Abstract
Pulmonary remodeling is characterized by the permanent and progressive loss of the normal alveolar architecture, especially the loss of alveolar epithelial and endothelial cells, persistent proliferation of activated fibroblasts, or myofibroblasts, and alteration of extracellular matrix. Hepatocyte growth factor (HGF) is a pleiotropic factor, which induces cellular motility, survival, proliferation, and morphogenesis, depending upon the cell type. In the adult, HGF has been demonstrated to play a critical role in tissue repair, including in the lung. Administration of HGF protein or ectopic expression of HGF has been demonstrated in animal models of pulmonary fibrosis to induce normal tissue repair and to prevent fibrotic remodeling. HGF-induced inhibition of fibrotic remodeling may occur via multiple direct and indirect mechanisms including the induction of cell survival and proliferation of pulmonary epithelial and endothelial cells, and the reduction of myofibroblast accumulation.
Keywords: hepatocyte growth factor, myofibroblast, alveolar epithelial cell, pulmonary fibrosis
Pathophysiology of pulmonary fibrosis
Pulmonary fibrosis is a disease characterized by the replacement of the lung tissue with scar tissue, resulting in the permanent loss of the normal alveolar architecture. The disease is usually progressive, and death is often the direct result of either respiratory insufficiency or right heart failure due to pulmonary hypertension. Pulmonary fibrosis can be directly induced by a variety of insults to lung tissue including exposure to drugs, organic or inorganic particles, bacterial or viral infection, or clinical irradiation for the treatment of cancer1, 2. The condition may also occur idiopathically1. Treatments for pulmonary fibrosis consist of anti-inflammatory and immunomodulatory agents, cytotoxic agents (eg, methotrexate, cyclophosphamide), antioxidants (eg, N-acetylcycteine), antifibrotic agents (eg, pirfenidone, colchicine), interferon-gamma 1β, and/or lung transplantation3, 4. The pulmonary fibrosis patient's response to treatment often depends on the etiology of the disease. However, currently available treatments are largely ineffective in halting the progression of the disease.
The progression of pulmonary fibrosis is believed to involve a failed or dysregulated injury response, which may be accompanied by inflammation5. An emerging view of lung remodeling suggests that the disease may develop as the result of repeated stimuli, with early cycles of injury to alveolar epithelial and endothelial cells, followed by inflammation and attempted repair, ultimately leading to aberrant wound healing and fibrosis2, 6.
Cellular alterations in pulmonary fibrosis
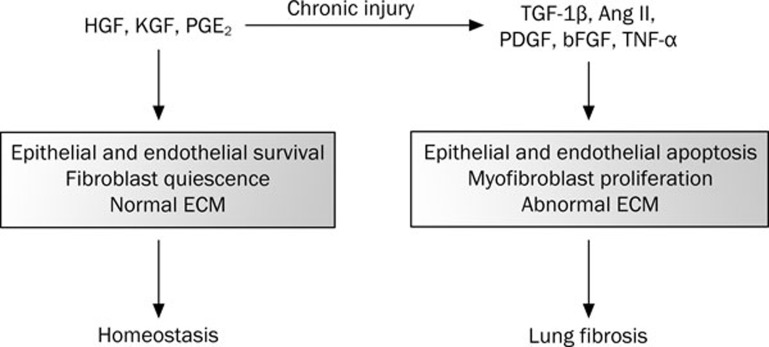
In pulmonary remodeling, the loss of the normal pulmonary architecture is characterized by: 1) the loss of alveolar epithelial and endothelial cells; 2) the persistent proliferation of activated fibroblasts, or myofibroblasts; and 3) the extensive alteration of the extracellular matrix (Figure 1). Two primary animal models have been developed for the study of experimentally-induced pulmonary fibrosis: thoracic irradiation and the profibrotic chemotherapy drug bleomycin. Both of these agents induce pulmonary fibrosis in humans with similar pathophysiology.
Figure 1.
