Abstract
Aim:
To explore whether glutathione (GSH) increased through Nrf-2 activation is involved in the cytoprotective effects of carnosol in HepG2 cells.
Methods:
Human hepatoma cell line HepG2 were exposed to rosemarry essential oil or carnosol. Cell viability was measured using an Alamar blue assay. The production of intracellular GSH was determined using monochlorobimane. The level of protein or mRNA was examined by Western blotting or RT-PCR, respectively.
Results:
Rosemarry essential oil (0.005%–0.02%) and carnosol (5 and 10 mol/L) increased the intracellular GSH levels and GSH synthesis enzyme subunit GCLC/GCLM expression. Rosemary essential oil and carnosol increased nuclear accumulation of Nrf2 and enhanced Nrf2-antioxidant responsive element (ARE)-reporter activity. Transfection of the treated cells with an Nrf2 siRNA construct blocks GCLC/GCLM induction. Furthermore, pretreatment of the HepG2 cells with essential oil and carnosol exerted significant cytoprotective effects against H2O2 or alcohol. In TNFα-treated cells, the nuclear translocation and transcriptional activity of NF-κB was abolished for 12 h following carnosol pretreatment. Cotreatment with GSH also suppressed NF-κB nuclear translocation, whereas cotreatment with BSO, a GSH synthesis blocker, blocked the inhibitory effects of carnosol.
Conclusion:
This study demonstrated that Nrf2 is involved in the cytoprotective effects by carnasol, which were at least partially mediated through increased GSH biosynthesis.
Keywords: carnosol, glutathione, Nrf2, NF-κB, human hepatoma cell line HepG2, cytoprotection
Introduction
Non-nutritional constituents in many foods may have beneficial health effects such as anti-inflammatory and anti-carcinogenic properties. Rosmarinus officinalis (rosemary) originates from southern Europe and is a commonly used herbal flavoring agent1. Rosemary extracts exhibit potent antioxidant activities that reduce lipid peroxidation, the production of reactive oxygen species (ROS), and inflammation2, 3. Carnosol is a diterpene derived from rosemary where it is found in considerable quantities; approximately 0.2%–1%, in dried rosemary4, and 10.3% in commercially available rosemary extracts5. Although carnosic acid is the major polyphenolic compound present in rosemary plants, carnosol, an oxidation product of carnosic acid, has stronger anti-inflammatory effects6. Several studies including ours had been reported previously that the cytoprotective effects of carnosol have a number of beneficial properties from a medicinal standpoint, including antioxidation, anti-inflammation and anti-cancer effects in various cell types7, 8, 9. However, details of the mechanisms underlying the hepatitic cytoprotection of carnosol and its regulation remain to be elucidated.
To protect and survive against a variety of environmental or intracellular stresses, mammalian cells have developed robust cellular defensive systems, including mechanisms that alleviate oxidative stress. Among the factors involved in these defense responses are components of detoxifying systems, including phase II drug metabolizing enzymes such as glutathione S-transferase, NAD(P)H: quinone oxidoreductase, and UDP-glucuronosyltransferase10, and anti-oxidant enzymes such as glutamine-cysteine ligase (GCL)11, 12. Previous studies have shown that these enzymes are coordinately regulated through transcription factor NF-E2-related factor-2 (Nrf2) activation in response to electrophiles10. Carnosol possesses high electrophilic activity and has been reported to activate Nrf2-related phase II detoxifying enzyme genes and antioxidant enzymes13, 14. GCL is one of the most readily induced anti-oxidant genes and is rate-limiting for glutathione (GSH) synthesis15. GSH is a tripeptide that functions in the detoxification of chemical substances and is therefore a major cellular antioxidative defense molecule. Increased intracellular GSH may thus provide cytoprotective effects under conditions of oxidative stress or inflammation. To more fully understand the mechanisms underlying this effect, in our current study we examined the cytoprotective characteristics of rosemary essential oil and carnosol in a human hepatoma cell line, HepG2. Our findings demonstrate that both substances induce GCL expression and also an elevation of the intracellular GSH levels. In addition, the increased GSH levels were found to be associated with the inhibition of TNFα-induced NF-κB nuclear accumulation.
Materials and methods
Materials
Bacterially derived TNFα was purchased from Calbiochem (San Diego, CA). The p3xARE/Luc vector was constructed as described previously16. Antibodies raised against Nrf2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p65 antibodies was obtained from Stressgen Biotechnologies (SB, San Diego, CA, USA). ECL reagents were purchased from Pierce (Rockford, IL, USA). Luciferase assay kits were purchased from Promega (Madison, WI, USA). Peroxidase-conjugated anti-rabbit and anti-mouse antibodies were obtained from Amersham (Arlington Heights, IL, USA) and nitrocellulose was obtained from Schleicher & Schuell (Dassel, Germany). All other reagents, including carnosol and rosemary essential oil were purchased from Sigma (St Louis, MO, USA).
Cell culture
HepG2 cells (ATCC HB-8065) were grown in high glucose Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were seeded at a density of 1.5 million cells per mL in T-25 culture flasks with 5 mL of complete medium and cultured at 37 °C with 5% carbon dioxide. The culture medium was then replaced with serum free DMEM and the cells were incubated for 12 h prior to experimental treatment.
GSH Assay
GSH levels were determined as described previously17. Briefly, cells were cultured at 37 °C in the presence or absence of the treatment reagents indicated in the corresponding figures, washed with PBS and incubated with monochlorobimane (MCB, 40 μmol/L) in the dark for 20 min at room temperature. After two further washes with PBS, the cells were solubilized with 1% SDS and 5 mmol/L Tris HCl (pH 7.4). Fluorescence was measured by spectrofluorophotometry (Shimadzu, Rf-5301PC), with excitation and emission wavelengths of 405 and 510 nm, respectively and samples were assayed in triplicate. The assay for detecting in vitro GSH levels was performed identically but without cell lysates. The levels of intracellular GSH were quantified using a GSH solution as a standard.
