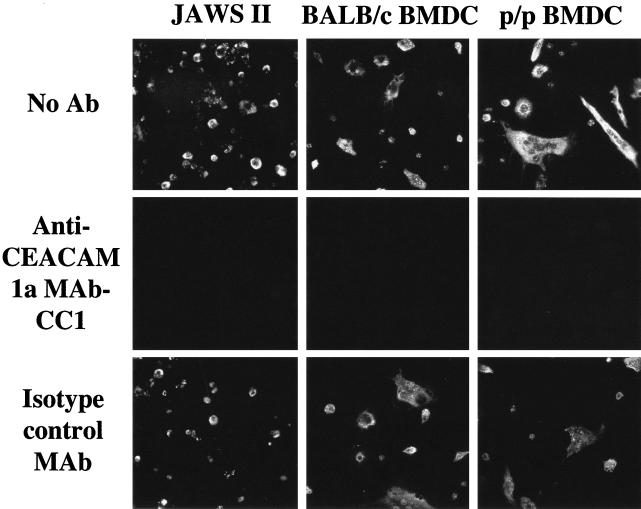
FIG. 3.
Infection of DC by MHV-A59 is mediated by the virus receptor, murine CEACAM1a. The JAWS II DC line and primary cultures of BALB/c and p/p BMDC were incubated with anti-CEACAM1a MAb CC1 that blocks binding of virus to the receptor, an isotype-matched control MAb, or with no antibody (No Ab) and inoculated with MHV-A59. Infected cells were detected 8 h after inoculation by immunofluorescence with antibody to the viral nucleocapsid protein.
DC actively acquire antigens through macropinocytosis, phagocytosis, and clathrin-mediated endocytosis. To determine if JAWS II DC and BMDC were infected with MHV-A59 via the CEACAM1a receptor or through a receptor-independent mechanism, we blocked the virus-binding domain of murine CEACAM1a with antireceptor MAb-CC1 (15). DC on coverslips were incubated with MAb-CC1 (12 μg/ml), an isotype control MAb (12 μg/ml), or medium alone for 1 h at 37°C and then inoculated with MHV-A59 (multiplicity of infection, 10 PFU/cell) in the presence of the same antibody during the 1-h virus adsorption period and subsequent incubation at 37°C. After 8 h, coverslips were fixed in methanol-acetic acid (3:1) for 10 min at −20°C, air dried, incubated with MAb against the nucleocapsid protein (kindly provided by Julian Leibowitz, Texas A & M University) and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin, and analyzed under a fluorescence microscope. Figure 3 shows that anti-CEACAM1a MAb blocked virus infection of the JAWS II DC and primary BMDC from BALB/c and p/p mice. Thus, MHV-A59 infects murine DC only by means of its specific CEACAM1a receptor and not by a DC-SIGN-mediated mechanism like that used by human immunodeficiency virus type 1, Ebola virus, human cytomegalovirus, and dengue virus (20, 22, 40, 41).
DC play a central role in the adaptive immune response to viruses. It is therefore understandable that for every stage in the differentiation of DC there is an example of a virus that interferes with DC function (36). Infection of DC by MHV may in part explain how the normal immune response can be modulated by MHV infection (9). Coronavirus infection could potentially disrupt DC function by several mechanisms: (i) killing immature DC in epithelial tissues and preventing DC from acquiring antigen; (ii) inhibiting DC migration by blocking trafficking signals through receptors such as CCR7; (iii) inhibiting DC-T-cell interactions in the lymph node by preventing expression of costimulatory molecules such as CD40, CD80, and CD86; (iv) transferring virus from DC in peripheral tissue to activated T cells in the lymph nodes (it has recently been shown that CEACAM1a is a very early surface marker on activated T cells [31]); and (v) altering cytokine profiles in infected DC to preferentially secrete Th1- or Th2-type cytokines, thereby skewing the T-cell response. Recently, treatment with anti-CEACAM1a MAb-CC1 was found to diminish delayed-type hypersensitivity in vitro and in vivo (31). Ligation of CEACAM1a on DC by MAb-CC1, the spike glycoprotein of MHV during infection, or by homophilic adhesion to CEACAM1a may alter signaling in DC, modulating the immune response (25, 31). Because CEACAM1a is expressed on both DC and activated T cells, it is uncertain whether the immunomodulating activity of MAb-CC1 is on the DC or the T cells or both.