Schematic diagram depicting the development of lung fibrosis following irreparable damage to lung cells. A number of pro-survival factors including HGF, KGF and Cox-2 normally promote survival of epithelial and endothelial cells, fibroblasts quiescence and normal regulation of extracellular matrix (ECM) which altogether results in homeostasis in the lung. Injuries such as bleomycin, radiation, and pro-fibrotic factors may cause epithelial and endothelial apoptosis as well as fibroblast activation and myofibroblast proliferation – events observed in the development of lung fibrosis.
Studies of lung fibrosis have demonstrated the presence of extensive and apparently progressive epithelial cell apoptosis, especially in regions adjacent to fibrotic foci7, 8, 9, 10. Endothelial cell apoptosis has been less studied but has also been identified as a prominent event in fibrotic human lung tissue9. In rodent models of experimental lung fibrosis, extensive apoptosis occurs, similarly to that observed in human lung fibrosis patients11, 12. Rodent models have also demonstrated lung microvascular and pulmonary artery endothelial cell injury and apoptosis11, 13, 14.
Pro-apoptotic factors are upregulated in fibrotic lung tissue. Lung fibrosis patient samples have increased levels of transforming growth factor β1 (TGF-β1) and angiotensin II (Ang II)15, 16, 17 that induce apoptosis and/or growth arrest in epithelial and endothelial cells18. Tumor necrosis factor-α (TNF-α), a ligand for the death receptors, as well as death receptors themselves, are increased in the lung tissue of patients with IPF19, 20, 21. Data indicate many of the same factors identified in human lung fibrosis are also increased in animal models of the disease22, 23, 24, 25, 26, 27, 28, 29. The imbalance of homeostatic factors created by increased production of pro-apoptotic factors is further exacerbated by a decrease in the production of factors that sustain epithelial and endothelial cell survival, including hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF)30, 31, 32, 33, 34. The inhibition of cellular apoptosis by a caspase inhibitor or by blocking Ang II signaling significantly mitigated fibrotic remodeling in mice treated with bleomycin35, 36. Specific inhibition of endothelial cell death was also demonstrated to prevent TGF-β1-induced fibrosis in a rat model of lung fibrosis14.
Activated fibroblasts, or myofibroblasts, a central topic in pulmonary fibrosis research, are thought to be a primary causative cell type in the progression of the disease37, 38, 39. Lung tissue from IPF patients contain increased levels in specific factors that support fibroblasts and/or mesenchymal cell growth including TGF-β1, basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), TNFα, interleukins-1β and -8, and insulin-like growth factor15, 16, 17, 21, 25, 40, 41, 42, 43, 44. At the same time, IPF lung tissue has reduced levels of factors that suppress fibroblast growth, such as cyclooxygenase-2 (COX-2) and its downstream product prostaglandin E2 (PGE2)45, 46. Myofibroblasts, either from patient sample, or from animal models of pulmonary fibrosis, have pathophysiological characteristics consistent with their key role in affecting alterations associated with fibrotic remodeling47. 1) They exhibit rapid proliferation and secrete autocrine factors including bFGF, PDGF, and TGF-β148, 49. 2) They display significant resistance to apoptosis, including that mediated by Fas50, 51, 52. 3) They are contractile and express α-smooth muscle actin, and these cells are highly motile38. And finally, 4) they significantly alter the extracellular milieu of the lung by secreting extracellular matrix proteins, including collagen types I and III, and by producing reactive oxygen species that contribute to the oxidative state of the lung in fibrosis and to the cross-linking of the extracellular matrix53, 54, 55, 56. Unlike normal fibroblasts that provide a supportive environment to the resident epithelial and endothelial tissues of the lung, myofibroblasts create a toxic environment for other lung cells. Myofibroblasts are a primary source of many pro-apoptotic factors that induce epithelial and endothelial cell death in lung fibrosis. Data from in vitro experiments using myofibroblasts cultured from fibrotic tissue indicate that these cells induce growth arrest and apoptosis in primary lung epithelial and endothelial cells35, 47.