Cell viability assay
Cell viability was performed using an Alamar blue assay kit (Serotec, Oxford, UK) in accordance with the manufacturer's instructions. This assay is based on the detection of metabolic activity in living cells using a redox indicator that changes from an oxidized (blue) to a reduced (red) form. The intensity of the red color is proportional to the viability of the cells, and is calculated by the difference in the absorbance values at 570 and at 600 nm and expressed as a percentage of the control.
RNA isolation and RT-PCR
Total cellular RNA was extracted using the phenol-guanidinium isothiocyanate method18. Equal amounts (5 μg) of RNA from the different treatments were then reverse-transcribed for 50 min at 42 °C using 50 units of Superscript II (Invitrogen, Carlsbad, CA). Amplifications of the cDNA were performed in 25 μL of PCR buffer (10 mmol/L Tris-HCl, 50 mmol/L KCl, 5 mmol/L MgCl2, and 0.1% Triton X-100, pH 9.0) containing 0.6 units of Taq DNA polymerase (Promega, Madison, WI) and 30 pmol of the specific primers GCLM forward, 5′-CAGCGAGGAGCTTCATGATTG-3′ reverse, 5′-TGATCACAGAATCCAGCTGTGC-3′ GCLC forward, 5′-GTTCTTGAAACTCTGCAAGAGAAG-3′ reverse, 5′-ATGGAGATGGTGTATTCTTGTCC-3′19 and GAPDH forward 5′-TATCGTGGAAGGACTCATGACC-3′ reverse 5′-TACATGGCAACTGTGAGGGG-3′. Reaction products were separated electrophoretically in a 2.5% agarose gel and stained with ethidium bromide.
Quantitative PCR
For transcript quantification purposes, real-time PCR was performed. Then 1 μL of the reverse-transcriptase product was used in a 25 μL volume reaction containing 200 μmol/L of each dNTP, 5 pmol of each primer, 1×PCR buffer II, 1.5 mmol/L MgCl2, 0.2×SYBR Green I (Molecular Probes), and 1.25 U of Amplitaq Gold (Applied Biosystems). Amplification was performed using ABI 7500 Real-Time PCR System (Applied Biosystems) programmed as 94 °C for 12 min followed by 40 cycles of (94 °C for 30 s, 57 °C for 20 s, 72 °C for 50 s). Variability in the initial quantities of cDNA was normalized to the internal control, GAPDH. A negative control was included in each set of experiments. Melting curve analysis was performed to enhance specificity of the amplification reaction, and the 7500 software was used to compare the amplification in the experimental samples during the log-linear phase.
Plasmids, transfections and luciferase assays
A NF-κB/Luc fragment containing tandem repeats of double-stranded oligonucleotides spanning the NF-κB binding site of ICAM-1: 5′-TGGAAATTCC-3′20, sense: 5′-CCCGGGTGGAAATTCCTGGAAATTCCTGGAAATTCCGGAGTCTAGA-3′, anti-sense: 5′-TCTAGACTCCGGAATTTCCAGGAATTTCCAGGAATTTCCACCCGGG-3′ was introduced into the pGL3 promoter plasmid (Promega, Madison, WI, USA). The HepG2 cells were then grown to 60%–80% confluence and transfected with a total of 1 μg of NF-κB/Luc or Nrf2/Luc16 using lipofectamine reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. For luciferase assays, the cell lysate was first mixed with luciferase substrate solution (Promega), and the resulting luciferase activity was measured in a luminometer. For each experiment, luciferase activity was determined in triplicate and normalized with respect to β-galactosidase activity levels.
Transient transfection with siRNA targeting Nrf2
An siRNA targeting human Nrf2 5′-UCCCGUUUGUAGAUGACAA-3′21 and a control siRNA 5′-GCAAGCUGACCCUGAAGUUCAU-3′ (non-sense) were purchased from Ambion (Austin, TX, USA). HepG2 cells were seeded onto 60-mm dishes, incubated for 24 h, and then transiently transfected with 100 nmol/L siRNA per dish at 90% confluence with Lipofectamine 2000. After 24 h of recovery in 10% serum medium, the cells were cultured in serum-free medium without serum for another 12 h prior to treatment.
Preparation of cytosolic and nuclear lysates
To separate the cytosolic and nuclear fractions from HepG2 cells, the cells were collected by scraping in cold PBS. The cell pellet was then lysed in 10 mmol/L HEPES, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L DTT, 0.5 mmol/L PMSF and 0.3% nonidet P-40. After 5 min of centrifugation (3000 rounds per minute at 4 °C) the supernatant was collected and designated as the cytosolic fraction. Nuclear proteins were then extracted from this preparation using a buffer containing 25% glycerol, 20 mmol/L HEPES, 0.6 mol/L KCl, 1.5 mmol/L MgCl2 and 0.2 mmol/L EDTA. Protein concentrations were determined using a protein assay DC system (Bio-Rad, Richmond, CA, USA).
Western blotting
Whole lysates of HepG2 were prepared as previously described22. A total of 1×106 cells were lysed on ice in lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and a protease inhibitor mixture) and whole-cell extracts were boiled for 5 min prior to separation on 10% SDS-PAGE, in which the protein samples were evenly loaded. The proteins were then transferred to a nitrocellulose filter in Tris-glycine buffer at 100 V for 1.5 h. The membranes were then blocked with PBS containing 5% nonfat milk and incubated with antibodies for two hours at 4 °C, with gentle shaking. The results were visualized by chemiluminescence using ECL (Pierce, Rockford, IL, USA), according to the manufacturer's instructions.
Statistical analysis
Overall treatment effects were examined by ANOVA. Post hoc analysis was also performed to detect differences between specific groups using the Dunnett's test (SPSS 12.0 software package, Chicago, IL, USA). A confidence limit of P<0.05 was considered to be significant.