The receptors for group I coronaviruses are aminopeptidase N (CD13) glycoproteins (11, 42-44). Like CEACAM1a, CD13 is expressed on DC (5). Therefore, it is possible that coronaviruses in group I, such as human respiratory coronavirus 229E, transmissible gastroenteritis virus of swine, and feline coronaviruses, can also modulate the immune response to infection by infecting DC. Recently, angiotensin-converting enzyme 2 ACE2 was found to be a receptor for the novel coronavirus (SARS-CoV) that causes SARS (26). It will be interesting to learn whether angiotensin-converting enzyme 2 is expressed on DC or whether SARS-CoV can interact with DC by another mechanism to modulate the immune response in SARS.
Acknowledgments
We thank the members of the laboratory of Cara Wilson, including Andrew Stubbs and Laura Sharp for assistance with isolation of murine BMDC, Trine Jorgensen for information on JAWS II cells, and David Wentworth and Larissa Thackray for helpful discussion.
This research was funded by NIH grant R01AI 25231.
REFERENCES
- 1.Abendroth, A., G. Morrow, A. L. Cunningham, and B. Slobedman. 2001. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J. Virol. 75:6183-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchemin, N., T. Chen, P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlson, M. Kuroki, S. H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. Watt, and W. Zimmermann. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243-249. [DOI] [PubMed] [Google Scholar]
- 3.Bender, A., M. Albert, A. Reddy, M. Feldman, B. Sauter, G. Kaplan, W. Hellman, and N. Bhardwaj. 1998. The distinctive features of influenza virus infection of dendritic cells. Immunobiology 198:552-567. [DOI] [PubMed] [Google Scholar]
- 4.Blau, D. M., C. Turbide, M. Tremblay, M. Olson, S. Letourneau, E. Michaliszyn, S. Jothy, K. V. Holmes, and N. Beauchemin. 2001. Targeted disruption of the Ceacam1 (MHVR) gene leads to reduced susceptibility of mice to mouse hepatitis virus infection. J. Virol. 75:8173-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordessoule, D., M. Jones, K. C. Gatter, and D. Y. Mason. 1993. Immunohistological patterns of myeloid antigens: tissue distribution of CD13, CD14, CD16, CD31, CD36, CD65, CD66 and CD67. Br. J. Haematol. 83:370-383. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D. S., M. Asanaka, K. Yokomori, F. Wang, S. B. Hwang, H. P. Li, and M. M. Lai. 1995. A pregnancy-specific glycoprotein is expressed in the brain and serves as a receptor for mouse hepatitis virus. Proc. Natl. Acad. Sci. USA 92:12095-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton, S. R., S. W. Barthold, and A. L. Smith. 1993. The cellular and molecular pathogenesis of coronaviruses. Lab. Anim. Sci. 43:15-28. [PubMed] [Google Scholar]
- 8.Coutelier, J. P., C. Godfraind, G. S. Dveksler, M. Wysocka, C. B. Cardellichio, H. Noel, and K. V. Holmes. 1994. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur. J. Immunol. 24:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cray, C., M. O. Mateo, and N. H. Altman. 1993. In vitro and long-term in vivo immune dysfunction after infection of BALB/c mice with mouse hepatitis virus strain A59. Lab. Anim. Sci. 43:169-174. [PubMed] [Google Scholar]
- 10.Cui, W., Y. Fan, W. Wu, F. Zhang, J. Y. Wang, and A. P. Ni. 2003. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 37:857-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmas, B., J. Gelfi, R. L'Haridon, L. K. Vogel, H. Sjostrom, O. Noren, and H. Laude. 1992. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 357:417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosten, C., S. Gunther, W. Preiser, S. Van Der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 13.Dveksler, G. S., C. W. Dieffenbach, C. B. Cardellichio, K. McCuaig, M. N. Pensiero, G. S. Jiang, N. Beauchemin, and K. V. Holmes. 1993. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J. Virol. 67:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dveksler, G. S., M. N. Pensiero, C. B. Cardellichio, R. K. Williams, G. S. Jiang, K. V. Holmes, and C. W. Dieffenbach. 1991. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J. Virol. 65:6881-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dveksler, G. S., M. N. Pensiero, C. W. Dieffenbach, C. B. Cardellichio, A. A. Basile, P. E. Elia, and K. V. Holmes. 1993. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc. Natl. Acad. Sci. USA 90:1716-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 17.Fouchier, R. A., T. Kuiken, M. Schutten, G. Van Amerongen, G. J. Van Doornum, B. G. Van Den Hoogen, M. Peiris, W. Lim, K. Stohr, and A. D. Osterhaus. 2003. Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frana, M. F., J. N. Behnke, L. S. Sturman, and K. V. Holmes. 1985. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J. Virol. 56:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geijtenbeek, T. B., and Y. van Kooyk. 2003. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr. Top. Microbiol. Immunol. 276:31-54. [DOI] [PubMed] [Google Scholar]
