Abstract
Aim:
To investigate the anticancer mechanism of a methoxyflavanone derivative, WJ9708012, highlighting its role on a crosstalk between endoplasmic reticulum (ER) and mitochondrial stress.
Methods:
Cell proliferation was examined using sulforhodamine B assay. Cell-cycle progression, Ca2+ mobilization and mitochondrial membrane potential (ΔΨm) were detected using flow cytometric analysis. Protein expression was detected using Western blot.
Results:
WJ9708012 displayed an antiproliferative and apoptotic activity in human hormone-refractory prostate cancer cells with IC50 values of 6.4 and 5.3 μmol/L in PC-3 and DU-145 cells. WJ9708012 induced a prompt increase of cytosolic Ca2+ level and activation of protein kinase C (PKC)-α. The cleavage of μ-calpain was also induced by WJ9708012. Furthermore, WJ9708012 induced cell-cycle arrest at G1-phase associated with down-regulation of cyclin D1, cyclin E and cyclin-dependent kinase-4 expressions. It also caused a rapid and time-dependent decrease of phosphorylation level of mTOR (Ser2448), 4E-BP1 (Thr37/Thr46/Thr70) and p70S6K (Thr389), indicating the inhibition of mTOR-mediated translational pathways. The ER stress was activated by the identification of up-regulated GADD153 and glucose-regulated protein-78 protein levels. The subsequent mitochondrial stress was also identified by the observation of a decreased Bcl-2 and Bcl-xL expressions, an increased truncated Bid and Bad and a loss of ΔΨm.
Conclusion:
WJ9708012 induces an increase of cytosolic Ca2+ concentration and activation of PKC-α. Subsequently, a crosstalk between ER stress and mitochondrial insult is induced, leading to the inhibition of mTOR pathways and arrest of the cell-cycle at G1 phase. The apoptosis is ultimately induced by a severe damage of mitochondrial function.
Keywords: methoxyflavanone, Ca2+ mobilization, protein kinase C-α, endoplasmic reticulum stress, mitochondrial stress
Introduction
Endoplasmic reticulum (ER) is the major organelle responsible for folding and maturation of transmembrane, secretory and ER-resident proteins. ER stress is originally an adaptive response that can be induced by various perturbations in normal ER function, including the accumulation of unfolded/misfolded proteins, lipid or glycolipid imbalances, or changes of ER Ca2+ homeostasis. ER stress triggers several signaling pathways to cope with the abnormal load in ER lumen. The unfolded protein response pathway causes an up-regulation of ER chaperones, such as GRP78 through C/EBP homologous transcription factor GADD153 and the ER overload response pathway induces the activation of nuclear factor κB (NF-κB), leading to production of cytokines1, 2. However, ER stress may also contribute to cell suicide when abnormalities become extensive. GADD153, NF-κB and caspase-12 have been implicated in the apoptotic regulation1, 3. Besides, ER stress can crossly react with mitochondrial stress, resulting in an increase of cytochrome c release and subsequent caspase-dependent apoptotic reaction4, 5. Recently, the ER stress caused by exogenously applied stimuli has been considered a potential strategy in anticancer approach6, 7.
Intracellular Ca2+ is an essential element in the control of cellular function and a lot of physical events. However, an overload of intracellular Ca2+ may cause stresses on target organelles, leading to oxidative stress and massive activation of a lot of enzymes and an ultimate cell death. ER and mitochondria play crucial roles in the maintenance of intracellular Ca2+ homeostasis and, therefore, regulate cell death8, 9, 10. The evidence suggests that sufficient extracellular Ca2+ influx causes Ca2+-activated ER stress, which contributes predominantly to apoptosis in many types of cells including prostate cancer cells11, 12. Notably, an increase of cytosolic Ca2+ can also impair protein processing, promoting ER stress and inhibiting translation pathways13, 14 that explain the down-regulation of cell-cycle regulators and arrest of the cell cycle by several anticancer agents13, 15. Ca2+ also serves as an effector communicating between ER- and mitochondria-mediated signaling cascades. Bcl-2 has been revealed to be associated with the ER and outer mitochondrial membrane10, 16 and plays a central role on negative regulation of Ca2+ homeostasis since Bcl-2 overexpression is capable of reducing the Ca2+ level released from the ER and diminishes cell apoptosis17. Bax and Bak, are two pro-apoptotic members of Bcl-2 family that impair mitochondrial function, also localize to the ER. Scorrano and colleagues provided evidence that both ER-released Ca2+ and the presence of mitochondrial Bax or Bak were required to fully restore apoptosis by several apoptotic stimuli18. Altogether, these data support that Ca2+ mobilization is a key regulator in the crosstalk between ER and mitochondria in propagating apoptotic signals.
Flavonoids are a family of polyphenolic phytochemicals including flavones and isoflavones. A large body of evidence shows that diet high in flavonoids is associated with the reduced incidence of some cancers19. Flavonoids are extensively studied to display anticancer activity through numerous signaling pathways5, 20, 21. Of note, most of the flavonoids exhibit antioxidant activities22. This activity has been implicated to reduce anticancer capability of flavonoids since reactive oxygen species (ROS) acts as important apoptotic mediators. It appears that ROS generation is important for stimulation of apoptotic cell death that is crucial protective effects in the body for killing cancer cells. This oxidative stress is also critical for effective cancer chemotherapy and radiation treatment23, 24. Furthermore, Salganik and colleagues reported that antioxidant-depleted diets, but not antioxidant-enriched diets, were capable of increasing ROS levels and dramatically increased apoptosis occurs within tumors25. To avoid the interruption of antioxidant effect, we synthesized methoxyflavanone derivatives and found that WJ9708012 [6-(3-Hydroxy-3-methylbutyl)-2′-(7-hydroxy-3,7-dimethyloctyl)-3′,4′,5,7-tetramethoxyflavanone] displayed effective anticancer activity without antioxidant effect in human hormone-refractory prostate cancer cells. The anticancer mechanisms of WJ9708012 were identified to be related to Ca2+ and protein kinase C (PKC)-α involved interaction of ER and mitochondria stresses. This study provides evidence that flavonoids, in the absence of antioxidant effect, may have potential as a cancer therapy against human prostate cancers.