Multiple cellular origins of myofibroblasts have been identified in pulmonary fibrosis. Originally, it was thought that resident lung fibroblasts provided the sole source for this pathological cell type. Myofibroblasts can be derived from fibroblasts through the process of transdifferentiation, believed to be driven by sustained over-expression of TGF-β1 in fibrotic tissue4, 38, 57. Myofibroblasts can also derive from alveolar type II pneumocytes through epithelial-mesenchymal transformation (EMT)58, 59, 60; this process, like transdifferentiation, is also induced by TGF-β12. A third potential source of myofibroblasts are the mesenchymal stem cells from adult bone marrow, which can be recruited to the injured lung61, 62, 63. Circulating fibrocytes are increased in IPF patients compared to healthy control subjects64, 65, and studies tracking bone marrow-derived fibroblasts suggest that fibrocytes may migrate to the lung and contribute to remodeling61, 63, 66. The inhibition of factors that induce myofibroblast transdifferentiation and EMT processes, such as TGF-β1 and Ang II, significantly attenuates the development of pulmonary fibrosis in animal models17, 26, 36, 67, 68, 69. Likewise, the inhibition of fibrocyte extravasation to the lungs, for instance by inhibiting CXCL12 signaling, was shown to reduce collagen deposition and fibrosis in mouse models2, 70.
Hepatocyte growth factor in normal and fibrotic tissue repair
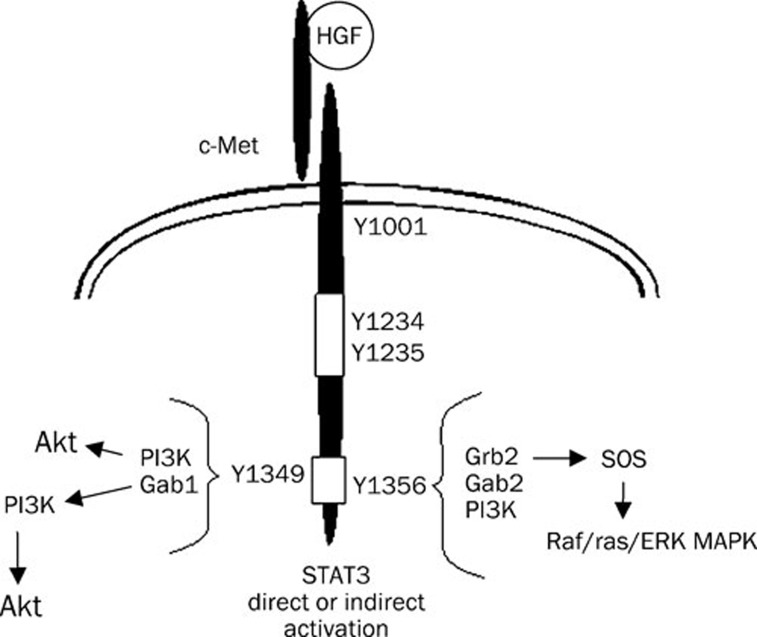
HGF is a paracrine factor produced by cells of mesenchymal origin (eg, fibroblasts and macrophages), while the HGF receptor, Met, is expressed by epithelial and endothelial cells71. HGF is a heterodimeric protein comprised of a 55–60 kDa α chain and a 32–34 kDa β chain linked by a single disulfide bond71. The Met receptor is a tyrosine kinase receptor with a single transmembrane spanning region and a conserved tyrosine kinase domain. Met is translated as a single polypeptide chain which is proteolytically cleaved to form a ∼145 kDa β heavy chain and a ∼35 kDa α light chain linked by a single disulfide bond71. The exclusion of Met expression from fibroblasts provides specificity for HGF-induced survival and proliferative activities on epithelial and endothelial cell types. Met contains a number of critical tyrosine residues that are phosphorylated in response to HGF binding (Figure 2)72. A juxtamembrane tyrosine (Y1001) is involved in down-regulation of Met following activation72. Two tyrosines in the kinase domain (Y1234 and Y1235) are required for kinase activity of the receptor73. Two other critical tyrosines (Y1349 and Y1356) are found in the carboxy terminal domain of Met, in the “multifunctional docking region”74. These latter phosphorylation sites are required for the association with multiple adaptor proteins and signaling molecules75.
Figure 2.