Results
Carnosol and essential oil of rosemary increase the intracellular GSH levels at non-cytotoxic concentrations
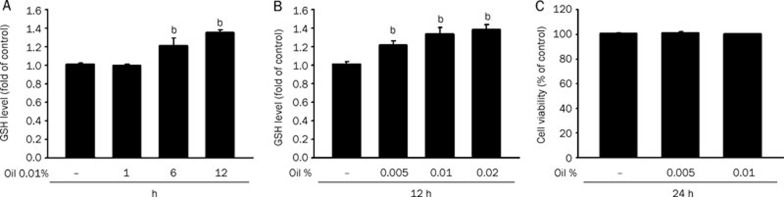
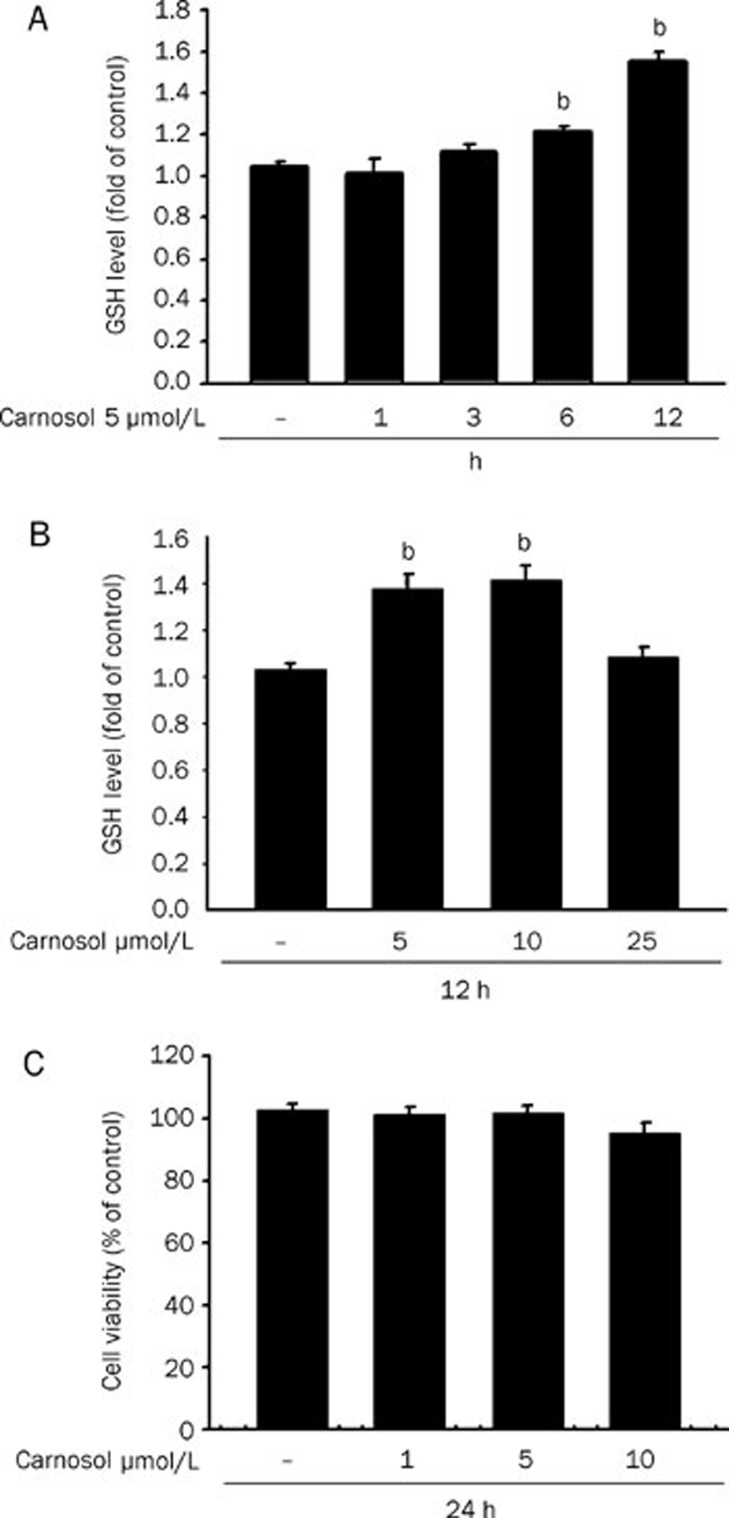
GSH is a well-studied tri-peptide and has numerous roles in protecting cells from oxidants and maintaining the cellular thiol redox status. We therefore tested the GSH levels in our current study in HepG2 cells exposed to essential oil or carnosol over a specific treatment period. As shown in Figure 1A and 2A, the GSH levels increased after 6 h of essential oil and carnosol treatment and persisted for over 12 h. Carnosol at 5 μmol/L increased GSH to near 160% of the starting levels after 12 h of treatment (Figure 2A). On the other hand, we subsequently detected a dose dependent increase in GSH levels following essential oil treatments for 12 h (Figure 1B and 2B). Significantly, an examination of the cytotoxic effects of these substances upon HepG2 cells using an Alamar blue assay indicated no adverse effects on cell viability upon exposure to 0.01% essential oil or 10 μmol/L carnosol (Figure 1C and 2C). Furthermore, there is no any detectable ROS production under 5 and 10 μmol/L carnosol treatment by using peroxide sensitive fluorescent probe 5-(and-6)-carboxy-2,7,dichlorodihydro fluorescein diacetate fluorescence assay (data not shown).
Figure 1.
The GSH levels are increased in HepG2 cells treated with rosemary essential oil. (A) The intracellular GSH levels of HepG2 cells incubated with 0.01% rosemary essential oil for 1, 6 and 12 h. Data values are expressed as a percentage of the untreated control, which was set at 100%. Results are the mean±SEM (n=3). bP<0.05 vs untreated cells. (B) The intracellular GSH levels of HepG2 cells incubated with the indicated doses of rosemary essential oil for 12 h. (C) Cell viability of HepG2 cells incubated with 0.005% and 0.01% rosemary essential oil for 24 h. Data are expressed as the mean±SEM of three independent experiments. No significant differences were found by ANOVA.
Figure 2.
