Abstract
Aim:
To investigate the protective or lethal role of autophagy and the effects of Salvianolic acid B (Sal B) on autophagy in starving myocytes.
Methods:
Cardiac myocytes were incubated under starvation conditions (GD) for 0, 1, 2, 3, and 6 h. Autophagic flux in starving cells was measured via chloroquine (3 μmol/L). After myocytes were treated with Sal B (50 μmol/L) in the presence or absence of chloroquine (3 μmol/L) under GD 3 h, the amount of LC3-II, the abundance of LC3-positive fluorescent dots in cells, cell viability and cellular ATP levels were determined using immunoblotting, immunofluorescence microscopy, MTT assay and luminometer, respectively. Moreover, electron microscopy (EM) and immunofluorescent duel labeling of LC3 and Caspase-8 were used to examine the characteristics of autophagy and apoptosis.
Results:
Immunoblot analysis showed that the amount of LC3-II in starving cells increased in a time-dependent manner accompanied by increased LC3-positive fluorescence and decreased cell viability and ATP content. Sal B (50 μmol/L) inhibited the increase in LC3-II, reduced the abundance of LC3 immunofluorescence and intensity of Caspase-8 fluorescence, and enhanced cellular viability and ATP levels in myocytes under GD 3 h, regardless of whether chloroquine was present.
Conclusion:
Autophagy induced by starvation for 3 h led to cell injury. Sal B protected starving cells by blocking the early stage of autophagic flux and inhibiting apoptosis that occurred during autophagy.
Keywords: Salvianolic acid B, autophagy, apoptosis, cardiac myocyte, starvation
Introduction
Autophagy is a highly conserved cellular mechanism that plays a key role in the turnover of long-lived proteins, RNA, and dysfunctional organelles. In starvation or stress conditions, autophagy represents an adaptive strategy by which cells clear damaged organelles and survive nutritional, bioenergetic stress1. It is becoming clear that autophagy might be more important in terminally differentiated cell types, such as cardiac myocytes2.
Basal autophagy activity is essential for the heart to maintain homeostasis3; however, increasing evidence suggests that autophagy constitutes a second mode of programmed cell death (Type II PCD) that is distinct from apoptosis (Type I PCD)4. Autophagy has been observed in both hypertrophied myocardium and failing myocardium, which is caused by dilated cardiomyopathy, vascular disease and ischemic heart disease5. Therefore, the regulation of autophagy by pharmacological approaches is a potential therapeutic strategy to treat heart diseases.
The autophagy-related genes (Atg) have been isolated and characterized in yeast and mammals6. In mammalian cells, LC3, one of the homologues of yeast Atg8, is modified via an ubiquitylation-like system. The carboxy-terminal region of LC3 is cleaved, generating a soluble form known as LC3-I and exposing a carboxy-terminal glycine essential for additional activity. LC3-I, in turn, is modified to a membrane-bound form, LC3-II (an LC3-phospholipid conjugate), by mammalian Atg7 and Atg3 homologues, prompting its localization to autophagosomes7. Thereafter, autophagosomes fuse with lysosomes, forming autolysosomes, to degrade engulfed cytosolic components. Thus, the amount of LC3-II in mammalian cells is a good early marker for the formation of autophagosomes7.
The cellular level of LC3-II and the presence of numerous autophagosomes are generally considered to be synonymous with increased autophagic activity. But autophagy is a dynamic process that reflects the formation of autophagosomes and their clearance subsequent to lysosomal fusion. The presence of numerous autophagosomes can reflect two different conditions: an increase in autophagosome formation or a decrease in autophagosome clearance due to frustrated autophagy. Frustrated autophagy is characterized by failure of lysosomal fusion, leading to the accumulation of autophagosomes, which may eventually cause catastrophic leakage of lysosomal proteases and cell death8.
We therefore used the lysosomal inhibitor chloroquine to measure autophagic flux, which reflected the dynamic process of autophagy and could help to estimate whether autophagy is frustrated during the process.
It has been reported that Sal B protects cardiac myocytes against ischemia-reperfusion injury through inhibition of platelet activation in cardiovascular endothelial cells9, regulation of the balance of the ET/NO10 and TXA2/PGI2 systems11 in coronary arteries, raising SOD activity12, and inhibition of calcium overload13, but not through the regulation of autophagy. To our knowledge, this study is the first to explore the potential mechanism by which Sal B mediates autophagy.
