Abstract
Aim:
To investigate whether the conjugation of magainin II (MG2), an antimicrobial peptides (AMPs), to the tumor-homing peptide bombesin could enhance its cytotoxicity in tumor cells.
Methods:
A magainin II-bombesin conjugate (MG2B) was constructed by attaching magainin II (MG2) to bombesin at its N-terminus. The peptides were synthesized using Fmoc-chemistry. The in vitro cytotoxicity of the peptide in cancer cells was quantitatively determined using the CCK-8 cell counting kit. Moreover, the in vivo antitumor effect of the peptide was determined in tumor xenograft models.
Results:
The IC50 of MG2B for cancer cells (10–15 μmol/L) was at least 10 times lower than the IC50 of unconjugated MG2 (125 μmol/L). Moreover, the binding affinity of MG2B for cancer cells was higher than that of unconjugated MG2. In contrast, conjugation to a bombesin analog lacking the receptor-binding domain failed to increase the cytotoxicity of MG2, suggesting that bombesin conjugation enhances the cytotoxicity of MG2 in cancer cells through improved binding. Indeed, MG2B selectively induced cell death in cancer cells in vitro with the IC50 ranging from 10 to 15 μmol/L, which was about 6–10 times lower than the IC50 for normal cells. MG2B (20 mg/kg per day, intratumorally injected for 5 d) also exhibited antitumor effects in mice bearing MCF-7 tumor grafts. The mean weights of tumor grafts in MG2B- and PBS-treated mice were 0.21±0.05 g and 0.59±0.12 g, respectively.
Conclusion:
The results suggest that conjugation of AMPs to bombesin might be an alternative approach for targeted cancer therapy.
Keywords: targeted cancer therapy, antimicrobial peptide, tumor-homing peptide, bombesin, magainin
Introduction
Conventional cancer chemotherapy is limited by the lack of specificity and multi-drug resistance. Thus, novel approaches to cancer therapy with a higher degree of selectivity for cancer cells and a lower risk for induction of resistance is desired1, 2. Recent attention has focused on membrane-active antimicrobial peptides (AMPs) with selective anticancer activity1, 3, 4. AMPs are positively charged molecules that exert their cytotoxicity by binding to the outer membrane of cancer cells, which contain 3%–9% anionic phosphatidylserine. In contrast, membranes of normal cells consist largely of zwitterionic phospholipids (neutral in net charge) and are therefore less attractive to cationic AMPs5. AMPs are selectively cytotoxic in cancer cells4 and are usually membrane-active, suggesting a low risk for induction of resistance4. Overall, AMPs are selective cytotoxic agents for cancer cells with therapeutic implications.
A great number of AMPs have been isolated from the skin secretions of amphibians4, 6, 7. Of these peptides, the best studied AMP is magainin II (MG2), which was isolated from the skin of the African clawed frog Xenopus laevis8. MG2 has been reported to be selectively cytotoxic for hematopoietic and solid tumor cells2, 9, 10. However, MG2 only exhibits obvious cytotoxicity in cancer cells at high concentrations. The average IC50 of MG2 against many cancer cell lines is greater than 100 μmol/L2, 9, 11. Most likely, this poor potency arises from the limited cell membrane-binding ability of MG2. Because electrostatic interactions between the peptide's positive charges and the negative charges on the cell membrane play a crucial role in the binding process4, one strategy for improving peptide binding is to increase the number of positive charges on the peptide by amino acid substitution. MG2 analogs with increased positive charges show enhanced membrane binding affinities12 and cytotoxicity9, 11. In addition, conjugation of the cationic KLA peptide (KLAKLAK)2 to a carrier peptide significantly enhanced its cytotoxicity in cancer cells13, 14. Consequently, conjugation of MG2 to a carrier peptide recognizing a specific surface marker on cancer cells might also improve its cytotoxicity.
Tumor-homing peptides can bind to tumor cells or tumor blood/lymphatic vessels with high affinity and specificity15, 16. In addition, many tumor-homing peptides can be internalized by tumor cells and accumulate in tumors at high concentrations17, 18. Therefore, these tumor-homing peptides are attractive drug carriers. Among these peptides, somatostatin and its analogs have been extensively investigated. The radiolabeled somatostatin analog 111In-DTPA-octreotide (111In-OctreoScan, Novartis) has been approved by the US Food and Drug Administration for scintigraphy in patients with NETs19. In addition, bombesin and its analogs also show great promise for tumor targeting. Bombesin is a 14-amino acid tumor-homing peptide that was isolated from frog skin20. Its receptors are overexpressed in a variety of common human cancers, such as neuroblastoma and small cell lung cancer, as well as cancers of the prostate, kidney, uterus, ovary, breast, pancreas, gastrointestinal tract, head and neck, and esophagus 21, 22. Bombesin can effectively deliver radioactive23 and chemotherapeutic agents24 to target cells due to its high affinity for these receptors. Because bombesin can specifically bind to its receptors with high affinity, the conjugation MG2 to bombesin might enhance the cytotoxicity of MG2 in cancer cells by improving its binding affinity.
In this paper, we first measured the binding of fluorescein isothiocyanate (FITC)-labeled bombesin to solid tumor cells, malignant hematopoietic cells, and normal cells. Subsequently, two conjugates were constructed by attaching MG2 to bombesin and a mutant form of bombesin lacking the receptor-binding domain. The cytotoxicity and binding ability of these peptides were further analyzed. Finally, the in vivo antitumor effect of the MG2-bombesin conjugate was evaluated. It was found that the conjugation to bombesin significantly enhanced the cytotoxicity of MG2 in cancer cells.
Materials and methods
Peptide synthesis
To probe bombesin-targeted delivery of MG2 to tumor cells, the MG2-bombesin conjugate (MG2B) was constructed by attaching MG2 to bombesin at its N-terminus. Another conjugate, MG2Ba, which contained MG2 and a bombesin analog lacking the C-terminal 8–14 amino acids, was also constructed. Because the C-terminal domain of bombesin is crucial for its receptor-binding activity25, the attachment of MG2 to the bombesin analog lacking the receptor-binding domain should not significantly improve its membrane-binding affinity. Unconjugated bombesin, unconjugated MG2, and an unrelated peptide (URP) were synthesized. All peptides (Table 1) were synthesized using Fmoc-chemistry (Genescript Inc, Nanjing, China). The purity of these peptides (>95%) was analyzed by reversed-phase high performance liquid chromatography, and the mass of the peptide was determined using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. FITC labels were linked to the N-terminus of peptides by introducing 5-carboxyfluorescein during the final synthesis cycle. All peptides were dissolved in isotonic phosphate-buffered saline (PBS: 137 mmol/L NaCl, 2.68 mmol/L KCl, 8.09 mmol/L Na2HPO4, 1.76 mmol/L KH2PO4, pH 7.4) and stored at −70 °C until further use.