Materials and methods
Materials
RPMI 1640 medium, fetal bovine serum (FBS), penicillin, streptomycin, and all other cell culture reagents were obtained from GIBCO/BRL Life Technologies (Grand Island, NY, USA). Antibodies to cyclin D1, cyclin E, cyclin-dependent kinase 4 (Cdk4), Bcl-2, Bcl-xL, Bak, Bax, Bid, Bad, glucose-regulated protein-78 (GRP78), cytochrome c, protein kinase C (PKC)-α, and anti-mouse and anti-rabbit IgGs were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Antibodies to AMP-activated protein kinase (AMPK)-α, phospho-AMPKαThr172, mammalian target of rapamycin (mTOR), phospho-mTORSer2448, phospho-p70S6KThr389, phospho-4E-BP1Thr70, phospho-4E-BP1Thr37/Thr46, procaspase-3, procaspase-7, PARP, GADD153 (growth arrest and DNA damage-inducible gene 153), apoptosis-inducing factor (AIF), endonuclease G, and α-tubulin were from Cell Signaling Technologies (Boston, MA, USA). Fluo-3/AM and 2′,7′-dichlorofluorescin diacetate (DCF-DA) were from Molecular Probes Inc (Eugene, OR, USA). Sulforhodamine B (SRB), propidium iodide (PI), phenylmethylsulfonylfluoride (PMSF), leupeptin, dithiothreitol, rhodamine 123, EDTA, carbonylcyanide-m-chlorophenylhydrazone (CCCP), W7, N-acetylcysteine (NAC), trolox, Go 6983, Compound C (6-[4-(2-Piperidin-1-ylethoxy)phenyl]-3-pyridin- 4-ylpyrazolo[1,5-a]pyrimidine) and all of the other chemical reagents were obtained from Sigma-Aldrich (St Louis, MO, USA). Ro-318220 was purchased from Calbiochem (La Jolla, CA, USA). WJ9708012 [6-(3-Hydroxy-3-methylbutyl)-2′-(7-hydroxy-3,7-dimethyloctyl)-3′,4′,5,7-tetramethoxyflavanone] (Figure 1A) was synthesized and provided by our colleagues (Dr Wei-jan HUANG). The purity is more than 95% by the examination using high-resolution MS and nuclear magnetic resonance.
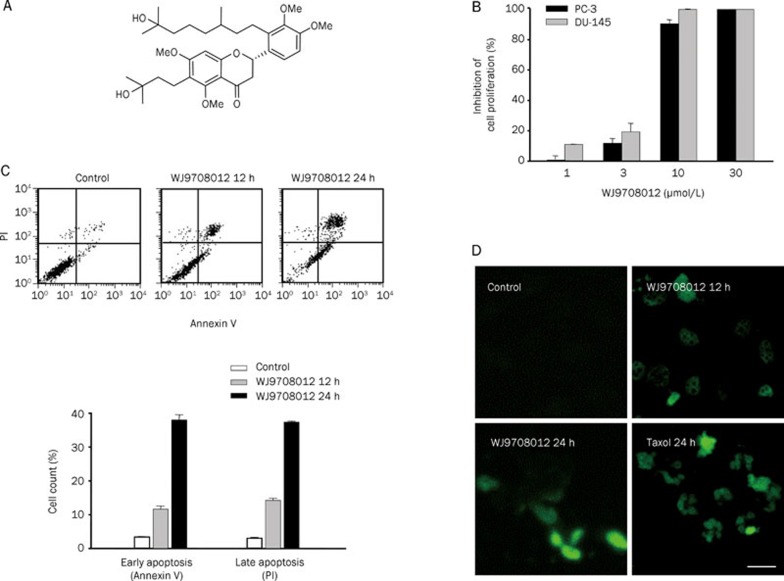
Figure 1.
Identification of anti-proliferative and apoptotic effects. (A) Chemical structure of WJ9708012. (B) The graded concentrations of WJ9708012 were added to cells for 48 h. Then, the cells were fixed and stained with SRB and the data were analyzed as described in the Materials and methods section. Data are expressed as mean±SEM of four determinations (each in triplicate). (C and D) PC-3 cells were treated with the indicated agent. Then, the cells were harvested for annexin V-PI double staining of apoptosis or in situ detection of apoptotic cells by using TUNEL apoptosis detection methods. Data are expressed as mean±SEM of three determinations. Scale bar, 20 μm.
Cell lines and cell culture
The cancer cell lines including PC-3 and DU-145 were from American Type Culture Collection (Rockville, MD, USA). The cells were cultured in RPMI-1640 medium with 10% FBS (v/v) and penicillin (100 units/mL)/streptomycin (100 μg/mL). Cultures were maintained in a humidified incubator at 37 °C in 5% CO2/95% air.
SRB assays
Cells were seeded in 96-well plates in medium with 5% FBS. After 24 h, cells were fixed with 10% trichloroacetic acid (TCA) to represent cell population at the time of compound addition (T0). After additional incubation of DMSO or WJ9708012 for 48 h, cells were fixed with 10% TCA and SRB at 0.4% (w/v) in 1% acetic acid was added to stain cells. Unbound SRB was washed out by 1% acetic acid and SRB bound cells were solubilized with 10 mmol/L Trizma base. The absorbance was read at a wavelength of 515 nm. Using the following absorbance measurements, such as time zero (T0), control growth (C), and cell growth in the presence of the compound (Tx), the percentage growth was calculated at each of the compound concentrations levels. Percentage growth inhibition was calculated as: 100–[(Tx–T0)/(C–T0)]×100. Growth inhibition of 50% (IC50) is determined at the compound concentration which results in 50% reduction of total protein increase in control cells during the compound incubation.