The goals of our study were the following: (1) to investigate the protective or lethal role of autophagy in starvation-induced cardiac myocytes using a time-course experiment; (2) to verify the effects of Sal B on autophagy in starving myocytes; and (3) to observe whether apoptosis occurs in myocytes during autophagy and determine the effect of Sal B on these autophagic myocytes, because there is crosstalk between the autophagic and apoptotic pathways14.
Materials and methods
Primary culture of neonatal rat ventricular myocytes
Primary cultures of ventricular cardiac myocytes were prepared from 1-day-old SD rats (Vital River Laboratory Animal Technology Co Ltd, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO) with 10% fetal bovine serum (FBS, GIBCO). Most cells beat spontaneously in a confluent monolayer 48–72 h after plating.
Starvation and drug treatment of cells
To obtain starvation conditions, cardiac myocytes were washed three times with phosphate-buffered saline and incubated in glucose-free, serum-free DMEM (GD, Invitrogen) at 37 °C for 0, 1, 2, 3, and 6 h, with cells cultured in nutrient-rich medium as a control. For drug treatment study, cells were treated with Sal B (50 μmol/L, National Institute for the Control of Pharmaceutical and Biological Products) with or without chloroquine (3 μmol/L, Sigma) under GD condition for 3 h. After treatment, cells were analyzed as described below. Incubation of cardiac myocytes with chloroquine (3 μmol/L) and/or Sal B (50 μmol/L) for 24 h under normal culture conditions did not result in cytotoxicity (data not shown).
Electron microscopy (EM)
Cardiac myocytes were centrifuged for collection, fixed with 4% glutaraldehyde, post-fixed with 1% osmium tetroxide, stained with uranyl acetate, and embedded in Epon. Ultrathin sections were observed using an electron microscope (Hitachi, H7500, Japan).
Cell viability of cardiac myocytes
Cell viability was measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, Amersco] assay. In brief, cardiac myocytes (1×105 per 100 μL) were seeded in 96-well plates. After treatment, 20 μL of MTT solution (5 mg/mL) was added to each well and incubated (37 °C, 5% CO2) for 4 h. Next, the media was removed and the wells were allowed to dry. The MTT metabolic product was resuspended in 200 μL of DMSO and placed on a shaking table for 5 min. At this point, the absorbance (optical density) was measured at 560 nm using a microplate reader.
Measurement of intracellular ATP content
Intracellular ATP content was measured using an ATP bioluminescent assay kit (Beyotime Institute of Biotechnology, China). Cells were lysed directly in somatic cell ATP-releasing agent, and the lysates were assayed according to the manufacturer's instructions using a 1:25 dilution of the ATP assay mix. Light emitted was measured using a luminometer. ATP content was calculated by comparison with a standard curve derived from known concentrations of ATP.
Immunoblot analyses
Following treatment, cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, China). Protein concentrations were determined using the BCA protein assay reagent (Beyotime Institute of Biotechnology, China). Equal protein amounts (10 μg) were electrophoresed in an sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to a polyvinylidene fluoride membrane, which was blocked with 3% BSA and incubated with rabbit anti-LC3 antibody (Sigma, St Louis, MO, USA) or rabbit anti-α-actin antibody (Beijing Biosynthesis Biotechnology, China) overnight at 4 °C. Membranes were then incubated with HRP-conjugated anti-rabbit IgG (Sigma, St Louis, MO, USA) for 1 h at room temperature. All primary antibodies were used at a dilution of 1:2000 and the secondary antibody was used at a dilution of 1:40 000. Blots were developed with ECL reagent (Thermo, USA) and exposed to film. Bands were analyzed using BIO-RAD Quantity One-4.6.2.
Immunofluorescence analysis
Cardiac myocytes growing on gelatinized coverslips were fixed with 100% ethanol for 30 min and blocked in immunostaining blocking buffer for 1 h at room temperature. The fixed cells were incubated with rabbit anti-LC3 antibody (1:100) at 4 °C overnight. Cellular LC3 protein was stained by incubating with FITC conjugated anti-rabbit IgG (1:150) (Zhongshan Goldbridge Biotechnology Co Ltd, China) for 1 h at room temperature. Nuclei were dyed with DAPI (Roche).