HGF/c-Met signal transduction. Two tyrosine phosphorylation sites (Y1349/Y1356) in the multi-functional docking domain interact with multiple adaptor proteins and signal transduction enzymes. STAT3 has been shown to bind directly to c-Met in some cell types, but the site has not been defined.
Signal transduction by HGF leads to a variety of biological responses including migration, proliferation and morphogenesis, especially branching tubulogenesis in specific cell types71. HGF is required for normal embryogenesis and development76, 77, including for the lung78. However, in the adult a primary function of HGF is tissue repair79. HGF promotes normal tissue regeneration and prevents fibrotic remodeling in the lung, heart, kidney, and liver80, 81, 82, 83, 84. HGF is expressed locally in response to injury in a number of tissues, including the lung, kidney, and liver82, 83, 85, 86, 87, 88. HGF is also produced in the lung in response to distal injuries, suggesting an endocrine function for tissue repair89.
The role of HGF in lung tissue repair has been well established82, 90. Studies indicate that HGF is elevated in the lung following injury. HGF mRNA levels are elevated in damaged lung tissue82, 91, and HGF protein levels are increased in bronchoalveolar fluid extracted from injured lungs92. The time course of HGF induction following lung injury correlates with proliferation of the alveolar epithelial cells82, 93 and lung vascular endothelial cells94. Administration of HGF neutralizing antibodies resulted in reduced DNA synthesis in alveolar epithelial cells after ischemia-reperfusion lung injury in rats95. Although HGF is increased in response to tissue injury, an inverse correlation has been identified for HGF expression during the development and/or progression of fibrosis in several tissues including the lung31, 96, 97. Lung tissue from patients with pulmonary fibrosis has reduced expression of factors that sustain epithelial and endothelial cell growth and survival, including HGF31. Lung fibroblasts isolated from IPF patients have decreased HGF expression and activation relative to fibroblasts from control patients30. In cell culture and animal models, suppression of HGF synthesis occurs in response to treatment with the pro-fibrotic factors TGF-β and Ang II98, 99, 100, 101.
Studies in animal models have provided strong evidence that HGF-induced lung repair prevents the induction of fibrotic remodeling. In vivo studies have shown that HGF potently mitigates the effects of acute and chronic lung injuries caused by oxidative stress and inflammation. Administration of HGF protein or adenoviral expression of HGF prevents fibrotic remodeling in several animal models of lung fibrosis91, 102, 103, 104. Transient in vivo expression of HGF, using non-viral plasmids, also prevents fibrotic lung remodeling. Using albumin-derived particles to transfect lung endothelial cells, in vivo transient transfection of HGF increased repair and prevented collagen deposition and remodeling in mice105, 106. Because HGF is secreted, it was reasoned that “nondiseased-organ-targeting gene transfer” could also be used to produce HGF protein, which would then reach the lung through the circulatory system107. Electrotransfer of an HGF-encoding plasmid into muscle tissue was also demonstrated to suppress bleomycin-induced fibrotic remodeling in mice107. Importantly, studies show that HGF has protective activity when given either simultaneously with or 7 d after administration of a pro-fibrotic treatment, suggesting that HGF is effective during both the initiation phase and the progressive phase of the disease102.
Because human patients are usually diagnosed only during the progressive phase of pulmonary fibrosis, the identification of factors effective during this phase of the disease is critical for development of treatments and cures.
HGF signaling to induce epithelial and endothelial survival and growth
Regeneration of normal epithelium and endothelium is critical to healthy repair following tissue injury. Thus, normal tissue repair requires factors, such as HGF, that specifically support growth in epithelial and endothelial cells, but not in myofibroblasts, may be required for antifibrotic tissue repair93, 108. HGF is mitogenic, motogenic, and induces survival in pulmonary endothelial and alveolar type II epithelial cells71, 109, 110, 111, 112, 113, 114. HGF also releases lung epithelial and capillary endothelial cells from growth arrest induced by the profibrotic factor TGF-β1115.