Annexin V-PI staining of apoptotic cell
Cells were cultured in 6-well plate for 24 h and then treated with vehicle or 30 μmol/L WJ9708012 for 12 or 24 h. After the treatment, cells were washed twice with phosphate-buffered saline (PBS) and stained with fluorescein isothiocyanate (FITC) annexin V and PI as per the manufacturer's directions (Annexin V-FITC Apoptosis Detection Kit, R&D Systems Inc, Minneapolis, MN, USA). The stained cells were evaluated by flow cytometry. The cells binding FITC-annexin V (excitation, 488 nm; emission, 520 nm) but not PI (excitation, 540 nm; emission, 630 nm) were termed annexin V-positive (early apoptosis) and the cells permeant to PI (regardless of FITC-annexin V binding) were deemed necrosis or late apoptosis26.
In situ labeling of apoptotic cells
In situ detection of apoptotic cells was performed using TUNEL (terminal dUTP nick-end labeling) apoptosis detection methods. The TUNEL method identifies apoptotic cells using TdT to transfer biotin-dUTP to the free 3′-OH of cleaved DNA. The biotin-labeled cleavage sites were then visualized by reaction with fluorescein conjugated avidin (avidin-FITC). After the treatment of cells with or without WJ9708012 (30 μmol/L) or taxol (0.1 μmol/L) for 12 or 24 h, the cells were washed, fixed and stained for apoptotic detection according to the protocol provided by the suppliers (Promega, Madison, WI, USA). The photomicrographs were obtained by a fluorescence microscopic examination (Nikon).
FACScan flow cytometric assay
After the indicated treatment, the cells were harvested by trypsinization, fixed with 70% (v/v) alcohol at 4 °C for 30 min and washed with PBS. After centrifugation, cells were incubated in 0.1 mL of phosphate-citric acid buffer (0.2 mol/L Na2HPO4, 0.1 mol/L citric acid, pH 7.8) for 30 min at room temperature. Then, the cells were centrifuged and resuspended with 0.5 mL PI solution containing Triton X-100 (0.1% v/v), RNase (100 μg/mL) and PI (80 μg/mL). DNA content was analyzed with FACScan and CellQuest software (Becton Dickinson, Mountain View, CA, USA).
Western blotting
After the treatment, cells were harvested with trypsinization, centrifuged and lysed in 0.1 mL of lysis buffer containing 10 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1 mmol/L EGTA, 1% Triton X-100, 1 mmol/L PMSF, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 50 mmol/L NaF and 100 μmol/L sodium orthovanadate. In some experiments, the mitochondrial/cytosol fractionation kit (Biovision, Mountain View, CA, USA) was used to separate mitochondrial and cytosolic fraction. Total protein was quantified, mixed with sample buffer and boiled at 90 °C for 5 min. Equal amount of protein (30 μg) was separated by electrophoresis in 8% or 12% SDS-PAGE, transferred to PVDF membranes and detected with specific antibodies. The immunoreactive proteins after incubation with appropriately labeled secondary antibody were detected with an enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, UK).
Measurement of intracellular Ca2+ level
Cells were pre-incubated with fluo 3-AM (5 μmol/L) for 30 min. Then, the cells were washed twice and incubated in fresh medium. Vehicle (0.1% DMSO) or WJ9708012 was added to the cells for the indicated times and the intracellular Ca2+ level was measured by flow cytometric analysis.
Measurement of mitochondrial membrane potential (ΔΨm) and ROS
Cells were treated without or with the agent for the indicated times. Thirty minutes before the termination of incubation, a rhodamine 123 solution (final concentration of 5 μmol/L, for ΔΨm measurement) or DCF-DA (final concentration of 10 μmol/L, for ROS measurement) was added to the cells and incubated for the last 30 min at 37 °C. The cells were finally harvested and the accumulation of rhodamine 123 or ROS was determined using FACScan flow cytometric analysis.
Immunofluorescence microscopic examination
Cells were seeded in 8-well chamber slides. After the compound treatment, the cells were fixed with 100% methanol at −20 °C for 5 min and incubated in 1% bovine serum albumin (BSA) containing 0.1% Triton X-100 at 37 °C for 30 min. The cells were washed twice with PBS for 5 min and incubated with primary antibodies at 37 °C for 1 h. The cells were washed three times with PBS for 10 min and incubated with FITC-conjugated secondary antibody at 37 °C for 40 min. The nuclei were recognized by the staining with DAPI (1 μg/mL). The labeled targets in cells were detected by a confocal laser microscopic system (Leica TCS SP2).
Data analysis
The compound was dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was 0.1% in cell culture media. Data are presented as the mean±SEM for the indicated number of separate experiments. Statistical analysis of data was performed with one-way analysis of variance (ANOVA) followed by a t-test and P-values less than 0.05 were considered significant.