Table 1. Peptides and sequences.
| Peptide | Sequence | Length (aa*) | Molecular Weight (Da) |
|---|---|---|---|
| MG2 | GIGKFLHSAKKFGKAFVGEIMNS | 23 | 2466 |
| Bombesin | QRLGNQWAVGHLM | 13 | 1509 |
| MG2B | GIGKFLHSAKKFGKAFVGEIMNSGGQRLGNQWAVGHLM | 38 | 4072 |
| MG2Ba | GIGKFLHSAKKFGKAFVGEIMNSGGQRLGNQ | 31 | 3277 |
| URP | DSHAKRHHGYKRKFHEKHHSHRGY | 24 | 3036 |
*: amino acid
Cell culture
Unless otherwise mentioned, all cells were purchased from the American Type Culture Collection, USA. The following cells were used in this experiment: human breast cancer cells (MCF-7 and ZR-75-30); human melanoma cells (A375, M14, and A875); human prostate cancer cells (DU145); human cervical cancer cells (HeLa); human lung adenocarcinoma cells (A549); human Burkitt's lymphoma cells (Raji); human promyelocytic leukemia cells (NB4); African green monkey kidney cells (Vero E6) and Ad5 transformed human embryonic kidney cells (Hek-293A); human fibroblast cells (HSF); and human vein endothelial cells (HUVECs). Human peripheral blood mononuclear cells (hPBMCs) were isolated from healthy volunteers by Percoll density gradient centrifugation. All of the cells were cultured in either Dulbecco's modified Eagle's medium (DMEM) or RPMI 1640 supplemented with 10% fetal bovine serum (GIBCO-BRL, USA), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a 5% CO2 humidified atmosphere.
Cellular peptide uptake
To observe cellular uptake of FITC-labeled peptide under a fluorescence microscope, 1×104 cells/well were seeded into 96-well plates and the cells were allowed to attach overnight. After being washed in PBS, the cells were further incubated with FITC-labeled peptides in 100 μL of medium supplemented with 2% bovine serum albumin (BSA) for 1 h at 37 °C. The cells were then washed and observed under a fluorescence microscope. To analyze cellular uptake of peptide by fluorescence-activated cell sorting (FACS), the adherent cells were first digested by the addition of 0.25% (w/v) trypsin in serum-free medium containing 0.02% of EDTA at 37 °C. After being washed with PBS, the cells (2×105) were incubated with 300 μL of FITC-labeled peptides at 37 °C for 1 h. The cells were then washed three times with 1 mL of PBS and analyzed by FACS.
Cytotoxicity assays
Assays were performed according to the methods of Laakkonen et al26 with some modifications. Adherent cells were plated in a 96-well plate at a density of 1×104 cells/well and allowed to attach overnight. The cells in suspension were collected and seeded in a 96-well plate (1×104 cells/well) immediately before use. After being washed once with PBS, 100 μL peptide diluted in serum-free medium supplemented with 2% BSA was added to the cells and further incubated at 37 °C for 2 h. Subsequently, 10 μL of CCK-8 solution was added to the wells and the absorbance was measured 4 h later at 450 nm. Cytotoxicity was expressed as the percentage of viable cells after treatment with peptide, which was calculated by assuming 100% survival with the PBS control. Each sample was processed in triplicate and the IC50 value was obtained from the respective cell viability curves.
The Live/Dead BacLight bacterial viability kit (Molecular Probe) was used as an additional method to evaluate the cytotoxicity of peptides. The DNA-binding SYTO 9 and propidium iodide (PI) are green and red dyes, respectively. The former, but not the latter, is membrane permeable. Based on their differences in membrane-permeability, the Syto 9 and PI mixture stained living cells green and dead cells red. After treatment with peptide for 2 h, the cells were then double stained with SYTO 9 and PI for 5 min in the dark followed by observation under a fluorescence microscope.
Detection of apoptosis using Annexin V and PI staining
Annexin V binds to phosphatidylserine exposed on the outer cell membrane during the early stages of apoptosis. Thus, double staining with FITC-Annexin V (appearing green) and propidium iodide (appearing red) is often used to detect cells undergoing apoptosis27. After treatment with peptide, the cells were then double stained with FITC-Annexin V and PI according to the manufacturer's instructions. Exposure of phosphatidylserine on the outer membrane of cells was first observed under a fluorescence microscope. Simultaneously, 3×104 events were analyzed by FACS. Cells stained with FITC-Annexin V alone (Annexin V+/PI−) were considered in early apoptosis, whereas those stained with both FITC-Annexin V and PI (Annexin V+/PI+) were considered in the advanced stages of apoptosis or necrosis. The FITC-Annexin V and PI double-negative (Annexin V−/PI−) cells were considered alive.
Detection of apoptosis by monitoring mitochondrial depolari-zation
The cells were treated with the peptides and stained with JC-1 dye (2 μmol/L) for 30 min at 37 °C in the dark, according to the manufacturer's instructions. The cells were subsequently washed twice with PBS and analyzed using FACS. To observe the mitochondria using a fluorescence microscope, the cells were double stained with JC-1 (showing mitochondria) and DAPI (showing nuclei). The JC-1 dye forms fluorescent red J-aggregates upon localization to healthy mitochondria, whereas the monomeric form of the dye fluoresces green in the cytoplasm. Consequently, a decrease in the ratio of red to green fluorescence reflects the loss of mitochondrial membrane potential27.
Detection of the caspase-mediated pathway in apoptosis
The assay was performed according to the method of Rege et al27. The cells were preincubated with the pan-caspase inhibitor z-VAD-Fmk (100 μmol/L) for 2 h and then treated with peptide for an additional 2 h. Cell viability was determined using the CCK-8 kit. The cytotoxicity of the peptides to cells that were preincubated with inhibitor and those that were not was compared.