HGF blocks apoptosis in lung epithelial and endothelial cells. The cell survival activities by HGF have been attributed to the activation of a number of anti-apoptotic signaling pathways112, 116, 117, 118, 119 although the specific anti-apoptotic mechanisms of HGF appear to differ among cell types118, 120. Three predominant pathways implicated in survival by HGF are ERK/MAPK, PI3K/Akt, and signal transducer and activator of transcription 3 (STAT3) (Figure 2)121. Although much of the research on HGF signaling for proliferation and survival has been performed on cancer cell types, some studies have investigated the mechanisms for HGF-induced survival and proliferation in primary lung cells.
In murine lung endothelial cells subjected to hypoxic stress followed by reoxygenation, a procedure that activates the extrinsic apoptotic pathway through the death inducing signaling complex (DISC) and caspase-8. HGF confers protection against extrinsic apoptosis through PI3K/Akt-dependent up-regulation of the caspase-8 inhibitor FLICE-like inhibiting protein (FLIP) and through down-regulation of DISC formation122. This report additionally showed that HGF inhibited Bax translocation into the mitochondria, also in an Akt-dependent manner122. An investigation of the effects of HGF on H2O2- and TNF-α-induced apoptosis in pulmonary epithelial cells demonstrated that survival of epithelial cells by HGF involved the activation of nuclear factor-kappa B (NF-κB)118. The mechanism by which HGF activates NF-κB in these cells is unknown.
Both cell culture and in vivo studies provide evidence that HGF regulates gene expression of the anti-apoptotic members of the Bcl-2 protein family. Studies of hypoxia-reoxygenation injury to endothelial cells demonstrate that HGF exerts Akt-dependent anti-apoptotic activity by enhancement of the expression of anti-apoptotic protein Bcl-xL118, 122. Investigation of HGF treatment prevented cellular apoptosis and increased Bcl-xL expression in mice following ischaemic reperfusion injury to the lung123.
HGF may also block fibrotic remodeling through indirect mechanisms, including the regulation of pro-fibrotic factors. As stated above, Ang II is a potent inducer of epithelial and endothelial cell apoptosis in lung fibrosis, and studies suggest that de novo generation of Ang II is required for FAS- and TNF-α induced apoptosis of alveolar epithelial cells in cell culture124, 125. The enzyme angiotensin converting enzyme (ACE) is required for the proteolytic activation of Ang II from its inactive precursor angiotensin I (Ang I), and bleomycin-induced fibrosis can be blocked in vivo using an ACE inhibitor or an Ang II receptor antagonist35. Our laboratory demonstrated that HGF reduces ACE expression in lung endothelial cell culture126. The down-regulation of ACE might provide a potential indirect mechanism for HGF reduction of lung cell apoptosis through Ang II suppression.
HGF inhibition of myofibroblast accumulation
Rodent models for lung fibrosis indicate that HGF treatment restricts myofibroblast recruitment. Three potential mechanisms for this effect of HGF are: 1) the induction of quiescence in lung fibroblasts and inhibition of transdifferentiation; 2) the inhibition of EMT of lung epithelial cells; and 3) induction of apoptosis in myofibroblasts. Direct inhibition of fibroblast transdifferentiation by HGF has not been demonstrated, but regulation of myofibroblast development may occur through indirect mechanism(s).
HGF reduces fibroblast activation to the myofibroblast phenotype. HGF may affect fibroblast activation indirectly through the regulation of lung endothelial cell expression of cyclooxygenase 2 (COX-2), a potent activator of prostaglandin E2 (PGE2) synthesis127, 128. PGE2 is secreted by pulmonary endothelial cells, induces fibroblast quiescence and is a potent inhibitor TGF-β1-induced fibroblast transdifferentiation57, 129. Our laboratory has shown that HGF regulates COX-2 expression in primary lung epithelial cells through Akt- and beta catenin-dependent up-regulation of COX-2 mRNA127. This suggests a possible mechanism for HGF-mediated COX-2 inhibition of fibroblast transdifferentiation.