Results
Anti-proliferative and apoptosis-inducing activities in prostate cancer cells
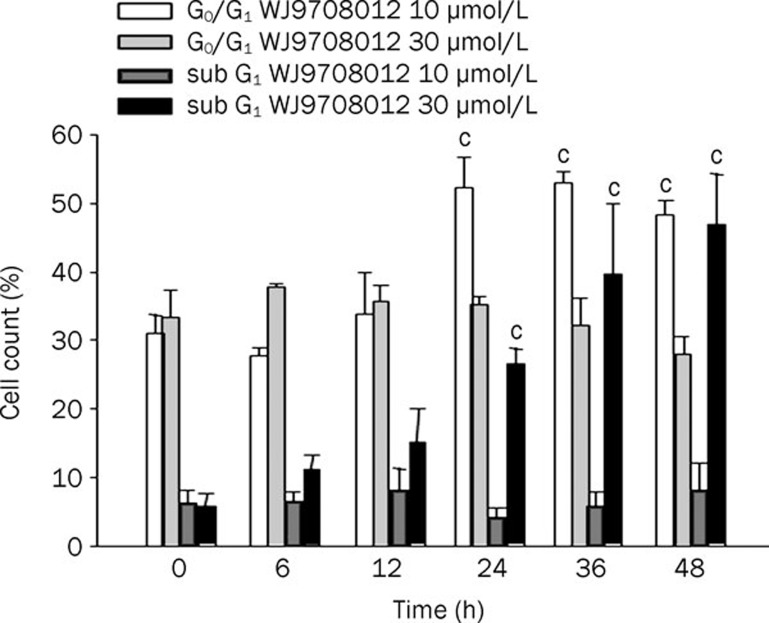
The antiproliferative effect of WJ9708012 was examined in human hormone-refractory prostate cancer PC-3 and DU-145 cell lines by SRB assay. WJ9708012 inhibited cell proliferation in a concentration-dependent manner with IC50 values of 6.4 and 5.3 μmol/L in PC-3 and DU-145 cells, respectively (Figure 1B). WJ9708012 also induced a time-dependent apoptotic cell death by the detection of positive annexin V-staining and TUNEL-positive cells (Figures 1C and 1D). Besides, the flow cytofluorometric analysis of cell cycle by PI staining showed that WJ9708012 induced an increase of cell population at G1 phase and a time-dependent apoptosis in PC-3 cells. The data revealed that the cells were more susceptible to arrest at G1 phase than the induction of apoptosis to WJ9708012 action (Figure 2).
Figure 2.
Effect of WJ9708012 on cell-cycle progression. PC-3 cells were treated without or with WJ9708012 for the indicated times. After the treatment, the cells were fixed and stained with propidium iodide to analyze DNA content by FACScan flow cytometer. Data are expressed as mean±SEM of three determinations. cP<0.01 compared with the respective control.
Effect of WJ9708012 on Ca2+ mobilization and PKC-α activity
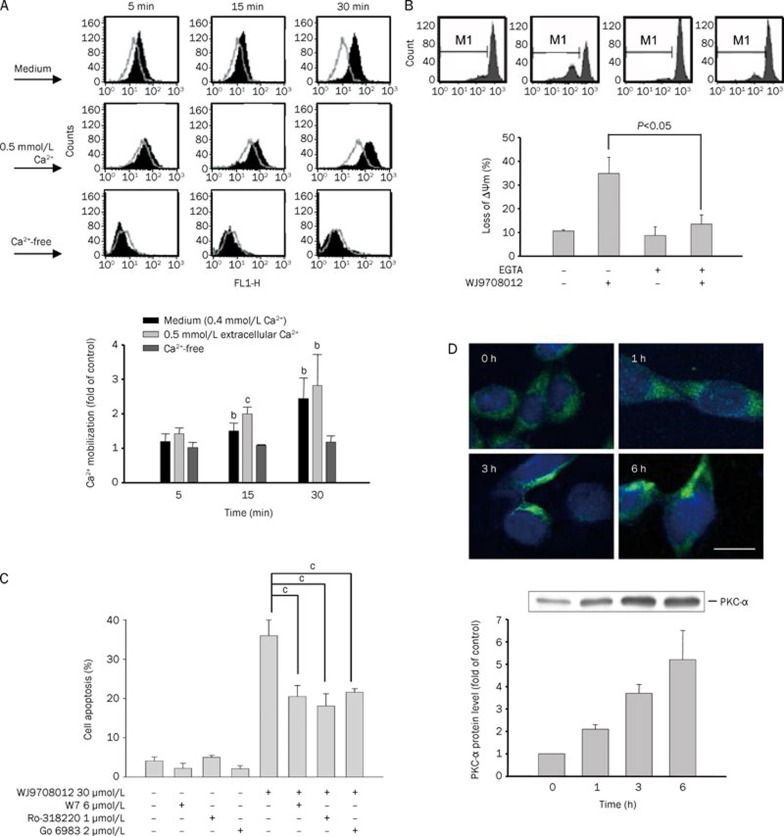
The Ca2+ mobilization was examined in the present work. The data in Figure 3A demonstrated that WJ9708012 triggered a significant increase of Ca2+ influx in extracellular Ca2+-containing medium other than Ca2+-free solution. In a parallel experiment, the Ca2+-free solution supplemented with EGTA significantly inhibited WJ9708012-induced loss of ΔΨm, indicating that the influx of extracellular Ca2+ served as an upstream initiator to cause cellular stresses (Figure 3B). Furthermore, several pharmacological inhibitors were used to study the related target proteins. As a result, W7 (a membrane-permeable calmodulin inhibitor), Ro-318220 and Go-6983 (PKC inhibitors) significantly reduced WJ9708012-induced apoptotic effect (Figure 3C). Since PKC-α is the only conventional (Ca2+-dependent) PKC isoform detected in PC-3 cells27, the effect of WJ9708012 on PKC-α translocation was identified. The immunofluorescence microscopic examination showed that PKC-α translocated to plasma membrane after the exposure of cells to WJ9708012. The data were confirmed by Western blot that detected the plasma membrane fraction and demonstrated a time-dependent increase of PKC-α protein expression (Figure 3D). The data suggest that WJ9708012 induces the activation of PKC-α in PC-3 cells.