For dual labeling studies, LC3 (1:100) and Caspase-8 (1:300) (Santa Cruz) primary antibodies were simultaneously added to cells followed by incubation with secondary antibodies labeled with FITC (1:150) for LC3 and Cy3 (1:500) (CW Biotechnology Co LTD, China) for Caspase-8.
Immunofluorescence of cells was visualized using an immunofluorescence microscope (Olympus IX81, Japan). Images were captured with a CCD camera (ROLERA-MGi PLUS), and image contrast was adjusted using Image-Pro Analyzer 6.3 software.
Statistical analysis
Data were expressed as means±SD. ANOVA was performed, and significance was assumed when the P value was less than 0.05.
Results
Endogenous LC3-II accumulation in starving cardiac myocytes led to cell injury in a time-dependent manner
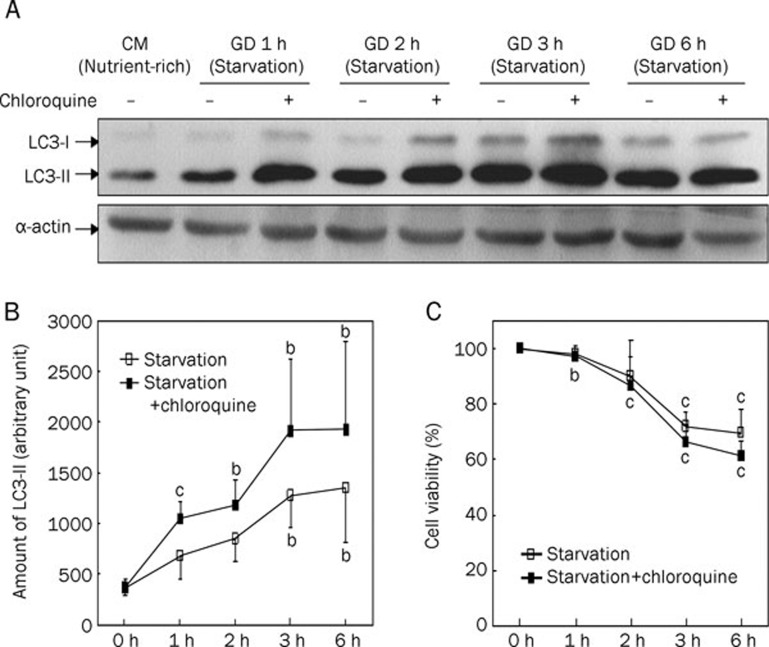
To investigate the starvation condition, cardiac myocytes were cultured under GD for 1, 2, 3, and 6 h, with myocytes cultured in nutrient-rich conditions (GD 0 h) serving as a control. Immunoblot analysis showed that the amount of LC3-II increased in a time-dependent manner in myocytes under starvation conditions (Figure 1A, 1B).
Figure 1.
The accumulation of LC3-II in cardiac myocytes under starvation condition led to cell injury in a time-dependent manner. For nutrient-rich condition, cells were cultured in completed medium (CM). For starvation condition, cells were incubated for 1, 2, 3, and 6 h in glucose-free, serum-free DMEM medium (GD) in presence (+) or absence (−) of lysosomal inhibitor chloroquine (3 μmol/L). The cells were lysed and endogenous LC3 in the lysates was recognized by immunoblotting with an anti-LC3 or anti-α-actin antibody (WB: anti-LC3 or anti-α-actin). A) Representative immunoblots of LC3. LC3-I, soluble form of LC3; LC3-II, membrane-bound form of LC3. Western blotting of α-actin showed equal loading of the samples. B) Densitometric analyses of endogenous LC3-II in cardiac myocytes (A). The relative amount of LC3-II was shown. C) Cell viability of cardiac myocytes under starvation conditions in time-course experiment. Data are mean of three culture preparations. bP<0.05, cP<0.01 vs CM/Starvation 0 h.