EMT is an important process during development and organogenesis, and HGF has been demonstrated to induce EMT under specific cellular conditions130, 131. However, EMT associated with fibrotic remodeling is negatively modulated by HGF96. Rat alveolar epithelial cells that were treated with TGF-β to induce EMT, HGF inhibits the expression of myofibroblast markers such as α-SMA, collagen type I, and fibronectin132. The inhibitory activity of HGF on EMT requires upregulation of Smad7 expression and its export from the nucleus to the cytoplasm. The export of Smad-7 to cytoplasmic compartment results in the inhibition of signal transduction by the TGF-β receptor132. HGF may also indirectly affect EMT processes. Endothelial nitric oxide attenuates EMT133. Increased nitric oxide results in the retention of epithelial morphology while inhibition of NOS leads to increased α-SMA expression and fibroblast-like morphology in TGF-β1-treated alveolar epithelial cells133. HGF stimulates activity of endothelial nitric oxide synthase (eNOS) via a PI3K/Akt-dependent pathway in endothelial cells134, 135.
Finally, it has been shown recently that HGF affects the viability of myofibroblasts through direct mechanisms. Although normal fibroblasts lack the HGF receptor Met, myofibroblasts taken from the fibrotic lungs of experimental animals have been shown to express Met136. In the Met-expressing myofibroblasts, HGF was shown to induce apoptosis in a caspase-dependent manner136. This apoptotic activity of HGF is associated with increased degradation of the extracellular matrix. Treatment of myofibroblasts with HGF increases in the activities of predominant enzymes involved in fibronectin degradation and a decrease in a fibronectin central cell binding domain which is involved in FAK phosphorylation; both of these activities lead to decreased survival of myofibroblasts136.
Conclusion
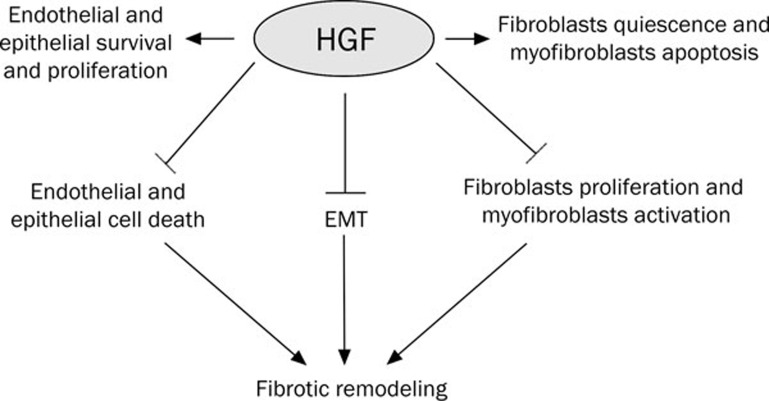
Findings from animal models of pulmonary fibrosis show that HGF can inhibit both the initiation and progression of lung fibrosis (Figure 3). However, the critical mechanism(s) for HGF protection of the lung from fibrotic remodeling and promotion of normal tissue regeneration remains poorly understood. HGF directly induces epithelial and endothelial proliferation and survival, and may indirectly modulate myofibroblast accumulation in the lung after injury. Despite the potential clinical applications for HGF for wound repair and prevention of fibrotic remodeling, its complex structure has precluded its development for clinical use. The future development and study of HGF mimetics and/or Met agonists may aid in the understanding of HGF mechanisms of tissue repair as well as provide potential therapies for treatment of lung fibrosis.
Figure 3.
HGF actions for the inhibition of fibrotic remodeling.
Acknowledgments
We thank Dr Usamah KAYYALI for critical reading of this article. Some of the authors are employees of the US Government, and this manuscript was prepared as part of their official duties. Title 17 USC §105 provides that 'Copyright protection under this title is not available for any work of the United States Government.' Title 17 USC §101 defined a US Government work as a work prepared by a military service member or employees of the US Government as part of that person's official duties. The views in this article are those of the authors and do not necessarily reflect the views, official policy, or position of the Uniformed Services University of the Health Sciences, Department of the Navy, Department of Defense, or the US Federal Government.