Increased GSH levels in HepG2 cells treated with carnosol. (A) The intracellular GSH levels of HepG2 cells incubated with 5 μmol/L carnosol for 1, 3, 6, and 12 h. Data values are expressed as a percentage of the untreated control, which was set at 100%. Results are presented as the mean±SEM (n=3). bP<0.05 vs untreated cells. (B) The intracellular GSH levels of HepG2 cells incubated with the indicated doses of carnosol for 12 h. (C) Cells were incubated with the indicated doses of carnosol for 24 h and cell viability was measured. Data are expressed as the mean±SEM of three independent experiments. No significant differences were found by ANOVA.
Upregulation of both GSH and GSH synthesis enzyme levels in HepG2 cells by carnosol and essential oil of rosemary
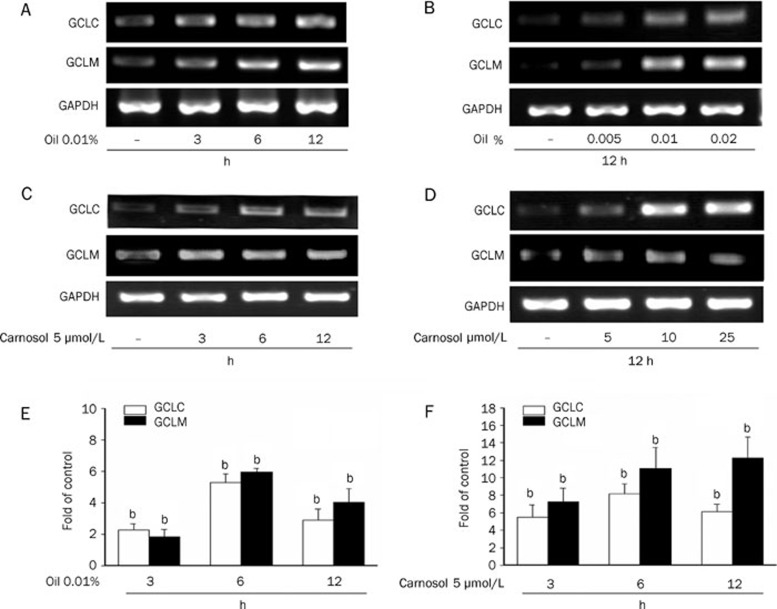
The rate-limiting enzyme in the de novo synthesis of GSH is glutamate-cysteine ligase (GCL), also known as γ-glutamylcysteine synthetase. GCL consists of a catalytic heavy subunit (GCLC) and a modulatory light subunit (GCLM). We assayed both GCLC and GCLM expression in essential oil and carnosol-treated HepG2 cells. Treatment with 0.01% essential oil increased both the GCLC and GCLM expression levels over the course of the incubation time (Figure 3A). As shown in Figure 3B, an increase in both the GCLC and GCLM mRNA levels was detected following essential oil treatments at 0.01% and 0.02% for 12 h. In a similar experiment, carnosol exposure increased both the GCLC and GCLM gene expression levels after three hours treatment (Figure 3C). In addition, carnosol at 5 μmol/L and 10 μmol/L concentrations was found to increase GCLC and GCLM gene expression over 12 h treatments (Figure 3D). To further determine the effect of 0.01% essential oil and carnosol, cells were incubated for different times. Quantitative PCR reactions were performed to amplify GCLC and GCLM, the production of GCLC and GCLM were increased significantly by 0.01% essential oil or carnosol as early as 3 h and reached a maximum by 6 h (Figure 3E and 3F).
Figure 3.
Upregulation of GSH synthesis enzyme levels in HepG2 cells by rosemary essential oil of and carnosol. The mRNA levels of GCLM and GCLC genes were assayed by RT-PCR in HepG2 cells. (A and C) Cells were treated with 0.01% rosemary essential oil or 5 μmol/L carnosol for the indicated time. (B and D) Cells were treated with the indicated doses of rosemary essential oil or carnosol for 12 h. (E and F) Cells were treated with 0.01% rosemary essential oil or 5 μmol/L carnosol for the indicated time. GCLM and GCLC were assayed by quantitative PCR. All samples were run in triplicate, the relative expression values were normalized to the expression value of GAPDH.