Figure 3.
Effect of WJ9708012 on Ca2+-related function and PKC-α membrane translocation. (A) PC-3 cells were treated with WJ9708012 (30 μmol/L) for 0 (control), 5, 15, or 30 min in various mediums containing different extracellular Ca2+ levels. After the treatment, the intracellular Ca2+ level was measured as described in the Materials and methods section. (B and C) PC-3 cells were pre-treated without or with EGTA (1 mmol/L, B) or the indicated agent for 30 min. WJ9708012 (30 μmol/L) was added for another 6 h (B) or 24 h (C). After the treatment, the flow cytometric analysis of rhodamine 123 or PI staining were used to detect the change of mitochondrial membrane potential (ΔΨm) or cell-cycle progression. Data are expressed as mean±SEM of three determinations. bP<0.05, cP<0.01 compared with the respective control. (D) PC-3 cells were treated without or with WJ9708012 (30 μmol/L) for the indicated time. After the treatment, the cells were fixed for the detection of PKC-α by confocal microscopic examination or the cells were harvested and the cell membrane was separated for the detection of PKC-α expression by Western blot. The data are representative of two independent experiments. The expressions were quantified using the computerized image analysis system ImageQuant (Amersham Biosciences). Scale bar, 20 μm.
Effect of WJ9708012 on several cellular stresses
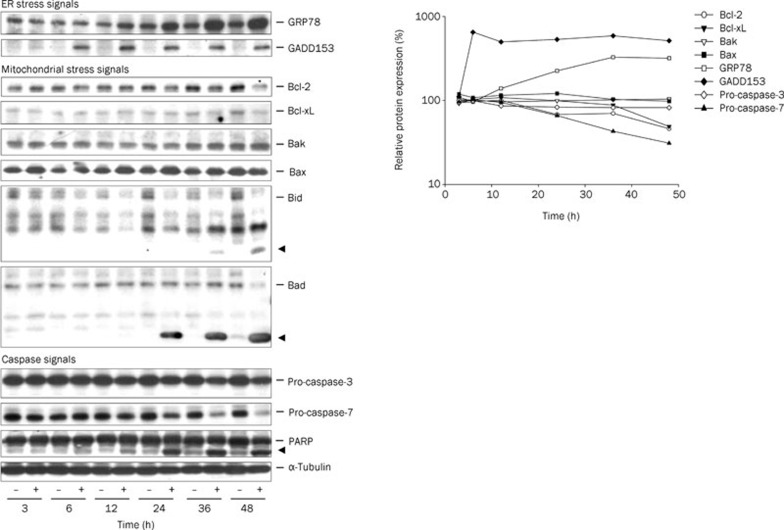
Numerous lines of evidence suggest that Ca2+ plays a central role not only on the induction of ER stress and mitochondrial insult but also on the communication of these two events8, 10, 11, 12, 16. Accordingly, several cellular stresses were determined in this study. The data demonstrated that WJ9708012 induced an early and sustained ER stress by the identification of up-regulated GADD153 and GRP78 protein levels (Figure 4). The mitochondrial stress was also induced by the alteration of mitochondria-related signals, including the down-regulation of Bcl-2 and Bcl-xL expressions and the degradation of Bid and Bad associated with the formation of their truncated active fragments (tBid and tBad), two more potent inducers of apoptosis than the wild-type proteins28, 29 (Figure 4). As expected, the activation of caspase-3 and caspase-7, and the cleavage of downstream effector PARP were induced by WJ9708012 (Figure 4).
Figure 4.
Effect of WJ9708012 on the expression of several ER and mitochondrial stress-related proteins. PC-3 cells were incubated in the absence or presence of WJ9708012 (30 μmol/L) for the indicated time. After the treatment, the cells were harvested and lysed for the detection of the indicated protein expressions by Western blot. The data are representative of three independent experiments. The expressions were quantified using the computerized image analysis system ImageQuant (Amersham Biosciences).
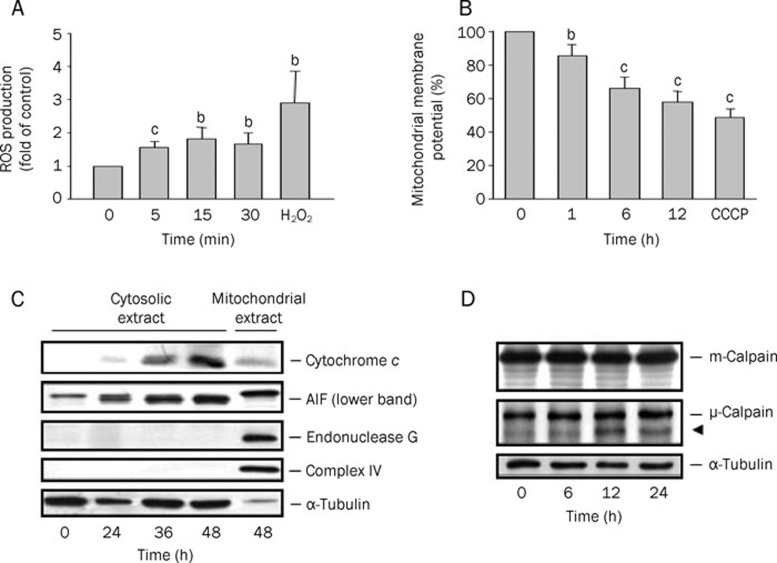
Effect of WJ9708012 on mitochondrial stress
Excessive generation of ROS renders a cell oxidatively stressed and impairs membrane proteins, leading to mitochondrial dysfunction and apoptotic cell death. A lot of flavonoids have been reported to exhibit antioxidant activities22, which reduce apoptotic capability of these compounds. WJ9708012 did not show antioxidant effect. Instead, it induced a moderate but significant increase of ROS production (Figure 5A), an effect prior to a loss of ΔΨm (Figure 5B). Cytochrome c, AIF and endonuclease G are three pro-apoptotic proteins and are frequently found in the cytosol after apoptotic induction. In this study, WJ9708012 induced a dramatic increase of cytosolic level of cytochrome c and AIF (Figure 5C) in the time comparable to the impact on Bcl-2 family proteins (Figure 4). Additionally, we examined if WJ9708012 played a role on calpain activity. As a result, the cleavage of μ-calpain other than m-calpain was induced by WJ9708012 (Figure 5D).