References
- Streiter R, Keane M, Stadiford T, Kunkel SL.Cytokine biology and the pathogenesis of interstitial lung diseaseLondon: BC Decker, Inc; 1998
- Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–70. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N, Collard HR, King TE., Jr Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:330–8. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–30. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res. 2002;3:3. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T, Hunninghake G. Idiopathic pulmonary fibrosis. N Engl J Med. 1996;345:517–25. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122:293S–98S. doi: 10.1378/chest.122.6_suppl.293s. [DOI] [PubMed] [Google Scholar]
- Penny DP, Rubin P. Specific early fine structural changes in the lung following irradiation. Int J Rad Oncology Biol Phys. 1977;2:1123–32. doi: 10.1016/0360-3016(77)90119-5. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kuwano K, Hagimoto N, Watanabe K, Matsuba T, Fujita M, et al. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol. 2002;198:388–96. doi: 10.1002/path.1208. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Gidea C, Bargout R, Bifero A, Ibarra-Sunga O, Papp M, et al. Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol. 1998;275:L1013–L17. doi: 10.1152/ajplung.1998.275.5.L1013. [DOI] [PubMed] [Google Scholar]
- Ward WF, Molteni A, Solliday NH, Jones GE. The relationship between endothelial dysfunction and collagen accumulation in irradiated rat lung. Int J Radiat Oncol Biol Phys. 1985;11:1985–90. doi: 10.1016/0360-3016(85)90281-0. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang H, Soledad-Conrad V, Zhuang J, Uhal B. Bleomycin-induced apoptosis of alveolar epithelial cells requires angiotensin synthesis de novo. Am J Physiol Lung Cell Mol Physiol. 2003;284:L501–7. doi: 10.1152/ajplung.00273.2002. [DOI] [PubMed] [Google Scholar]
- Zhang H, Lawson WE, Polosukhin VV, Pozzi A, Blackwell TS, Litingtung Y, et al. Inhibitor of differentiation 1 promotes endothelial survival in a bleomycin model of lung injury in mice. Am J Pathol. 2007;171:1113–26. doi: 10.2353/ajpath.2007.070226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, et al. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119:1298–311. doi: 10.1172/JCI36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Broekelmann T, Limper A, Colby T, McDonald J. Transfomring growth factor β1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–46. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T, et al. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L156–64. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- Lee YH, Mungunsukh O, Tutino RL, Marquez AP, Day RM. Angiotensin II-induced apoptosis requires SHP-2 regulation of nucleolin and Bcl-xL in primary lung endothelial cells. J Cell Sci. 2010;123:1634–43. doi: 10.1242/jcs.063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhagen MW, Schrum S, Zissel G, Zipfel PF, Schlaak M, Muller-Quernheim J. Increased expression of proinflammatory chemokines in bronchoalveolar lavage cells of patients with progressing idiopathic pulmonary fibrosis and sarcoidosis. J Investig Med. 1998;46:223–31. [PubMed] [Google Scholar]
- Homolka J, Ziegenhagen MW, Gaede KI, Entzian P, Zissel G, Muller-Quernheim J. Systemic immune cell activation in a subgroup of patients with idiopathic pulmonary fibrosis. Respiration. 2003;70:262–9. doi: 10.1159/000072007. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Ribaux C, Karpuz V, Grau GE, Kapanci Y. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am J Pathol. 1993;143:651–5. [PMC free article] [PubMed] [Google Scholar]
- Kuwano K, Miyazaki H, Hagimoto N, Kawasaki M, Fujita M, Kunitake R, et al. The involvement of Fas-Fas ligand pathway in fibrosing lung diseases. Am J Respir Cell Mol Biol. 1999;20:53–60. doi: 10.1165/ajrcmb.20.1.2941. [DOI] [PubMed] [Google Scholar]
- Cavarra E, Carraro F, Fineschi S, Naldini A, Bartalesi B, Pucci A, et al. Early response to bleomycin is characterized by different cytokine and cytokine receptor profiles in lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1186–92. doi: 10.1152/ajplung.00170.2004. [DOI] [PubMed] [Google Scholar]
- Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, et al. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997;150:981–91. [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Sime PJ, Wu T, Warshamana GS, Pociask D, Tsai SY, et al. Transforming growth factor-beta(1) overexpression in tumor necrosis factor-alpha receptor knockout mice induces fibroproliferative lung disease. Am J Respir Cell Mol Biol. 2001;25:3–7. doi: 10.1165/ajrcmb.25.1.4481. [DOI] [PubMed] [Google Scholar]