Effects of carnosol upon Nrf2 activation
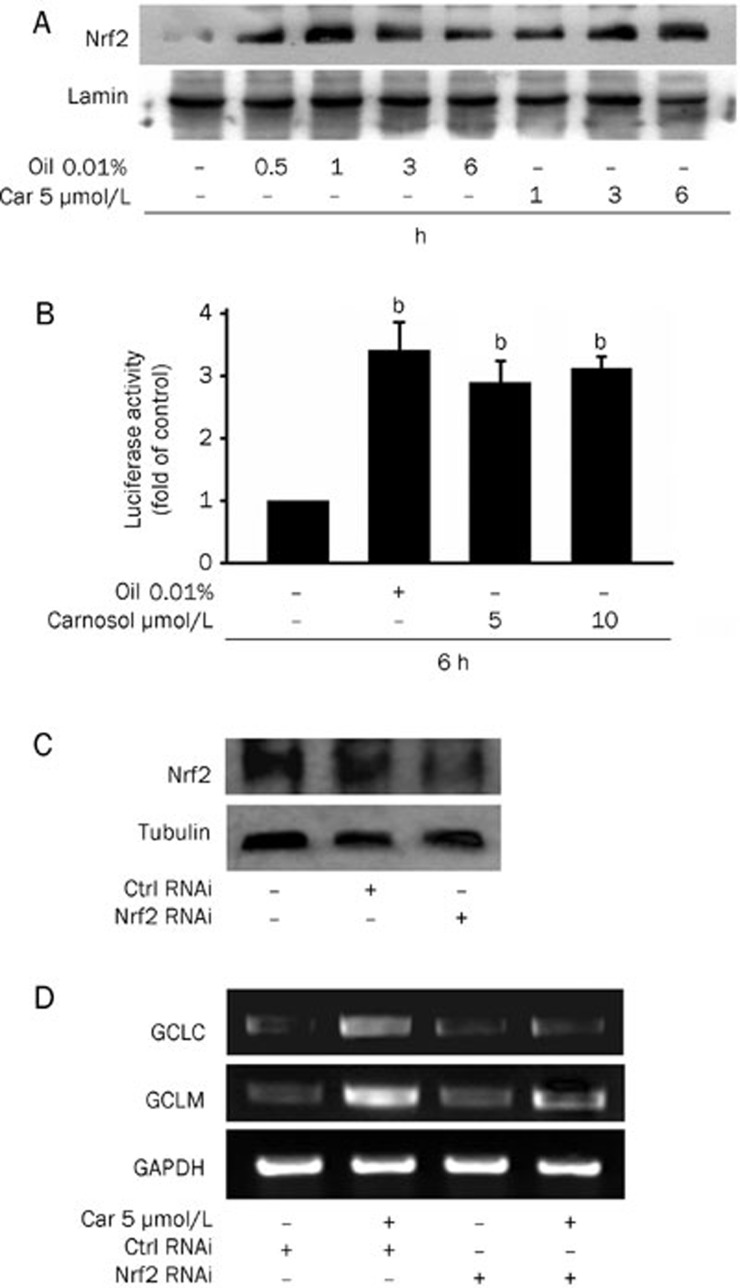
In our present study, we have found that 0.01% rosemary essential oil and carnosol (5 μmol/L) increase the level of Nrf2 in the nucleus of HepG2 cells. We thus evaluated the specificity of carnosol for Nrf2 binding site, antioxidant response element (ARE) sequences, in our current study by transfecting HepG2 cells with luciferase reporter constructs harboring this element. Cells treated with 0.01% essential oil, and 5 μmol/L or 10 μmol/L carnosol, indeed displayed increased ARE-luciferase activity (Figure 4B). To further assess the mediating role of Nrf2 in the inhibitory effects of carnosol, a more targeted inhibition of Nrf2 using siRNA was undertaken. Cells were transfected with Nrf2 siRNA to reduce the Nrf2 protein level (Figure 4C) and this abolished the induction of both GCLC and GCLM expression following 12 h of carnosol pretreatment (Figure 4D). These findings suggested that carnosol promotes GCLC and GCLM expression via the activation of the Nrf2 pathway.
Figure 4.
Effects of carnosol upon Nrf2 activation. (A) Nuclear extracts from HepG2 cells were prepared after treatment with 0.01% rosemary essential oil and 5 μmol/L carnosol for the indicated time periods. Immunoblots of nuclear lysates were then probed with Nrf2 specific antibodies. The nuclear lamin band intensities indicate equal loading of each well. (B) Cells were transfected with the ARE-luciferase construct (ARE) and then stimulated with 0.01% rosemary essential oil, 5 or 10 μmol/L carnosol. The cells were then lysed and analyzed for luciferase activity. Induction is indicated by an increase in the normalized luciferase activity in the treated HepG2 cells, relative to the control. Results are the means±SEM from at least three separate experiments. bP<0.05 vs untreated HepG2 cells. (C) HepG2 cells were transfected with control or Nrf2 siRNA for 36 h and the intracellular protein levels of Nrf2 were determined by Western blotting. (D) HepG2 cells were transfected with control or Nrf2 siRNA for 36 h and then exposed to 5 μmol/L carnosol for 12 h. The mRNA levels of GCLM and GCLC genes were the determined by RT-PCR.
The protective effects of carnosol and rosemary essential oil against oxidative stress and ethanol
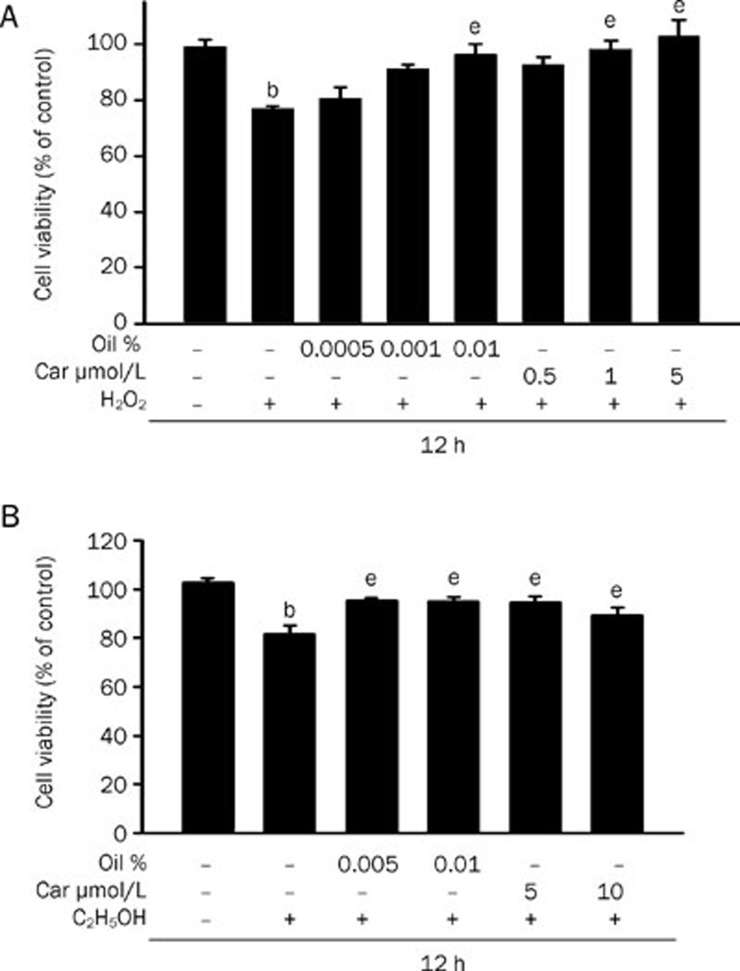
We investigated the effects of rosemary essential oil and carnosol on H2O2-induced cytotoxicity and as shown in Figure 5A, it was found that HepG2 cells treated with 3 mmol/L H2O2 showed significantly reduced cell viability. In contrast, this cytotoxicity was significantly reduced in cells pretreated with 0.01% essential oil or 5 μmol/L carnosol.