Figure 5.
Effect of WJ9708012 on mitochondria-related function and calpain cleavage. PC-3 cells were treated without or with WJ9708012 (30 μmol/L) for the indicated time. After the treatment, the ROS production (A) or mitochondrial membrane potential (B) were measured as described in the Materials and methods section. Data are expressed as mean±SEM of three to five determinations. bP<0.05, cP<0.01 compared with the respective control. (C) The cells were harvested. The cytosolic and mitochondrial fractions were obtained for the detection of the indicated protein expressions by Western blot. (D) The detection of calpain cleavage by Western blot. The data are representative of two independent experiments.
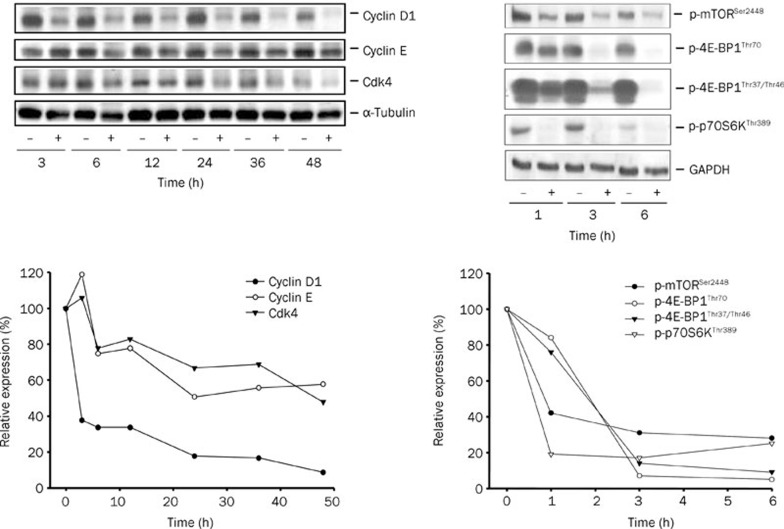
Effect of WJ9708012 on the expression of cell-cycle regulators and mTOR pathway signals
The cell-cycle progression is regulated by periodic activation of various Cdk/cyclin complexes. Accordingly, several cyclins and Cdks that regulate G1 phase of the cell cycle were examined. The data demonstrated that the protein expressions of cyclin D1, cyclin E and Cdk4 were down-regulated by WJ9708012 (Figure 6) that correlated with the cell-cycle arrest at G1 phase. The data also showed a rapid down-regulation of cyclin D1 expression reflecting a short half-life of this protein (Figure 6). To examine if translational signaling pathways were affected by WJ9708012-induced stress, we detected several related protein expressions and the phosphorylation state, including mTOR, 4E-BP1 and p70S6K. mTOR, a serine/threonine kinase, is a key regulator of protein translation/synthesis. The phosphorylation of mTOR at Ser2448 is a marker of mTOR activation, which transmits positive signals to activate p70S6K (phosphorylation at Thr389) and inactivate the translation repressor protein 4E-BP1 through sequential phosphorylation at Thr37/Thr46 and Thr70. The data showed that WJ9708012 induced a rapid and time-dependent decrease of phosphorylation level of mTOR (Ser2448), 4E-BP1 (Thr70 and Thr37/Thr46) and p70S6K (Thr389), suggesting the inhibition of mTOR-mediated translational pathways (Figure 6).
Figure 6.
Effect of WJ9708012 on several protein expressions. PC-3 cells were treated without or with WJ9708012 (30 μmol/L) for the indicated time. After the treatment, the cells were harvested and lysed for the detection of the indicated protein expressions by Western blot. The expressions were quantified using the computerized image analysis system ImageQuant (Amersham Biosciences).
Discussion
The signaling pathways that control cell proliferation have been extensively studied in recent decades. Cell cycle progression is delicately controlled in cells responsive to diverse mitogenic signals, which stimulate sequential signaling cascades and regulate various cell cycle proteins through transcriptional control, posttranslational modifications and protein degradation. Cells are prone to arrest of the cell cycle in response to a variety of cellular stresses, including radiation, chemicals, oxidants and metabolite signals. This study showed that WJ9708012, a methoxyflavanone derivative, posed a cellular stress, leading to an arrest of the cell cycle at G1 phase and a subsequent apoptosis in prostate cancer cells. The impact on several G1-phase regulatory proteins was observed during the stress response, such as the down-regulation of cyclin D1, cyclin E and Cdk4. The data provided evidence that WJ9708012 reduced the protein levels of these regulators through an inhibition of mTOR-mediated translational pathways. Of note, cyclin D1 was the most susceptible protein to this inhibitory activity. It can be explained by the impairment of cyclin D1 stability since it has been suggested that a constitutive activation of phosphatidyl inositol 3-kinase/Akt that keeps glycogen synthase kinase (GSK)-3β inhibited increases cyclin D1 stability. Rapamycin, an mTOR inhibitor, blocks the inhibitory action on (GSK)-3β, leading to a nuclear export of cyclin D1 and a decrease of cyclin D1 stability30.