It was not evident whether the observed increase in LC3-II was due to active autophagy or low autophagic flux (ie impaired fusion with lysosomes). Therefore, we used chloroquine, a lysosomal inhibitor that inhibits lysosome-autophagosome fusion, to measure autophagic flux. In the presence of chloroquine (3 μmol/L), endogenous LC3-II dramatically increased in starving cells (Figure 1A, 1B). The amount of LC3-II in cells treated with chloroquine was about 1.5-fold higher than in untreated control cells at 3 h. These results suggest that autophagy was successfully induced, and LC3-II was rapidly degraded by lysosomal hydrolases, indicating high autophagic flux under starvation conditions.
We also examined cell viability over a time-course. As shown in Figure 1C, the viability of cells subjected to 1-h starvation was similar to that of cells in nutrient-rich conditions. However, 2-h starvation resulted in a slight decrease in cell viability, and 3-h starvation resulted in cell viability that was obviously decreased, indicating that starving for 3 h could be lethal to myocytes. Chloroquine interrupted the autophagy process by inhibiting autophagosome-lysosome fusion and led to more serious cell injury.
In the following experiments, we investigated the effects of Sal B on autophagy regulation in cardiac myocytes subjected to starvation for 3 h.
Sal B inhibited the accumulation of LC3-II and LC3-positive fluorescence dots in starving cardiac myocytes
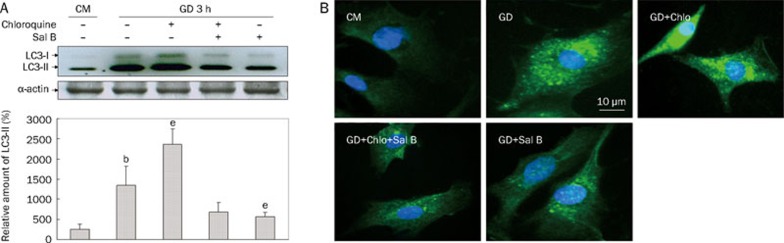
Based on the results of the time-course experiment above, we investigated the regulatory effects of Sal B on autophagy in myocytes subjected to 3-h starvation (Figure 2). In this experiment, immunoblot analysis showed that starvation caused an increase in endogenous LC3-II. This increase was augmented by coadministration of chloroquine, indicating that the increase in LC3-II under starvation conditions was due to increased flux, not diminished clearance. The increased flux resulting from starvation could be blocked by Sal B (Figure 2A).
Figure 2.
Sal B decreased the accumulation of LC3-II and LC3-positive fluorescence dots in starving cardiac myocyes. Cells were cultured in GD 3 h in presence (+) or absence (−) of chloroquine (Chlo, 3 μmol/L) or Sal B (50 μmol/L) with cells in CM as control. Cell lysates were subjected to immunoblots for detection of LC3-I, LC3-II, and α-actin. (A) Representative immunoblots of LC3 and the relative amount of LC3-II were shown. Western blotting of α-actin showed equal loading of the samples. (B) Representative images of LC3-positive fluorescence. Green fluorescence presented LC3-FITC staining of autophagosomes. Blue fluorescence indicated DAPI-stained nucleus. Data are mean of three culture preparations. bP<0.05 vs CM, eP<0.05 vs GD.
Although the accumulation of endogenous LC3-II under starvation was confirmed, its intracellular localization was not evident. Using immunofluorescence microscopy, we examined the intracellular localization of endogenous LC3 in cardiac myocytes during starvation-induced autophagy in the presence or absence of chloroquine. As shown in Figure 2B, the immunofluorescence of LC3-FITC was seen as weak and diffuse cytoplasmic staining in cells cultured in nutrient-rich conditions. Strong punctate staining of LC3-FITC was induced in cells under starvation conditions. This staining appeared as multiple distinct dots connected together, localized more abundantly in the cytoplasm than in the nucleus. Because chloroquine inhibits autophagosome-lysosome fusion during autophagy, we observed that LC3-positive punctate staining was significantly increased under starvation conditions. Sal B-treated starving myocytes presented fewer fluorescent LC3 dots in the presence or absence of chloroquine, indicating that Sal B blocked the autophagic flux.