Figure 5.
The protective effects of rosemary essential oil and carnosol against oxidative stress and alcohol. Cells were initially preincubated for 12 h in the presence of the indicated doses of rosemary essential oil or carnosol. The medium was then removed and the cells were exposed to 3 mmol/L H2O2 for 12 h. Cell viability was measured spectrophotometrically using an Alamar blue assay. Data are expressed as the mean±SEM of at least three independent experiments. bP<0.05 vs control; eP<0.05 vs H2O2 alone. (B) Cells were initially preincubated for 12 h in the presence of the indicated doses of essential oil or carnosol. The medium was then removed and the cells were exposed to 50 mmol/L ethanol for 12 h. Data are expressed as the mean±SEM of at least three independent experiments. bP<0.05 vs control; eP<0.05 vs ethanol alone.
It is well-known that excess alcohol in the liver induces hepatotoxicity. To evaluate whether essential oil and carnosol had protective effects in this regard, we examined their effects upon cell viability in the presence of excess alcohol. The addition of 50 mmol/L ethanol to the culture medium tended to reduce cell viability (Figure 5B). In the presence of essential oil or carnosol however, no loss of cell viability was evident upon exposure to excess alcohol (Figure 5B). These results suggest that the protection against damage from oxidative stress or excess alcohol is dependent on an increased GSH level.
Carnosol inhibits TNFα-induced NF-κB activation via upregulated GSH
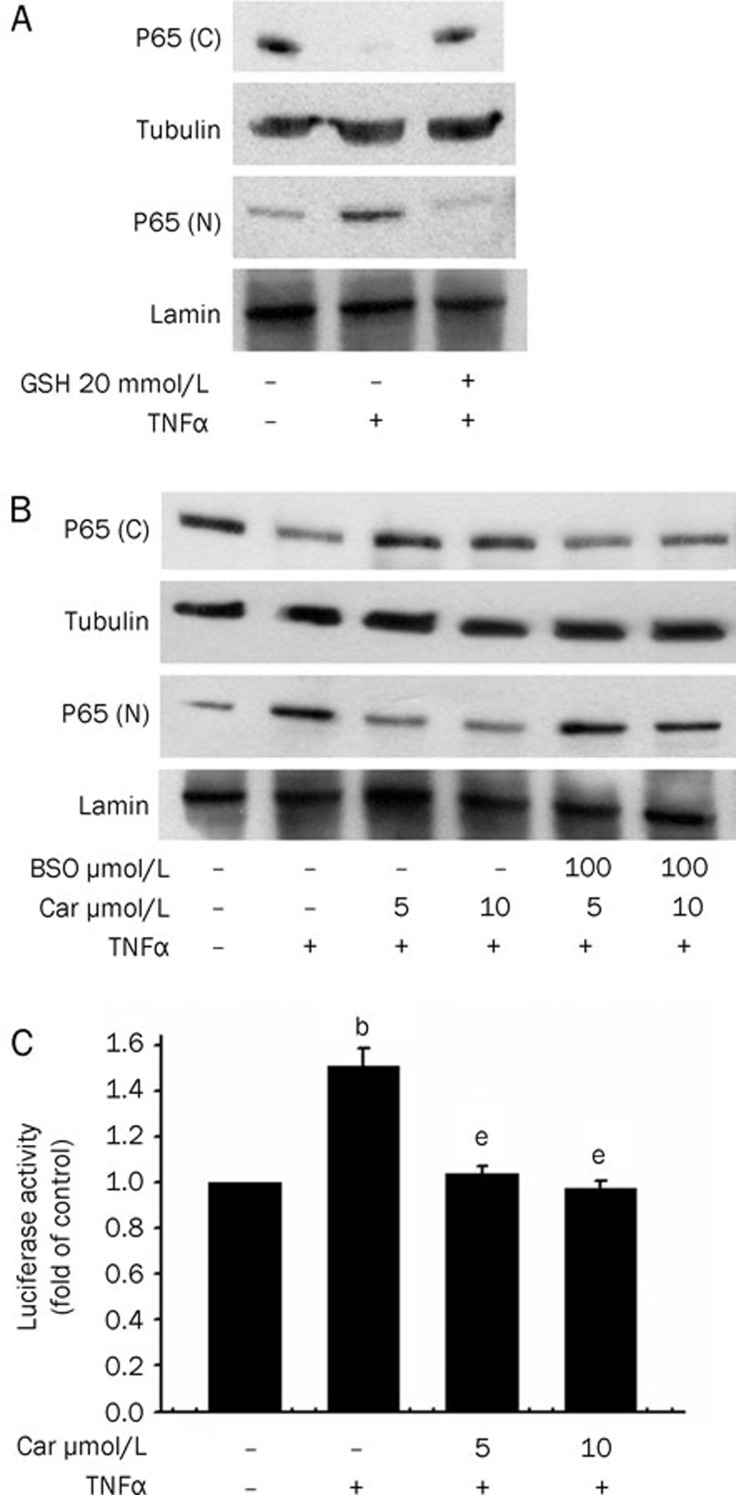
To evaluate its effects on NF-κB activation in HepG2 cells, cells were treated with GSH for 30 min before TNFα stimulation. As shown in Figure 6A, GSH indeed inhibits TNFα-induced NF-κB nuclear accumulation. We next investigated the possible role of GSH in mediating the inhibitory effects of carnosol in HepG2 cells. Pretreatment of cells with buthionine sulfoximine (BSO), a specific inhibitor of γ-glutamyl cysteine synthetase at concentrations of 100 μmol/L abolished the suppressive effects of carnosol upon NF-κB activation (Figure 6B). Furthermore, we tested whether carnosol inhibits TNFα-induced P65 activation at the transcriptional level. Following pretreatment for 12 h and a luciferase assay (Figure 6C), we found that TNFα-induced NF-κB activation was indeed inhibited. The results of these experiments indicate that the inhibitory effects of carnosol are the result of increased cellular GSH levels.
Figure 6.