Tumor xenograft model
All protocols for the animal models were approved by the University Animal Care and Use Committee. Six to eight-week-old female nude mice were obtained from the University Animal Center. About 1×107 MCF-7 cells suspended in 100 μL of saline were injected subcutaneously into the right flank of mice. At the onset of a palpable tumor (about 50–100 mm3), 18 mice were divided into three groups that were intratumorally injected with 20 mg/kg MG2B, MG2 or an equivalent volume of PBS every day for a total of five days. The tumor volume (mm3) was calculated as length×width2×0.5. At the end of the experiment, all animals were killed and the tumor masses were measured.
To probe the death of tumor cells in vivo, a single dose of peptide (200 μg, 100 μL) was injected into the tumor graft (<1000 mm3). The same volume of PBS was intratumorally injected into the mice of the control group. Sixteen hours post-injection, the mice were killed and the tumor grafts were excised, paraffin-embedded, sectioned, and stained with hematoxylin/eosin (H&E) to examine the histological architecture. Simultaneously, terminal nucleotidyl transferase–mediated nick end labeling (TUNEL) staining (Invitrogen) was used to examine the apoptosis of tumor cells.
Statistical analysis
Results are presented as the mean±SD of at least three experiments. Statistical comparisons were made using Student's t test.
Results
Bombesin specifically binds to tumor cells
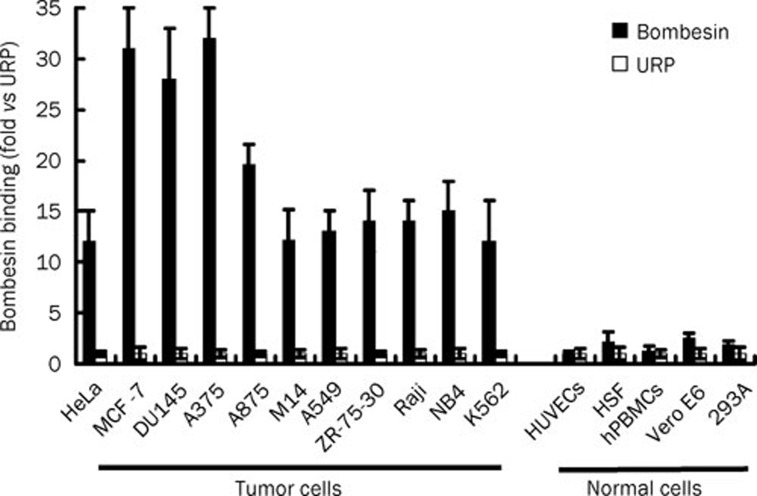
As shown in Figure 1, bombesin bound 28–32 times more to MCF-7, DU145, and A375 cells than did the negative control peptide URP. The amount of bombesin bound to HeLa, A875, M14, A549, ZR-75-30, Raji, NB4, and K562 is about 12–18 times greater than the amount of the negative control peptide URP. In contrast, both bombesin and URP bound to normal cells, including HUVECs, HSF, hPBMCs, Vero E6, and 293A at similarly low levels. These data indicate that bombesin can bind to tumor cells with high affinity.
Figure 1.
Binding of bombesin to tumor cells and normal cells. In preparation, about 2×105 cells were collected and incubated with the FITC-labeled bombesin peptide (10 μmol/L, 300 μL) at 37 °C for 1 h. After being washed with PBS, 3×104 cells were analyzed using FACS in each assay. The FITC-labeled URP peptide was used as a control. The binding of bombesin is shown relative to the binding of the negative control peptide URP, for which the value of mean fluorescence was set to 1. The data are expressed as means±SD.
Bombesin enhances cytotoxicity of MG2 in tumor cells
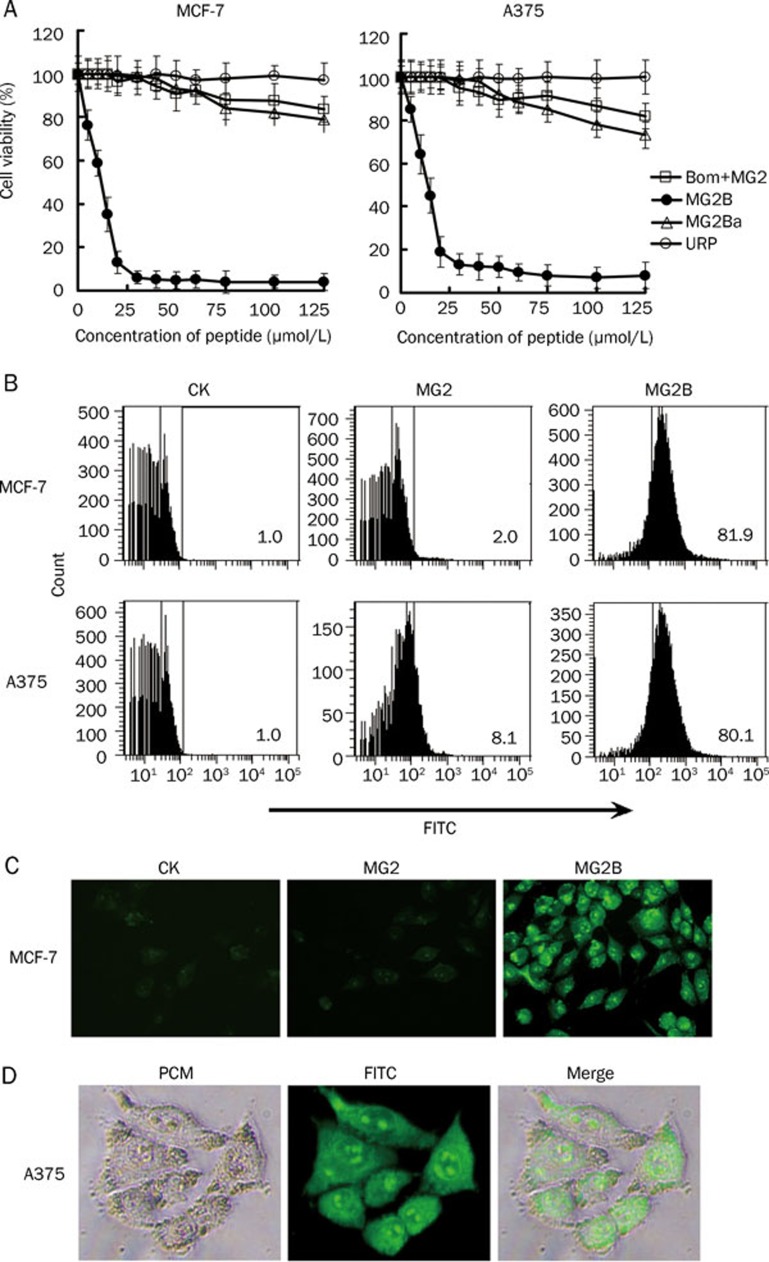
The unconjugated MG2 peptide only showed mild cytotoxicity in cancer cells at high concentrations. As shown in Figure 2A, the mixture containing unconjugated MG2 and unconjugated bombesin induced about 20% cell death in MCF-7 and A375 cells at 125 μmol/L. The IC50 of the unconjugated MG2 for these cells was over 200 μmol/L. However, the MG2-bombesin conjugate MG2B induced approximately 30%–40% cell death in MCF-7 and A375 cells at a low concentration of 10 μmol/L. Moreover, MG2B induced over 80% cell death in these cells when the concentration was increased to 20 μmol/L. The IC50 of the MG2B peptide for MCF-7 and A375 cells was within the range of 10–15 μmol/L, which is about 13–16 times lower than that for unconjugated MG2 in these cells. However, another conjugate MG2Ba, in which the MG2 peptide was conjugated to a bombesin analog lacking 8–14 amino acids at the C-terminus of bombesin, was similar to unconjugated MG2 in terms of cytotoxicity in MCF-7 and A375 cells (Figure 2A). These results suggest that the attachment to bombesin, but not the bombesin analog lacking the receptor-binding domain, at its N-terminus significantly increases the cytotoxicity of MG2 in tumor cells.