Together with the decrease of immunofluorescent LC3 puncta in the cells, the reduction of endogenous LC3-II observed by immunoblot indicated that Sal B blocked the autophagic flux in cardiac myocytes and inhibited autophagy induced by starvation.
Sal B enhanced cell viability and cellular ATP content of starving myocytes
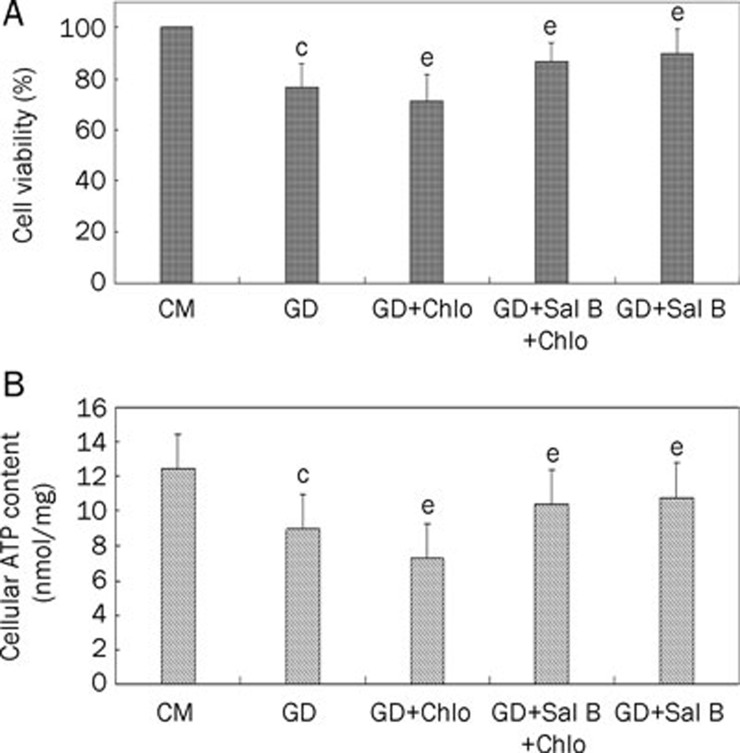
To verify the protective role of Sal B on autophagy-induced myocytes, cell viability and cellular ATP content were assayed. Under starvation conditions, cell viability and ATP content were significantly decreased in the presence of chloroquine. Sal B increased the ATP content and viability of starving cells regardless of the presence or absence of chloroquine (Figure 3).
Figure 3.
Sal B enhanced cell viability and cellular ATP content of starving myocytes. Cells were treated with CM or GD for 3 h in presence or absence of chloroquine (Chlo, 3 μmol/L) or Sal B (50 μmol/L). (A) Cell viability. Percentage of viability was shown. (B) Cellular ATP content. Data shown are from three independent experiments. cP<0.01 vs CM; eP<0.05 vs GD.
Our findings indicate that Sal B protected starving cardiac myocytes by inhibiting autophagy.
Evidence of autophagy and apoptosis in starving cardiac myocytes
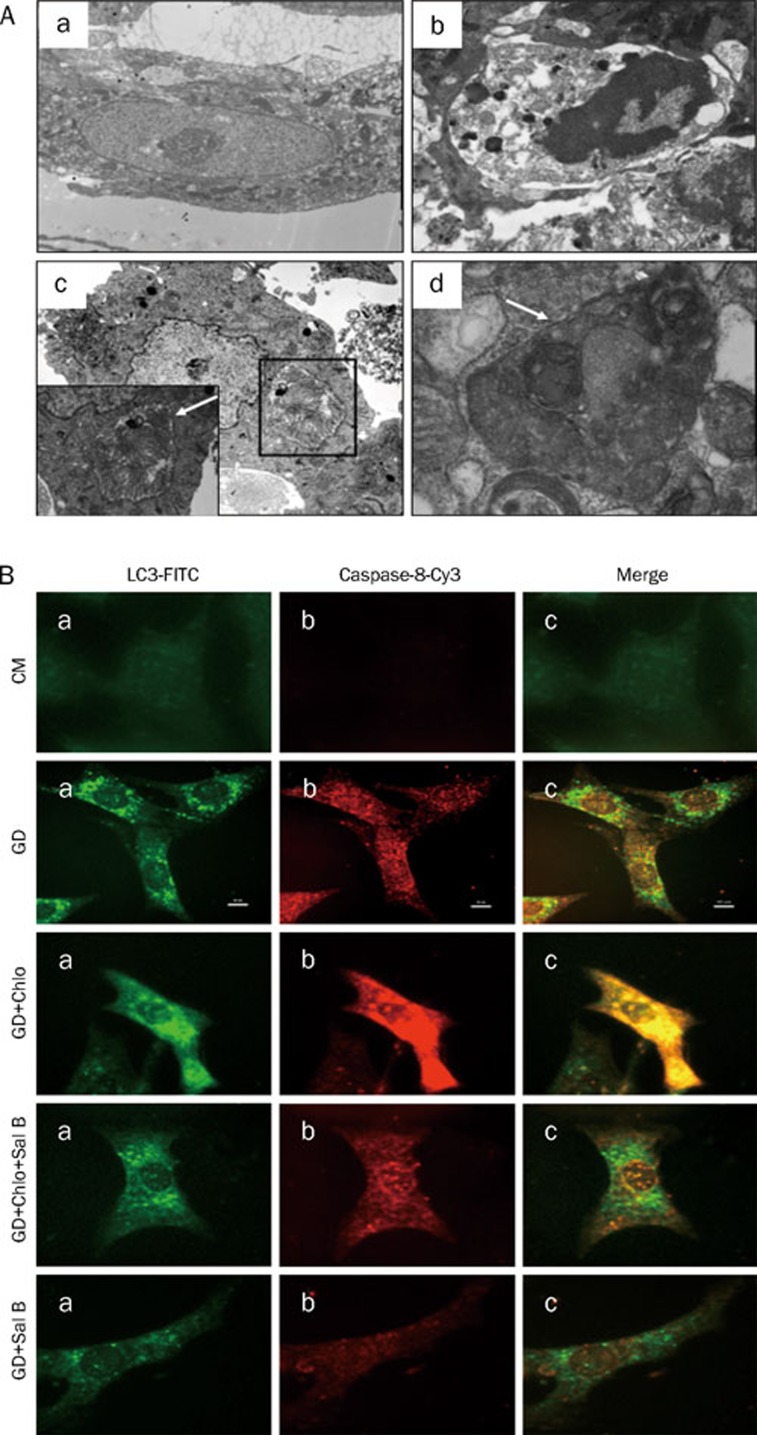