Carnosol inhibits TNFα-induced NF-κB activation via increased GSH. (A) Cells were pretreated with 20 mmol/L GSH for 30 min and then stimulated with 100 U/mL TNFα. Nuclear (N) and cytosolic (C) extracts were then prepared and subjected to Western blot analysis with p65 antibodies. The nuclear lamin band intensities indicate equal loading of each well. (B) Cells were pretreated with 5 or 10 μmol/L carnosol for 12 h and then stimulated with 100 U/mL TNFα. Nuclear (N) and cytosolic (C) extracts were again prepared and subjected to Western blot analysis with p65 antibodies. (C) HepG2 cells were co-transfected with the NF-κB luciferase reporter construct and β-galactosidase for 16 h. Cells were then exposed to 5 μmol/L carnosol for 12 h and to 100 U/mL TNFα for a further 6 h. Luciferase activity was normalized against β-galactosidase activity; the untreated value was taken as 1. bP<0.05 vs untreated HepG2 cells; eP<0.05 vs TNFα alone (mean±SEM).
Discussion
GSH is a well-studied tri-peptide and has numerous roles in protecting cells from oxidants and maintaining the cellular thiol redox status23, 24. It was found in our previous study that cinnamaldehyde increases the cellular GSH levels in HepG2 cells after 9 h of exposure25. Carnosol is an electrophilic phytochemical present in the rosemary herb and our present study provides new evidence that both rosemary essential oil and carnasol enhance the GSH levels in HepG2 cells by upregulating the expression of GCLC and GCLM at non-cytotoxic concentrations. Our present experiments also show that the translocation of Nrf2 into the nucleus following treatment with rosemary essential oil and carnosol is associated with increases in its ARE transcriptional activity. We further demonstrate that Nrf-2 siRNA abolishes the induction of GCLC and GCLM by carnosol. Hence, the presence of activated Nrf-2 is required for the protective effects of carnosol. These protective effects of both rosemary essential oil and carnosol were manifested by the maintenance of cell viability under conditions of oxidative stress and following ethanol treatment. In addition, the inhibitory effects of carnosol upon TNFα-induced NF-κB activation are mediated through an increase in the intracellular GSH level.
Oxidative stress occurs when the redox equilibrium, which is the ability of cells to protect against damage caused by production of free radicals, is disrupted. Oxidative stress has been implicated as a major cause of cellular injuries in a variety of human diseases. Previous studies have found that polyphenolic compounds have intrinsic antioxidant characteristics and protect cells against oxidative cell damage via a Michael acceptor function. These compounds include curcumin, phenylethyl isothiocyanate, epigallocatechin gallate, and other green tea polyphenols7. Recently, much attention has focused on the upregulation of phase II detoxifying and antioxidant enzymes via the activation of the Nrf2 transcription factor15, 26. Carnosol, a diterpene derived from the rosemary herb, is a representative member of a family of plant-derived phenols13. Our present experiments show that carnosol induces Nrf2-mediated GCLC and GCLM expression, which in turn controls GSH synthesis27. Furthermore, our present data demonstrate that carnosol induces Nrf2 translocation and activates ARE-luciferase promoter activity, indicating that it directly induces Nrf2 via its ARE.
Recent studies have demonstrated that phytochemicals including resveratrol28, sulforaphane29, and epigallocatechin 3-O-gallate30, induce a GSH increase in HepG2 cells and thus play an important role in cytoprotection. Tumor necrosis factor-α (TNFα) is an inflammatory cytokine that causes liver cell injury by generating oxidative stress31. Since glutathione (GSH) is a key cellular antioxidant that detoxifies reactive oxygen species, we next examined the effects of carnosol-increased GSH in TNFα-treated cells. TNFα stimulation has been shown to activate NF-κB signaling pathways32. We found in our current analyses that carnosol increases the GSH levels after six hours of treatment, and that BSO abolishes p65 translocation after a 12-h incubation with carnosol. In addition, treatment with GSH alone also inhibits p65 translocation. Thus, an increased GSH level is essential for carnosol-induced anti-inflammatory effects. Previous studies have demonstrated that changes in the thiol redox state of the cell might affect the posttranslational modification of p65, including the phosphorylation of critical residues shown to contribute to the nuclear import of this molecule33, 34. Moreover, a recent study has revealed that a number of redox-sensitive transcription factors are modified by GSH and thereby inhibit their function35. The p65 modification by GSH is thus implicated as an inhibitory mechanism by which carnosol regulates NF-κB activation through the increase in GSH levels.
Our present data thus reveal that carnosol induces cytoprotective mechanisms that may contribute to its putative beneficial effects in suppressing the liver cell response to oxidative stress, alcohol or cytokines during inflammation. Our present results thus expand our understanding of the role of phytochemicals in cytoprotection and potentially assist in the identification of new therapeutic strategies for diseases caused by oxidative damage and other environmental stresses.
Author contribution
Chien-chung CHEN analyzed data and contributed analytic tools. Hui-ling CHEN performed research. Chia-wen HSIEH designed research. Yi-ling YANG contributed analytic tools. Being-sun WUNG designed research, analyzed data and wrote the paper.
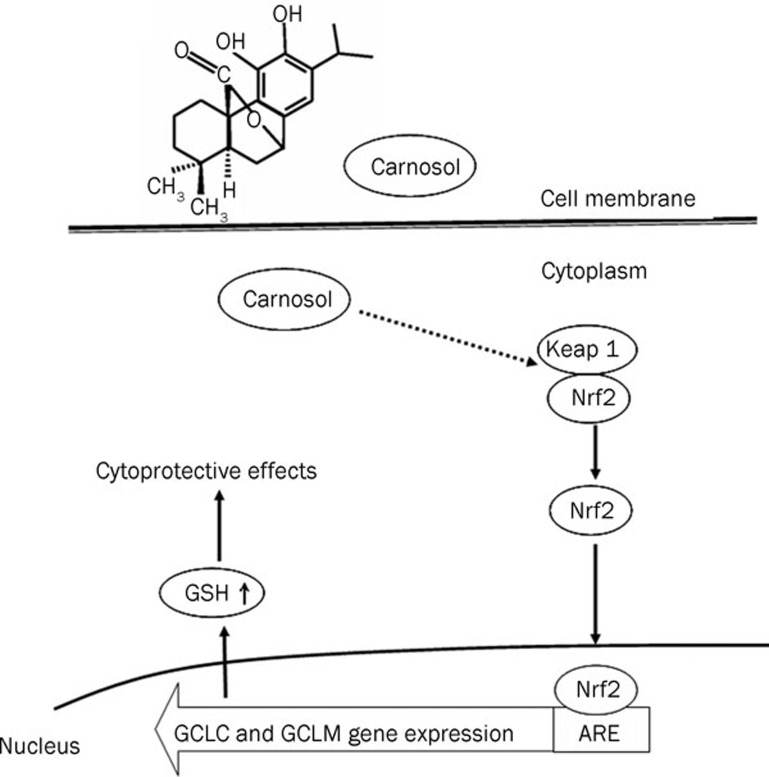
Figure 7.