- 21.Godfraind, C., S. G. Langreth, C. B. Cardellichio, R. Knobler, J. P. Coutelier, M. Dubois-Dalcq, and K. V. Holmes. 1995. Tissue and cellular distribution of an adhesion molecule in the carcinoembryonic antigen family that serves as a receptor for mouse hepatitis virus. Lab. Investig. 73:615-627. [PubMed] [Google Scholar]
- 22.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 23.Huang, J. Q., C. Turbide, E. Daniels, S. Jothy, and N. Beauchemin. 1990. Spatiotemporal expression of murine carcinoembryonic antigen (CEA) gene family members during mouse embryogenesis. Development 110:573-588. [DOI] [PubMed] [Google Scholar]
- 24.Jones, C. A., M. Fernandez, K. Herc, L. Bosnjak, M. Miranda-Saksena, R. A. Boadle, and A. Cunningham. 2003. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J. Virol. 77:11139-11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kammerer, R., D. Stober, B. B. Singer, B. Obrink, and J. Reimann. 2001. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J. Immunol. 166:6537-6544. [DOI] [PubMed] [Google Scholar]
- 26.Li, W., M. J. Moore, N. Vasilieva, J. Sui, S. K. Wong, M. A. Berne, M. Somasundaran, J. L. Sullivan, K. Luzuriaga, T. C. Greenough, H. Choe, and M. Farzan. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marra, M. A., S. J. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404. [DOI] [PubMed] [Google Scholar]
- 29.Moller, M. J., R. Kammerer, F. Grunert, and S. von Kleist. 1996. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int. J. Cancer 65:740-745. [DOI] [PubMed] [Google Scholar]
- 30.Morrow, G., B. Slobedman, A. L. Cunningham, and A. Abendroth. 2003. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J. Virol. 77:4950-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima, A., H. Iijima, M. F. Neurath, T. Nagaishi, E. E. Nieuwenhuis, R. Raychowdhury, J. Glickman, D. M. Blau, S. Russell, K. V. Holmes, and R. S. Blumberg. 2002. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J. Immunol. 168:1028-1035. [DOI] [PubMed] [Google Scholar]
- 32.Nedellec, P., G. S. Dveksler, E. Daniels, C. Turbide, B. Chow, A. A. Basile, K. V. Holmes, and N. Beauchemin. 1994. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J. Virol. 68:4525-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen, C. W. 1993. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 36:1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plotnicky-Gilquin, H., D. Cyblat, J. P. Aubry, Y. Delneste, A. Blaecke, J. Y. Bonnefoy, N. Corvaia, and P. Jeannin. 2001. Differential effects of parainfluenza virus type 3 on human monocytes and dendritic cells. Virology 285:82-90. [DOI] [PubMed] [Google Scholar]
- 35.Pollara, G., K. Speidel, L. Samady, M. Rajpopat, Y. McGrath, J. Ledermann, R. S. Coffin, D. R. Katz, and B. Chain. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J. Infect. Dis. 187:165-178. [DOI] [PubMed] [Google Scholar]
- 36.Rescigno, M., and P. Borrow. 2001. The host-pathogen interaction: new themes from dendritic cell biology. Cell 106:267-270. [DOI] [PubMed] [Google Scholar]
- 37.Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M.-H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394-1399. [DOI] [PubMed]
- 38.Servet-Delprat, C., P. O. Vidalain, H. Valentin, and C. Rabourdin-Combe. 2003. Measles virus and dendritic cell functions: how specific response cohabits with immunosuppression. Curr. Top. Microbiol. Immunol. 276:103-123. [DOI] [PubMed] [Google Scholar]
- 39.Sevilla, N., S. Kunz, D. McGavern, and M. B. Oldstone. 2003. Infection of dendritic cells by lymphocytic choriomeningitis virus. Curr. Top. Microbiol. Immunol. 276:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 41.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, W. Sun, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tresnan, D. B., and K. V. Holmes. 1998. Feline aminopeptidase N is a receptor for all group I coronaviruses. Adv. Exp. Med. Biol. 440:69-75. [DOI] [PubMed] [Google Scholar]
- 43.Tresnan, D. B., R. Levis, and K. V. Holmes. 1996. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 70:8669-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeager, C. L., R. A. Ashmun, R. K. Williams, C. B. Cardellichio, L. H. Shapiro, A. T. Look, and K. V. Holmes. 1992. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokomori, K., and M. M. Lai. 1992. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J. Virol. 66:6194-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokomori, K., and M. M. Lai. 1992. The receptor for mouse hepatitis virus in the resistant mouse strain SJL is functional: implications for the requirement of a second factor for viral infection. J. Virol. 66:6931-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yrlid, U., and M. J. Wick. 2002. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J. Immunol. 169:108-116. [DOI] [PubMed] [Google Scholar]