Given the molecular connections between autophagy and apoptosis, we used EM to evaluate these two processes by examining characteristic structures. As shown in Figure 4A, different types of autophagosomes, which contained heterogeneous organelles ranging from mitochondria to multivesicular bodies surrounded by a sequestering membrane, were observed in myocytes under GD for 3 h (Figure 4, Ac–Ad). In addition to autophagosomes, apoptosis was observed in starving cardiac myocytes (Figure 4, Ab) with altered nuclear morphology, including chromatin condensation and fragmentation and cell shrinkage.
Figure 4.
Evidences of autophagy and apoptosis in starving cardiac myocytes. (A) Representative electron micrograph image of autophagy and apoptosis. Cells were cultured in CM or GD for 3 h. (a) Cardiac myocyte under CM (×20 000). (b) Apoptosis morphology in myocyte under GD for 3 h (×8000). (c) Autophagosome, dysfunctional mitochondrias are sequestrated in a doubl emembrane-bound vesicle (×4000). (d) Autophagosome, cytoplasmic proteins and dysfunctional mitochondrias are sequestrated in a doublemembrane-bound vesicle (×10 000). Autophagosomes are indicated by arrows. (B) Representative images of LC3-Capase-8 dual labeling. Myocytes were cultured in CM or GD for 3 h in presence or absence of chloroquine (Chlo, 3 μmol/L) or Sal B (50 μmol/L) as indicated. The cells were fixed and permeabilized with digitonin, and endogenous LC3 and Caspase-8 were recognized using FITC-conjugated or Cy3-conjugated antibody respectively.