Figure 2.
Enhancement of MG2-induced cytotoxicity in tumor cells by attachment to bombesin at its N-terminus. (A) Comparison of cytotoxicity of the MG2-bombesin conjugate, MG2B, and unconjugated MG2 in MCF-7 and A375 cells. Solution (100 μL) containing increasing concentrations (0–125 μmol/L) of peptide was added to cells (1×104 cells/well) and further incubated at 37 °C for 2 h. Subsequently, cell viability was determined using the cell counting reagent CCK-8. Results were expressed as the percentage of viable cells after treatment with peptide, which was calculated assuming 100% survival with the PBS control. Bom+MG2: unconjugated bombesin plus unconjugated MG2; MG2B: MG2-bombesin conjugate; MG2Ba: MG2-bombesin analog conjugate; URP: a negative control peptide. (B) Different binding affinities of MG2B and unconjugated MG2 to MCF-7 and A375 cells. About 1×105 cells were collected and incubated with FITC-labeled peptide (40 μmol/L, 300 μL) at 37 °C for 1 h. After being washed with PBS, the cells were analyzed by FACS. The URP peptide was used as a negative control. The percentage of positive cells is indicated. (C) Cellular translocation of MG2B in MCF-7 cells. The cells were seeded into 96-well plates (1×104 cells/well) and allowed to attach overnight. After incubation with FITC-labeled peptide (20 μmol/L, 100 μL) and being washed once with PBS, the cells were observed under a fluorescence microscope (original magnification, ×160). (D) Distribution of FITC-labeled MG2B in MCF-7 cells. After incubation with FITC-labeled peptide, the cells were observed under a phase-contrast microscope (PCM) and a fluorescence microscope (original magnification, ×320).
Further analysis demonstrated that MG2B is different than unconjugated MG2 in its binding ability. Figure 2B shows that 20 μmol/L of unconjugated MG2 bound to 2% MCF-7 and 8.1% A375 cells, respectively. In contrast, MG2B bound over 80% of both cells at the same concentration, as determined by FACS (Figure 2B). MG2B and unconjugated MG2 are different in efficacy of cellular translocation. Compared to unconjugated MG2 peptide, MG2B was internalized to a higher extent by MCF-7 cells (Figure 2C). In addition, the internalized MG2B peptide was uniformly distributed in the cytoplasm and rich in the nucleolus of MCF-7 cells (Figure 2D). The cytotoxicity of MG2B and MG2 was closely related to their binding and cellular translocation efficacy. These data suggest that conjugated bombesin contributes to the enhancement of cytotoxicity through improved binding and cellular translocation of MG2.
The MG2-bombesin conjugate MG2B selectively induces cell death in tumor cells
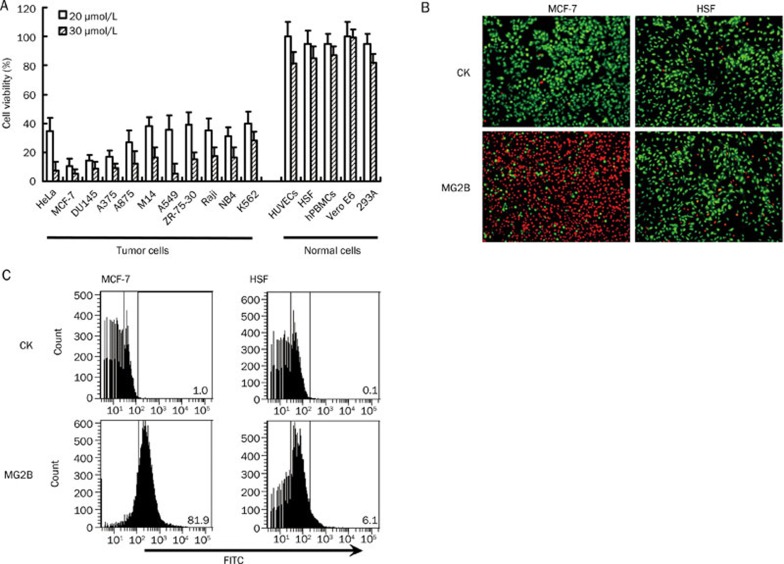
Cell viability assays using CCK-8 demonstrated that MCF-7, DU145, and A375 cells were most sensitive to MG2B. As shown in Figure 3A, the survival rates of these three cells were lower than 20% after treatment with MG2B at 20 μmol/L for 2 h. Other tumor cells including HeLa, A875, M14, A549, ZR-75-30, Raji, NB4, and K562 were also sensitive to MG2B. Twenty μmol/L MG2B induced approximately 60%–70% cell death in these tumor cells. When the concentration of MG2B was increased to 30 μmol/L, the survival rates of these tumor cells were reduced by 80%–90%. The IC50 of MG2B for these tumor cells was within the range of 10–15 μmol/L. However, normal cells including HUVECs, HSF, hPBMCs, Vero E6, and 293A were relatively resistant to MG2B. The IC50 of MG2B for these normal cells was within the range of 80 to 100 μmol/L, which is about 6–10 times higher than the IC50 of MG2B for tumor cells.