Ca2+ is a double-edged sword in physiological control of cellular function as well as an inducer of cell death. Calcium has been implicated in propagating apoptotic signaling cascades in many types of cancer cells including prostate cancers31. However, a massive and acute influx of Ca2+ may cause cell necrosis without programmed death mechanism that is not an appropriate anticancer strategy. Classical PKC isoforms, including PKC-α, -βI, -βII, and -γ, are Ca2+-dependent and are activated by diacylglycerol and phorbol esters through the cysteine-rich C1 domains32. Classical PKC isoforms are also well-known downstream effectors of Ca2+ in signaling cell proliferation, differentiation and apoptosis. PKC-α is the predominant isoform in many types of cancer cells33. Depending on the tumor type and different treatment, contradictory results have been described in PKC-α-mediated cell survival and apoptosis. For example, PKC-α knockout mice have been demonstrated to increase the tendency of developing spontaneous intestinal tumors34. In contrast, Varga and the colleagues provided the data showing that the expression of PKC-α gradually increased with increasing tumor grades35 The data in this study showed that WJ9708012 induced an increase of intracellular Ca2+ level through extracellular origin. The increased Ca2+ subsequently induced cell apoptosis through a PKC-α-dependent pathway. Our data also showed the induction of ER stress to WJ9708012. Recent studies reveal that PKC is involved in the induction of GRP7836, a major ER chaperone and a crucial regulator of ER homeostasis. Furthermore, PKC-α has been reported to mediate growth arrest in human embryonal rhabdomyosarcoma cells by inducing an activation of c-Jun N-terminal protein kinases (JNKs), p38 kinase and extracellular signal-regulated kinases (ERKs)37, which are involved in the induction of ER stress36, 38. These reports support the present study that WJ9708012 activates PKC-α which in turn, directly or indirectly, induces ER stress in PC-3 cells.
Permeabilization of the outer mitochondrial membrane and release of pro-apoptotic proteins are the most extensively characterized pathways to cell apoptosis and make the mitochondria an attractive target for cancer chemotherapy39. Recently, a crosstalk between ER and mitochondria that reinforces the efficacy and efficiency of anticancer agents has been widely addressed8. Several pathways have been suggested to connect the interaction between ER and mitochondria. The electron tomographic examination shows that the ER network forms close or direct contacts with the mitochondria8, 40, 41. Several studies also reveal that ER may communicate with mitochondria via mitochondria-associated membranes41. In addition to direct network connection, a large body of evidence points to the Ca2+ as a central player that is released from the ER store and, in turn, causes a load in mitochondria, leading to necessary biochemical activities. However, an overload of mitochondrial Ca2+ induces the release of apoptotic proteins from the mitochondria, including cytochrome c, AIF and endonuclease G. Cytochrome c is a central co-factor in intrinsic apoptotic pathways. AIF and endonuclease G are also responsible for the nuclear DNA fragmentation upon numerous apoptotic stimuli8, 29, 39. In this study, WJ9708012 induced an increase of intracellular Ca2+ and ER stress, leading to the down-regulation of Bcl-2 and Bcl-xL expressions and a dramatic increase of cytosolic level of cytochrome c and AIF. The data reveal a crosstalk between ER and mitochondria in communicating WJ9708012-mediated apoptotic signaling pathways.
Calpains are consisted of a class of intracellular cysteine proteases that are activated by Ca2+ 42. μ-Calpain (calpain-I) and m-calpain (calpain-II) are two major forms that are activated by micromolar and millimolar concentrations of Ca2+, respectively. Several Bcl-2 family members, such as Bcl-2, Bid, and Bcl-xL, are reported to be processed by calpain43. Furthermore, calpain-mediated cleavage of Bid to an active fragment has been implicated in mitochondrial permeabilization and cell death44. Recently, Polster and the colleagues used isolated mitochondria model and demonstrated that μ-calpain induced the cleavage and release of AIF29. In this study, WJ9708012 also induced the cleavage and release of AIF. The effect may be explained by the activation of μ-calpain.
Taken together, the data suggest that WJ9708012 induces an increase of cytosolic Ca2+ concentration and the activation of PKC-α in PC-3 cells. Subsequently, a crosstalk between ER stress and mitochondrial insult is induced, leading to the inhibition of mTOR translational pathways and arrest of the cell cycle at G1 phase. Moreover, the summation of WJ9708012-mediated stress results in the down-regulation of Bcl-2 and Bcl-xL protein expressions and an increased release of cytochrome c and AIF, leading to apoptotic cell death. The data also demonstrate that WJ9708012 is an effective methoxyflavanone worth further investigation and development.
Author contribution
Ting-chun KUO performed the study; Wei-jan HUANG synthesized and provided the compound WJ9708012; Jih-hwa GUH designed the study and prepared the manuscript.
Acknowledgments
This work was supported by a research grant of the National Science Council of the Taiwan, China (NSC 98-2323-B-002-006). Facilities provided by grants from the National Science Council of the Republic of China (NSC 98-2323-B-002-001) are also acknowledged.