Dual fluorescent labeling of LC3 and Caspase-8 demonstrated the presence of autophagy and apoptosis in starving cardiac myocytes (Figure 4B, 4C). In control myocytes, green fluorescent staining of LC3 was diffusely localized to the cytoplasm, with or without weak Caspase-8 fluorescence. However, 3 h after GD, punctuate LC3 fluorescence was present, with some of these dots connected together, accompanied by intense Caspase-8 fluorescence in the cytoplasm and the nucleus. LC3 and caspase-8 exhibited a positive correlation. Chloroquine enhanced the number of fluorescent LC3 puncta and the intensity of Caspase-8 signal. Starving myocytes treated with Sal B alone or co-treated with chloroquine exhibited fewer LC3 puncta and less intense Capase-8 fluorescence (Figure 4B).
Together, these data indicate that apoptosis occurred in autophagy-induced myocytes. Sal B demonstrated inhibitive effects on autophagy and apoptosis.
Discussion
Whether autophagy plays a protective role or a lethal role in ischemic myocardium remains controversial. Several studies reported a role for autophagy in programmed cell death type II (nonapoptotic death) in heart disease15, 16 and indicated that excessive autophagy could lead to cell death. Valentim et al17 showed that inhibition of autophagy, by both genetic and pharmacological inhibition of Beclin 1, reduced cell death in cardiomyocytes subjected to simulated ischemia-reperfusion. In line with this evidence, heterozygous Beclin 1+/− mice exhibited a reduction in autophagy and apoptosis and reduced infarct size in the heart after ischemia-reperfusion injury18. Matsui et al18 found that autophagy might be protective during ischemia, but might play a detrimental role during reperfusion. Taken together, these studies suggest that autophagy is typically stimulated by nutrient starvation and has dual roles in the heart. The conditions under which autophagy provides protection or induces cell death depend on the intensity of insult and duration of autophagy19.
In our study, we used nutrient starvation in cultured cardiac myocytes to mimic myocardium ischemia in vitro. In this model, we first studied autophagy intensity over four timepoints. According to immunoblot analysis, the amount of LC3-II was markedly increased in a time-dependent manner.
Increased levels of LC3-II alone do not constitute conclusive evidence of autophagy without considering lysosomal turnover of LC3-II. An increase in the amount of endogenous LC3-II could be due either to active autophagy or to frustrated autophagy. In the setting of frustrated autophagy, autophagosomes form and engulf targets but cannot fuse with lysosomes and clear their contents. This may elicit an acute and significant inflammatory response, or it may induce self-digestion and cell death8.
In order to distinguish active autophagy and frustrated autophagy from each other, we assayed autophagic flux using lysosomal inhibitors. Flux reflects the dynamic process of autophagosome formation, engulfment, and lysosomal fusion. Chloroquine, a lysosomal inhibitor, is used to verify that increased numbers of autophagosomes reflect increased flux20. Comparing the number of autophagosomes in the presence and absence of chloroquine enables one to distinguish between increased autophagosome formation and decreased clearance; thus, it can provide a quantitative index of autophagic flux. In our time-course experiment, the amount of LC3-II increased in a time-dependent manner during autophagy. Administration of chloroquine to starving cardiac myocytes further enhanced the accumulation of LC3-II, indicating high autophagic flux in cells under starvation without chloroquine, in which autophagy was successfully induced and the LC3-II was rapidly degraded by lysosomal hydrolases.
To investigate whether the accumulation of LC3-II is beneficial or harmful to starving cells, cell viability was measured. We found that cells subjected to starvation for 1 or 2 h could maintain relatively high viability; however, when the insult of starving lasted 3 h, cell viability dramatically decreased, indicating that autophagy had surpassed its capacity to protect cardiac myocytes against starvation stress.
Next we examined the effect of Sal B on autophagy under starvation conditions lasting 3 h. The amount of LC3-II in starving myocytes increased, reflecting the activation of autophagy. The coadministration of chloroquine provided us with additional information about high autophagic flux in cells. Sal B decreased the amount of LC3-II in starving myocytes in the presence or absence of chloroquine, which could be interpreted to mean that Sal B inhibited the early stage of autophagy and blocked autophagic flux because Sal B inhibited the formation of autophagosomes. If this were the case, the accumulation of LC3 would not have occurred, even in the presence of chloroquine.