Figure 3.
Selective cytotoxicity of MG2B in cancer cells. (A) Comparison of cytotoxicity in MG2B in tumor cells and normal cells. Increasing concentrations of peptide in 100 μL serum-free medium supplemented with 2% BSA was added into cells (1×104 cells/well) and incubated at 37 °C for 2 h. Cell viability was determined using the CCK-8 reagent. (B) Live/dead assays for MG2B-treated MCF-7 tumor cells and normal HSF cells. After treatment with peptide (20 μmol/L, 100 μL), the cells (1×104 cells/well) were double stained with Syto 9 and PI. The Syto 9 and PI mixture shows live cells in green and dead cell reading red, respectively. (C) Cellular uptake of MG2B in tumor cells MCF-7 and normal cells HSF. About 1×105 cells were collected and incubated with FITC-labeled peptide (40 μmol/L, 300 μL) at 37 °C for 1 h. After being washed with PBS, the cells were analyzed by FACS.
The selective cytotoxicity of MG2B in tumor cells was also proven by live/dead assays. As shown in Figure 3B, over 80% MCF-7 cells were indicated as dead (appearing red) after treatment with 20 μmol/L MG2B. However, over 90% of normal HSF cells were indicated as live (appearing green) after treatment with MG2B at the same concentration. These results demonstrate that tumor cells, but not normal cells, are sensitive to MG2B. The selectivity of MG2B in other tumor cells and normal cells was also detected using the live/dead assay (data not shown). FACS analysis demonstrated that MG2B bound to tumor cells with greater affinity than to normal cells. Figure 3C shows that 20 μmol/L MG2B binds to 81.9% of MCF-7 tumor cells compared to 6.1% of normal HSF cells. These results suggest that the selectivity of MG2B in tumor cells and normal cells is closely related to its binding preference in these cells.
Involvement of the caspase-dependent pathway in MG2B-induced cell death
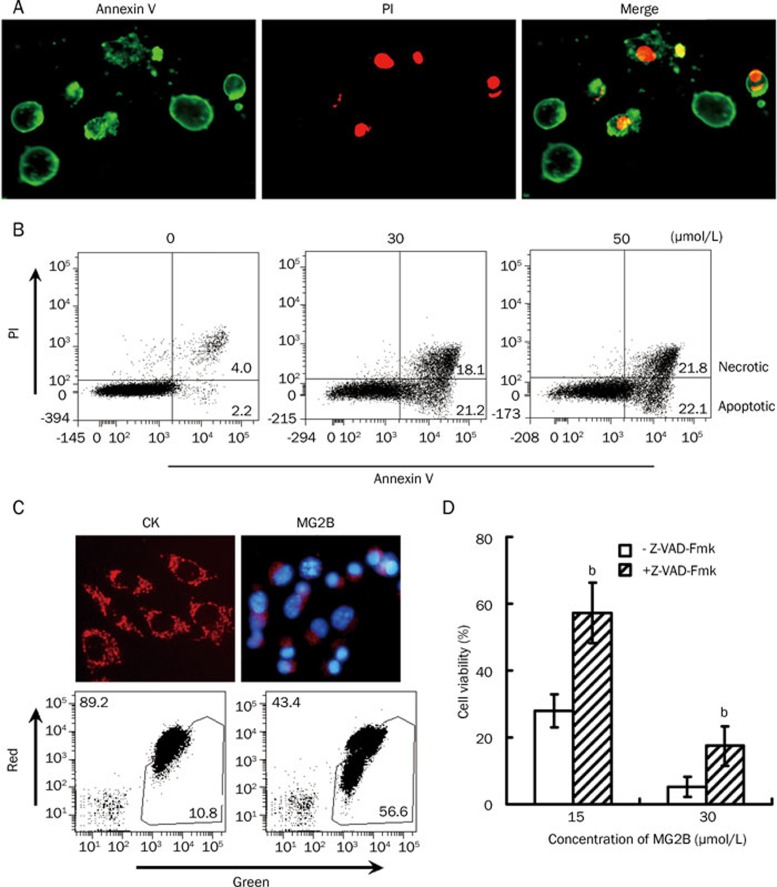
Under phase contrast microscopy, we observed that MG2B-dependent cell death in adherent tumor cells was accompanied by condensed nuclei, rounding, cell detachment, and clumping (data not shown). After treatment with peptide and double staining with Annexin V and PI, we observed many Annexin V+/PI+ and Annexin V+/PI- MCF-7 cells under fluorescence microscopy (Figure 4A). Annexin V+/PI+ cells were considered necrotic cells or apoptotic cells at an advanced stage of apoptosis. Annexin V+/PI- cells were considered apoptotic cells at an early stage of apoptosis. Consequently, the observation of Annexin V+/PI- cells demonstrates that MG2B induced apoptosis in MCF-7 cells. Further FACS analysis also revealed numerous Annexin V+/PI- cells in MG2B-treated MCF-7 cells. The percentage ratio of apoptotic/necrotic cells resulting from 0, 30, and 50 μmol/L MG2B was 2.2/4.0, 21.2/18.1, and 22.1/21.8, respectively (Figure 4B). JC-1 staining showed rich granule-like mitochondria (indicating healthy mitochondria) in cells treated with PBS. However, the MG2B-induced cell death was accompanied by the disappearance of the granule-like mitochondria in most peptide-treated cells (Figure 4C). Simultaneously, significant loss in mitochondrial membrane potentials in MG2B-treated cells was reflected by changes in the ratio of red/green fluorescence cells from 89.2/10.8 to 43.4/56.6 (Figure 4C). Moreover, preincubation of cells with the pan-caspase inhibitor z-VAD-Fmk before the addition of peptide reduced MG2B-induced cell death by 15%–30% (Figure 4D). These results demonstrate that MG2B induces caspase-dependent apoptosis in tumor cells.
Figure 4.