References
- Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–14. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–80. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendyk J, Michalak M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim Pol. 2005;52:381–95. [PubMed] [Google Scholar]
- Momoi T. Caspases involved in ER stress-mediated cell death. J Chem Neuroanat. 2004;28:101–5. doi: 10.1016/j.jchemneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Yeh TC, Chiang PC, Li TK, Hsu JL, Lin CJ, Wang SW, et al. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol. 2007;73:782–92. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Boelens J, Lust S, Offner F, Bracke ME, Vanhoecke BW. The endoplasmic reticulum: a target for new anticancer drugs. In Vivo. 2007;21:215–26. [PubMed] [Google Scholar]
- Linder S, Shoshan MC. Lysosomes and endoplasmic reticulum: targets for improved, selective anticancer therapy. Drug Resist Updat. 2005;8:199–204. doi: 10.1016/j.drup.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–18. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchini E, Siviero R, Giorgi C, Rizzuto R, Pinton P. Mitochondrial calcium signaling: message of life and death. Ital J Biochem. 2007;56:235–42. [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–18. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- Pyrko P, Kardosh A, Liu YT, Soriano N, Xiong W, Chow RH, et al. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 2007;6:1262–75. doi: 10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- Akamatsu K, Shibata MA, Ito Y, Sohma Y, Azuma H, Otsuki Y. Riluzole induces apoptotic cell death in human prostate cancer cells via endoplasmic reticulum stress. Anticancer Res. 2009;29:2195–204. [PubMed] [Google Scholar]
- Pyrko P, Kardosh A, Schönthal AH. Celecoxib transiently inhibits cellular protein synthesis. Biochem Pharmacol. 2008;75:395–404. doi: 10.1016/j.bcp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–63. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Jakobsen CH, Størvold GL, Bremseth H, Follestad T, Sand K, Mack M, et al. DHA induces ER stress and growth arrest in human colon cancer cells: associations with cholesterol and calcium homeostasis. J Lipid Res. 2008;49:2089–100. doi: 10.1194/jlr.M700389-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Terada K, Gotoh T, Mori M. In vitro analysis of Bcl-2 proteins in mitochondria and endoplasmic reticulum: similarities in anti-apoptotic functions and differences in regulation. Exp Cell Res. 2007;313:3767–78. doi: 10.1016/j.yexcr.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci USA. 1994;91:6569–73. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–9. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65:995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–80. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Szczepanski M, Lee YJ. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem Pharmacol. 2008;75:2345–55. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, Deinzer ML, et al. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J Agric Food Chem. 2000;48:3876–84. doi: 10.1021/jf0002995. [DOI] [PubMed] [Google Scholar]
- Verhaegen S, McGowan AJ, Brophy AR, Fernandes RS, Cotter TG. Inhibition of apoptosis by antioxidants in the human HL-60 leukemia cell line. Biochem Pharmacol. 1995;50:1021–9. doi: 10.1016/0006-2952(95)00233-p. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Antioxidants suppress apoptosis. J Nutr. 2004;134:3179S–80S. doi: 10.1093/jn/134.11.3179S. [DOI] [PubMed] [Google Scholar]
- Salganik RI, Albright CD, Rodgers J, Kim J, Zeisel SH, Sivashinskiy MS, et al. Dietary antioxidant depletion: enhancement of tumor apoptosis and inhibition of brain tumor growth in transgenic mice. Carcinogenesis. 2000;21:909–14. doi: 10.1093/carcin/21.5.909. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Long DD, Goodwin SM, Lee C. Transforming growth factor-beta1 inhibits membrane association of protein kinase C alpha in a human prostate cancer cell line, PC3. Endocrinology. 1997;138:4657–64. doi: 10.1210/endo.138.11.5531. [DOI] [PubMed] [Google Scholar]
- Condorelli F, Salomoni P, Cotteret S, Cesi V, Srinivasula SM, Alnemri ES, et al. Caspase cleavage enhances the apoptosis-inducing effects of BAD. Mol Cell Biol. 2001;21:3025–36. doi: 10.1128/MCB.21.9.3025-3036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Basañez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–54. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Dal Col J, Dolcetti R. GSK-3beta inhibition: at the crossroad between Akt and mTOR constitutive activation to enhance cyclin D1 protein stability in mantle cell lymphoma. Cell Cycle. 2008;7:2813–6. doi: 10.4161/cc.7.18.6733. [DOI] [PubMed] [Google Scholar]
- Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793:1105–9. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Geiger M, Wrulich OA, Jenny M, Schwaiger W, Grunicke HH, Uberall F. Defining the human targets of phorbol ester and diacylglycerol. Curr Opin Mol Ther. 2003;5:631–41. [PubMed] [Google Scholar]
- Martiny-Baron G, Fabbro D. Classical PKC isoforms in cancer. Pharmacol Res. 2007;55:477–86. doi: 10.1016/j.phrs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Oster H, Leitges M. Protein kinase C-α but not PKC-ζ suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 2006;66:6955–63. doi: 10.1158/0008-5472.CAN-06-0268. [DOI] [PubMed] [Google Scholar]
- Varga A, Czifra G, Tallai B, Nemeth T, Kovacs I, Kovacsand L, et al. Tumor grade-dependent alterations in the protein kinaseC isoform pattern in urinary bladder carcinomas. Eur Urol. 2004;46:462–5. doi: 10.1016/j.eururo.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Song MS, Park YK, Lee JH, Park K. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase C-epsilon/ERK/AP-1 signaling cascade. Cancer Res. 2001;61:8322–30. [PubMed] [Google Scholar]
- Mauro A, Ciccarelli C, De Cesaris P, Scoglio A, Bouché M, Molinaro M, et al. PKCα-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J Cell Sci. 2002;115:3587–99. doi: 10.1242/jcs.00037. [DOI] [PubMed] [Google Scholar]
- Lien YC, Kung HN, Lu KS, Jeng CJ, Chau YP. Involvement of endoplasmic reticulum stress and activation of MAP kinases in beta-lapachone-induced human prostate cancer cell apoptosis. Histol Histopathol. 2008;23:1299–308. doi: 10.14670/HH-23.1299. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria as targets for cancer chemotherapy. Semin Cancer Biol. 2009;19:57–66. doi: 10.1016/j.semcancer.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci USA. 2001;98:2399–406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–56. [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gil-Parrado S, Fernández-Montalván A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem. 2002;277:27217–26. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–8. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]