Starving cells presented many distinct puncta of LC3 fluorescence, which increased in abundance in the presence of chloroquine. After Sal B treatment, starving cells exhibited a diminished pattern of LC3 fluorescence. These results, consistent with our immunoblot results, suggest that Sal B may have suppressive effects on autophagy. Thereafter, we investigated the protective role of Sal B on starving cells. We showed that Sal B improved the cell viability and ATP content of starving cells in the presence or absence of chloroquine.
As previously reported, there is crosstalk between autophagic and apoptotic pathways14. The interplay between autophagic and apoptotic pathways is emerging as a crucial decision-making process in determining the initiation of programmed cell death14. Therefore, we attempted to determine whether apoptosis occurs in autophagy-induced cardiac myocytes. First, autophagy and apoptosis features were observed by EM in cardiac myocytes under starvation conditions. Next, we studied the effects of Sal B on cardiac myocytes under starvation conditions. To the best of our knowledge, we are the first to demonstrate the coexistence of autophagy and apoptosis by using immunofluorescent dual labeling. The results of co-labeling with LC3 and Caspase-8 confirm that cells underwent apoptosis during starvation-induced autophagy. Sal B reduced the accumulation of LC3 fluorescence and suppressed the expression of Caspase-8 in starving cells, indicating that Sal B inhibited both autophagy and apoptosis in starving cells.
Thus, our results demonstrate that autophagy overcomes the capacity of cardiac myocytes to maintain homeostasis when subjected to starvation for 3 h, and Sal B protected starving cells by blocking the early stage of autophagic flux and inhibiting autophagy.
As for autophagy and apoptosis observed in starving myocytes, this work was merely a primary study. Future experiments are needed to determine the relationship of autophagy and apoptosis in starvation conditions and the mechanism of Sal B suppressive effects on both autophagy and apoptosis.
Author contribution
Xiao HAN, Jian-xun LIU and Xin-zhi LI designed the research and revised the manuscript; Xiao HAN performed the research and analyzed the data.
Acknowledgments
This work was supported by the Key Project of the National Natural Science Foundation of China (No 30830118); Key Projectof the China Ministry of Science & Technology (No 2009ZX09301-005-2-11 and 2009ZX09303-003).
References
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao XH, Naomoto Y, Hao HF, Watanabe N, Sakurama K, Noma K, et al. Autophagy: Can it become a potential therapeutic target. Int J Mol Med. 2010;25:493–503. doi: 10.3892/ijmm_00000369. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, et al. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–15. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamaguchi O, Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ Res. 2008;103:343–51. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–6. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BH, Fu W, Li Z. Protective mechanism of Salviol acid B against acute myocardial ischemia/reperfusion injury in rats. J Tianjin Univ Tradit Chin Med. 2004;23:132–3. [Google Scholar]
- Zhang L, Yuan DP, Xu L, Jiang BP, Fang TH. Protective mechanism of Salvianolic acid B on myocardial ischemia-reperfusion injury of rats. Tradit Chin Drug Res Clin Pharmacol. 2008;19:467–9. [Google Scholar]
- Jin SM, Zhao GF, Fan YC. Effect of salvianolic acid B on endothel in release and TXA2-PGI2 system in myocardial ischemia reperfusion injury in rats. Chin J Gerontol. 2004;24:127–8. [Google Scholar]
- Zhao GF, Zhang HX. Protective effects of Salviol acid B against myocardial ischemia/reperfusion injury in rats. J Liaoning Univ Tradit Chin Med. 2004;6:55–6. [Google Scholar]
- Zhao GF, Li ZT, Fan YC. Effect of Salvianolic acid B on free calcium concentration in hypoxia and reoxygenation cardiac muscle cell. Mod J Integr Tradit Chin West Med. 2004;13:19–20. [Google Scholar]
- Kazuhiko N, Osamu Y, Kinya O. Crosstalk between autophagy and apotosis in heart disease. Circ Res. 2008;103:343–51. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- Dorn GW. 2ndApoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodeling. Cardiovasc Res. 2009;81:465–73. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentim L, Laurence KM, Townsend PA, Carrol CJ, Soond S, Scarabelli TM, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–52. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- Kang C, Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82–4. doi: 10.4161/auto.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, et al. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322–9. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]