MG2B induced caspase-dependent apoptosis in tumor cells. (A) Microscope view of MG2B-induced apoptotic cells. After treatment with MG2B, the cells were double stained with FITC-Annexin V (Green) and PI (Red) and then observed under a fluorescence microscope. Annexin V+/PI− cells were considered apoptotic cells at early stages of apoptosis. Annexin V+/PI+ cells were considered apoptotic cells at an advanced stage of apoptosis or necrotic cells. (B) FACS analysis for MG2B-induced apoptotic cells. About 1×105 cells were pooled and incubated with 0, 30, and 50 μmol/L MG2B at 37 °C for 1 h. Peptide-treated cells were then double stained with FITC-Annexin V and PI and assayed by FACS. The percentage of apoptotic (Annexin V+/PI−) and necrotic (Annexin V+/PI+) cells is indicated. (C) MG2B-induced loss of mitochondrial membrane potential in MCF-7 cells. To observe the mitochondria in cells, the adherent MCF-7 cells (1×104 cells/well) were incubated with 100 μL MG2B (20 μmol/L) at 37 °C for 2 h followed by staining with JC-1 and observed under a fluorescence microscope. To analyze the mitochondrial membrane potential by FACS, 1×105cells were collected and incubated with 100 μL MG2B (0, 75 μmol/L) at 37 °C for 1 h followed by staining with JC-1 and washing with PBS. The percentage of red and green cells is indicated. (D) Inhibition of the MG2B-induced cell death by pan-caspase inhibitor. Cells (1×104cells/well) were preincubated with 100 μmol/L of pan-caspase inhibitor z-VAD-Fmk for 2 h at 37 °C before addition of peptide. The cytotoxicity of MG2B (15–30 μmol/L) in MCF-7 cells was then determined using the CCK-8 reagent. The cytotoxicity of MG2B in MCF7 cells in the presence of z-VAD-Fmk is significantly (bP<0.05) different from the cytotoxicity of MG2B in the absence of z-VAD-Fmk.
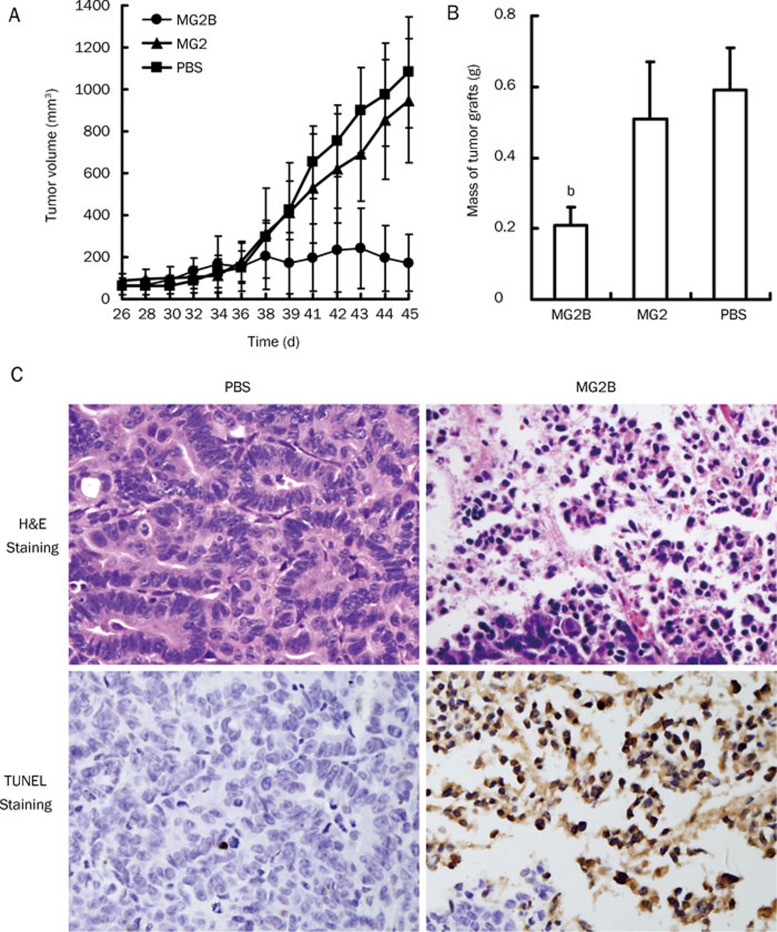
MG2B suppresses tumor growth in vivo
Figure 5A shows that MG2B exerts an obvious antitumor effect in vivo. When the average volume of tumor masses reached approximately 50–100 mm3, MG2B or MG2 was intratumorally injected into mice bearing a MCF-7 tumor graft at 20 mg/kg every day for a total of five days. The mice in the control group were injected with an equal volume of PBS. Rapid growth of tumor grafts in PBS-injected mice was observed as early as 10 days post-injection. However, tumor grafts in MG2B-injected mice grew slowly over the course of the experiment. Beginning on day 14 post-injection, the mean volume of tumor grafts in MG2B-treated mice was significantly different (P<0.01) from the tumor volume of MG2- and PBS-treated mice. At the end of this experiment, the average volume of tumor grafts in MG2B-injected mice was about 172±136 mm3, compared to 944±295 mm3 and 1081±264 mm3 in MG2- and PBS-treated mice, respectively (Figure 5A). The tumor masses excised from MG2B-treated mice were smaller than the tumor masses of PBS-treated mice. The mean weights of tumor masses in MG2B-treated mice, MG2-treated mice and PBS-treated mice were 0.21±0.05 g, 0.51±0.16 g and 0.59±0.12 g, respectively (Figure 5B).
Figure 5.
Suppression of tumor growth by MG2B. (A) Growth curve of MCF-7 tumor grafts in mice administered with MG2B. (B) Tumor masses excised from mice in treatment group and control group. The average mass of tumor grafts from MG2B-treated mice is significantly (bP<0.05) different than the average mass in PBS-treated mice and in MG2-treated mice. Tumors were established in BALB/c nude mice by subcutaneous injection of 100 μL of saline containing 1×107 MCF-7 cells. MG2B peptide (20 mg/kg) was intratumorally injected into mice bearing tumor grafts every other day for a total of 5 days. The mice in the control group were injected with equivalent volumes of PBS. The tumor volume (mm3) was calculated as length×width2×0.5. (C) Histological examination of MG2B-induced necrosis in vivo. Peptide (200 μg, in 100 μL PBS) was intratumorally injected into mice bearing MCF-7 xenografts (<1000 mm3). Approximately 16 h later, the tumor masses were excised. We then performed H&E and TUNEL staining on paraffin-embedded tumor tissues. Original magnification: ×400.
Further histological examination demonstrated that MG2B caused extensive necrosis in tumor cells. As shown in Figure 5C, H&E staining shows a large number of cells with a condensed and fragmented nucleus in tumor tissues at the injection sites. Rich TUNEL-positive stained cells were also observed in these tumor tissues (Figure 5C). These results demonstrate that MG2B induces apoptosis of tumor cells in vivo. However, no obvious histological damage was observed in the liver, kidney, and heart of mice injected with the peptides (data not shown).