Proposed model of the protective effects of carnosol via Nrf2 activation.
Acknowledgments
This work was supported in part by grants from Saint Martin De Porres Hospital and (97-2321-B-415-002) from the National Science Council, Taiwan, China. We thank Yuan-Jun LIN, for assistance in selected experiments.
References
- Zeng HH, Tu PF, Zhou K, Wang H, Wang BH, Lu JF. Antioxidant properties of phenolic diterpenes from Rosmarinus officinalis. Acta Pharmacol Sin. 2001;22:1094–8. [PubMed] [Google Scholar]
- Aruoma OI, Spencer JP, Rossi R, Aeschbach R, Khan A, Mahmood N, et al. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs. Food Chem Toxicol. 1996;34:449–56. doi: 10.1016/0278-6915(96)00004-x. [DOI] [PubMed] [Google Scholar]
- Peng CH, Su JD, Chyau CC, Sung TY, Ho SS, Peng CC, et al. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci Biotechnol Biochem. 2007;71:2223–32. doi: 10.1271/bbb.70199. [DOI] [PubMed] [Google Scholar]
- del Baño MJ, Lorente J, Castillo J, Benavente-Garcia O, del Rio JA, Ortuño A, et al. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis antioxidant activity. J Agric Food Chem. 2003;51:4247–53. doi: 10.1021/jf0300745. [DOI] [PubMed] [Google Scholar]
- Frankel EN, Huang SW, Aeschbach R, Prior E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid carnosol and rosmarinic acid in bulk oil and oil-in-water emulsion. J Agric Food Chem. 1996;44:131–5. [Google Scholar]
- Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, Hörnig C, et al. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91–7. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983–91. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- Sotelo-Félix JI, Martinez-Fong D, Muriel De la Torre P. Protective effect of carnosol on CCl(4)-induced acute liver damage in rats. Eur J Gastroenterol Hepatol. 2002;14:1001–6. doi: 10.1097/00042737-200209000-00011. [DOI] [PubMed] [Google Scholar]
- Lian KC, Chuang JJ, Hsieh CW, Wung BS, Huang GD, Jian TY, et al. Dual mechanisms of NF-κB inhibition in carnosol-treated endothelial cells. Toxicol Appl Pharmacol. 2010;245:21–35. doi: 10.1016/j.taap.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–9. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–307. [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, et al. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical Carnosol. J Biol Chem. 2004;279:8919–29. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, et al. Carnosic acid protects neuronal HT22 cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–5. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radical Biol Med. 2004;37:433–41. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889–97. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Kamencic H, Lyon A, Paterson PG, Juurlink BH. Monochlorobimane fluorometric method to measure tissue glutathione. Anal Biochem. 2000;286:35–7. doi: 10.1006/abio.2000.4765. [DOI] [PubMed] [Google Scholar]
- Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein-1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- Neurohr C, Lenz AG, Ding I, Leuchte H, Kolbe T, Behr J. Glutamate-cysteine ligase modulatory subunit in BAL alveolar macrophages of healthy smokers. Eur Respir J. 2003;22:82–7. doi: 10.1183/09031936.03.00080403. [DOI] [PubMed] [Google Scholar]
- van de Stolpe A, Caldenhoven E, Stade BG, Koenderman L, Raaijmakers JA, Johnson JP, et al. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J Biol Chem. 1994;269:6185–92. [PubMed] [Google Scholar]
- Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–84. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wung BS, Cheng JJ, Chao YJ, Hsieh HJ, Wang DL. The modulation of Ras-Raf-ERK pathway by reactive oxygen species is involved in cyclic strain-induced early growth response-1 gene expression in endothelial cells. Circ Res. 1999;84:804–12. doi: 10.1161/01.res.84.7.804. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–26. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Sen CK. Cellular thiols and redox-regulated signal transduction. Curr Top Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun YW, Wung BS. Cinnamaldehyde inhibits the tumor necrosis factor-alpha-induced expression of cell adhesion molecules in endothelial cells by suppressing NF-kappaB activation: effects upon IkappaB and Nrf2. Toxicol Appl Pharmacol. 2008;229:161–71. doi: 10.1016/j.taap.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997;272:7445–54. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMPK-mediated GSK3{beta} inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–95. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- Kim BR, Hu R, Keum YS, Hebbar V, Shen G, Nair SS, et al. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003;63:7520–5. [PubMed] [Google Scholar]
- Murakami C, Hirakawa Y, Inui H, Nakano Y, Yoshida H. Effects of epigallocatechin 3-O-gallate on cellular antioxidative system in HepG2 cells. J Nutr Sci Vitaminol (Tokyo) 2002;48:89–94. doi: 10.3177/jnsv.48.89. [DOI] [PubMed] [Google Scholar]
- Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–8. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor-kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–15. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, et al. Transient and selective NF-kappaB p65 serine 536 phosphorylation induced by T cell costimulation is mediated by IkappaB kinase beta and controls the kinetics of p65 Nuclear Import. J Immunol. 2004;172:6336–44. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein glutathionylation. Antioxid Redox Signal. 2008;10:1941–88. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]