Discussion
Here, we report the enhanced cytotoxicity of the MG2-bombesin conjugate MG2B compared to the unconjugated MG2 peptide. The IC50 of the MG2B for cancer cells was at least 10 times lower than the IC50 of unconjugated MG2 for these cells. The enhancement of cytotoxicity of MG2 is closely related to its improved binding affinity. In contrast, conjugation of MG2 to a bombesin analog lacking the receptor-binding domain failed to increase its cytotoxicity. These results demonstrate that conjugated bombesin enhances binding and thus enhances cytotoxicity of MG2 in cancer cells. Due to the specificity of conjugated bombesin, MG2B showed selective cytotoxicity in cancer cells by inducing caspase-dependent apoptosis in vitro. Furthermore, MG2B exerted obvious antitumor effects in an animal model. Our data suggest that conjugation of cationic AMPs to bombesin increases their cytotoxicity in cancer cells and thus present a potential novel approach to targeted cancer therapy.
Membrane-active AMPs are potential cancer therapies due to their low risk for induction of resistance4. Electrostatic interactions between the peptide's positive charges and the membrane's negative charges contribute to the binding of AMPs to the cell membrane, which is crucial for the cytotoxicity of these peptides in cancer cells4. Increasing the number of positive charges by amino acid substitution is an effective way to enhance the membrane-binding ability and cytotoxicity of AMPs9, 11, 12. In this work, we tried an alternative method to improve the anticancer activity of AMPs by conjugating the peptide to a tumor-homing peptide that can bind to its receptors.
We first constructed an MG2-bombesin conjugate MG2B by attaching MG2 to bombesin at its N-terminus. As expected, MG2B showed greater cytotoxicity in cancer cells compared to unconjugated MG2 peptide. We found that the IC50 of MG2B for cancer cells was within the range of 10–15 μmol/L, which is over 10 times lower than the IC50 of unconjugated MG2 (Figure 2A). Further investigation demonstrated that conjugated bombesin contributes to the binding affinity of MG2B to cancer cells. MG2B, but not unconjugated MG2, can bind and enter about 80% of cancer cells at a concentration of 20 μmol/L (Figure 2B). These results suggest that conjugation to bombesin at its N-terminus enhances the cytotoxicity of MG2 by increasing its membrane binding/translocation ability. However, conjugation of MG2 to a bombesin analog lacking the C-terminal 8–14 amino acids (WAVGHLM) failed to increase the cytotoxicity of MG2 in cancer cells. The C-terminal domain of bombesin is critical for its receptor binding affinity25. Therefore, conjugation of MG2 to a bombesin analog lacking this receptor-binding domain does not enhance cytotoxicity in cancer cells.
Bombesin receptors are overexpressed in numerous human cancer cells. Spectrum analysis demonstrates that the cytotoxicity of the MG2-bombesin conjugate MG2B in cancer cells and normal cells is positively related to the binding affinity of bombesin to these cells. Bombesin binds to cancer cells with 10–30 times greater affinity than to normal cells (Figure 1). The IC50 of MG2B for cancer cells was about 6–10 times lower than the IC50 for normal cells (Figure 3A). Because the conjugation to a bombesin analog lacking receptor-binding domain failed to enhance the cytotoxicity of MG2 (Figure 2A), we conclude that bombesin conjugation enhances the cytotoxicity and selectivity of MG2B. Bombesin shows high affinity to its receptors including the neuromedin B preferring receptor (BB1), gastrin-releasing peptide preferring receptor (BB2), orphan bombesin receptor subtype-3 (BB3), and BB4 receptor21. Therefore, it is possible for bombesin to enhance the cytotoxicity of MG2 by improving its ability to bind to the membrane of cancer cells. During the preparation of this paper, we also constructed many other AMPs-bombesin conjugates including attachment of the cationic KLA peptide27, BMAP27(1–18)28, and BMAP28(1–18)29 to bombesin. We found that all AMPs-bombesin conjugates enhanced the binding affinity and cytotoxicity in cancer cells. Moreover, bombesin receptors are overexpressed in a variety of primary cancer cells21, 22. Therefore, a bombesin-directed peptide such as MG2B might be potential target in spontaneous tumors.
Because the MG2 has been suggested to form pores on cell membranes30, the MG2-bombesin conjugate MG2B might induce necrosis in cancer cells by cell membrane-disruption. After treatment with the MG2B peptide, numerous necrotic cells (Annexin V+/PI+) with compromised membranes were observed under a microscope (Figure 4A) or detected by FACS analysis (Figure 4B). Although Annexin V+/PI+ cells might be apoptotic cells at advanced stages of apoptosis, the possibility of MG2B to induce necrosis in cancer cells cannot be excluded. Some cationic AMPs also induce apoptosis by binding to the mitochondrial membrane4. Imura et al observed that internalized MG2 peptide mainly accumulates in the mitochondria30. Westerhoff et al showed that MG2 can dissipate the membrane potential of isolated rat mitochondria31. These observations suggest that MG2 might also trigger the apoptosis in cancer cells through mitochondrial disruption. In our experiment, we observed numerous cells at an early stage of apoptosis (Annexin V+/PI−) in MG2B-treated cells (Figure 4A and 4B). MG2B-induced cell death was accompanied by the disappearance of healthy granule-like mitochondria and the loss of mitochondrial membrane potential (Figure 4C). Moreover, the involvement of caspase-dependent pathways in MG2B-induced cell death was revealed using a pan-caspase inhibitor (Figure 4D). These results demonstrate that MG2B induces caspase-dependent apoptosis by triggering mitochondrial disruption in cancer cells. Due to the rapid disruption of mitochondria by MG2B, it is difficult for us to capture a picture with high quality to show the peptide accumulation in mitochondria with a mitochondrial trace marker. However, it is understandable that the uniform distribution of MG2B in the cytoplasm (Figure 2D) allows it to bind and disrupt mitochondria.
To date, 65 AMPs have been identified as anticancer peptides32. In addition to MG2, many other cationic AMPs such as Cecropin A and Cecropin B also exhibit weak cytotoxicity in cancer cells33. Conjugation of these peptides to tumor-homing peptides might also enhance their cytotoxicity. Compared to antibody-directed immunotoxins with high molecular weights34, a tumor-homing peptide may offer advantages in the penetration of tumors. In addition, the peptide can be synthesized economically by chemical synthesis, thus avoiding the use of potentially hazardous biomolecules. Moreover, D-amino acids might be used to enhance the resistance of peptides to proteolytic degradation and increase its in vivo efficiency.
Conclusions
Magainin II shows weak cytotoxic efficacy in tumor cells due to its limited membrane binding affinity. The tumor-homing peptide bombesin can bind specifically to tumor cells with high affinity. Thus, conjugation to bombesin at its N-terminus significantly enhanced the cytotoxicity of magainin II in tumor cells through improved cell binding. Moreover, the magainin II-bombesin conjugate MG2B selectively induces cell death in cultured tumor cells. MG2B exerts promising antitumor effects in vivo. These results suggest that more novel approaches to targeted cancer therapy might be developed by the conjugation of antimicrobial peptides to the tumor-homing peptide bombesin.
Author contribution
Shan LIU performed most experiments. Hao YANG analyzed the peptide-induced apoptosis. Lin WAN and Hua-wei CAI contributed to the in vivo experiments. Sheng-fu LI cultured many cells. You-ping LI and Jing-qiu CHENG analyzed the data. Xiao-feng LU designed the experiments and wrote the paper.
Acknowledgments
This work was supported by the National Basic Research Program of China (2009CB522401), and the National Natural Science Foundation of China (81072566).
References
- Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2006;15:933–46. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, et al. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol. 2006;50:141–7. doi: 10.1016/j.eururo.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Bhutia SK, Maiti TK. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008;26:210–7. doi: 10.1016/j.tibtech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778:357–75. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papo N, Seger D, Makovitzki A, Kalchenko V, Eshhar Z, Degani H, et al. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res. 2006;66:5371–8. doi: 10.1158/0008-5472.CAN-05-4569. [DOI] [PubMed] [Google Scholar]
- Conlon JM, Kolodziejek J, Nowotny N. Antimicrobial peptides from the skins of North American frogs. Biochim Biophys Acta. 2009;1788:1556–63. doi: 10.1016/j.bbamem.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Fernandez DI, Gehman JD, Separovic F. Membrane interactions of antimicrobial peptides from Australian frogs. Biochim Biophys Acta. 2009;1788:1630–8. doi: 10.1016/j.bbamem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–53. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci USA. 1991;88:3792–6. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L, Zasloff M.Potential therapeutic applications of magainins and other antimicrobial agents of animal origin Ciba Found Symp 1994186197–216.discussion 216–23. [DOI] [PubMed] [Google Scholar]
- Baker MA, Maloy WL, Zasloff M, Jacob LS. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993;53:3052–7. [PubMed] [Google Scholar]
- Matsuzaki K, Nakamura A, Murase O, Sugishita K, Fujii N, Miyajima K. Modulation of magainin 2-lipid bilayer interactions by peptide charge. Biochemistry. 1997;36:2104–11. doi: 10.1021/bi961870p. [DOI] [PubMed] [Google Scholar]
- Liu Y, Steiniger SC, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4:435–47. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Takahashi S, Kamikatahira H, Kuroki Y, Jaalouk DE, O'Brien S, et al. Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J Biol Chem. 2008;283:11752–62. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakkonen P, Zhang L, Ruoslahti E. Peptide targeting of tumor lymph vessels. Ann N Y Acad Sci. 2008;1131:37–43. doi: 10.1196/annals.1413.003. [DOI] [PubMed] [Google Scholar]
- Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112:2628–35. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanziger D, Beck-Sickinger AG. Radiometal targeted tumor diagnosis and therapy with peptide hormones. Curr Pharm Des. 2008;14:2385–400. doi: 10.2174/138161208785777397. [DOI] [PubMed] [Google Scholar]
- Khan IU, Beck-Sickinger AG. Targeted tumor diagnosis and therapy with peptide hormones as radiopharmaceuticals. Anticancer Agents Med Chem. 2008;8:186–99. doi: 10.2174/187152008783497046. [DOI] [PubMed] [Google Scholar]
- Okarvi SM. Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev. 2008;34:13–26. doi: 10.1016/j.ctrv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, two analogous active peptides from the skin of the European amphibians Bombesina and Alytes. Experientia. 1971;27:166–9. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8:1139–46. [PubMed] [Google Scholar]
- Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann Oncol. 2007;18:1457–66. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- de Visser M, Verwijnen SM, de Jong M. Update: improvement strategies for peptide receptor scintigraphy and radionuclide therapy. Cancer Biother Radiopharm. 2008;23:137–57. doi: 10.1089/cbr.2007.0435. [DOI] [PubMed] [Google Scholar]
- Engel JB, Keller G, Schally AV, Halmos G, Hammann B, Nagy A. Effective inhibition of experimental human ovarian cancers with a targeted cytotoxic bombesin analogue AN-215. Clin Cancer Res. 2005;11:2408–15. doi: 10.1158/1078-0432.CCR-04-1670. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32:733–40. doi: 10.1016/j.nucmedbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Laakkonen P, Akerman ME, Biliran H, Yang M, Ferrer F, Karpanen T, et al. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc Natl Acad Sci USA. 2004;101:9381–6. doi: 10.1073/pnas.0403317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege K, Patel SJ, Megeed Z, Yarmush ML. Amphipathic peptide-based fusion peptides and immunoconjugates for the targeted ablation of prostate cancer cells. Cancer Res. 2007;67:6368–75. doi: 10.1158/0008-5472.CAN-06-3658. [DOI] [PubMed] [Google Scholar]
- Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem. 1996;271:28375–81. doi: 10.1074/jbc.271.45.28375. [DOI] [PubMed] [Google Scholar]
- Risso A, Braidot E, Sordano MC, Vianello A, Macrì F, Skerlavaj B, et al. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol. 2002;22:1926–35. doi: 10.1128/MCB.22.6.1926-1935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura Y, Choda N, Matsuzaki K. Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys J. 2008;95:5757–65. doi: 10.1529/biophysj.108.133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff HV, Hendler RW, Zasloff M, Juretić D. Interactions between a new class of eukaryotic antimicrobial agents and isolated rat liver mitochondria. Biochim Biophys Acta. 1989;975:361–9. doi: 10.1016/s0005-2728(89)80344-5. [DOI] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–7. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, et al. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008;8:5. doi: 10.1186/1471-2490-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–37. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]