- Li X, Zhuang J, Rayford H, Zhang H, Shu R, Uhal BD. Attenuation of bleomycin-induced pulmonary fibrosis by intratracheal administration of antisense oligonucleotides against angiotensinogen mRNA. Curr Pharm Des. 2007;13:1257–68. doi: 10.2174/138161207780618867. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, et al. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;280:L39–49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shannon JM, Morikawa O, Gauldie J, Hara N, Mason RJ. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol. 2003;29:669–76. doi: 10.1165/rcmb.2002-0046OC. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Hagimoto N, Tanaka T, Kawasaki M, Kunitake R, Miyazaki H, et al. Expression of apoptosis-regulatory genes in epithelial cells in pulmonary fibrosis in mice. J Pathol. 2000;190:221–9. doi: 10.1002/(SICI)1096-9896(200002)190:2<221::AID-PATH495>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Marchand-Adam S, Fabre A, Mailleux AA, Marchal J, Quesnel C, Kataoka H, et al. Defect of pro-hepatocyte growth factor activation by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:58–66. doi: 10.1164/rccm.200507-1074OC. [DOI] [PubMed] [Google Scholar]
- Marchand-Adam S, Marchal J, Cohen M, Soler P, Gerard B, Castier Y, et al. Defect of hepatocyte growth factor secretion by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:1156–61. doi: 10.1164/rccm.200212-1514OC. [DOI] [PubMed] [Google Scholar]
- Marchand-Adam S, Plantier L, Bernuau D, Legrand A, Cohen M, Marchal J, et al. Keratinocyte growth factor expression by fibroblasts in pulmonary fibrosis: poor response to interleukin-1beta. Am J Respir Cell Mol Biol. 2005;32:470–7. doi: 10.1165/rcmb.2004-0205OC. [DOI] [PubMed] [Google Scholar]
- Zhang F, Nielsen LD, Lucas JJ, Mason RJ. Transforming growth factor-beta antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. Am J Respir Cell Mol Biol. 2004;31:679–86. doi: 10.1165/rcmb.2004-0182OC. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Elborn JS, Ennis M. Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L69–76. doi: 10.1152/ajplung.00299.2006. [DOI] [PubMed] [Google Scholar]
- Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal B. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279:L143–51. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- Chen FP, Gong LK, Zhang L, Wang H, Qi XM, Wu XF, et al. Early lung injury contributes to lung fibrosis via AT1 receptor in rats. Acta Pharmacol Sin. 2007;28:227–37. doi: 10.1111/j.1745-7254.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Phan SH. Role of the myofibroblast in pulmonary fibrosis. Kidney Int Suppl. 1996;54:S46–8. [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:527–37. [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 2008;5:338–42. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17:1220–7. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- Pan LH, Ohtani H, Yamauchi K, Nagura H. Co-expression of TNF alpha and IL-1 beta in human acute pulmonary fibrotic diseases: an immunohistochemical analysis. Pathol Int. 1996;46:91–9. doi: 10.1111/j.1440-1827.1996.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Martinet Y, Menard O, Vaillant P, Vignaud JM, Martinet N. Cytokines in human lung fibrosis. Arch Toxicol Suppl. 1996;18:127–39. doi: 10.1007/978-3-642-61105-6_14. [DOI] [PubMed] [Google Scholar]
- Martinet Y, Rom WN, Grotendorst GR, Martin GR, Crystal RG. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987;317:202–9. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Zhang J, Laboy M, Lasky JA. Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L182–8. doi: 10.1152/ajplung.00083.2003. [DOI] [PubMed] [Google Scholar]
- Petkova DK, Clelland CA, Ronan JE, Lewis S, Knox AJ. Reduced expression of cyclooxygenase (COX) in idiopathic pulmonary fibrosis and sarcoidosis. Histopathology. 2003;43:381–6. doi: 10.1046/j.1365-2559.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–8. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–8. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des. 2007;13:1247–56. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, et al. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–67. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, et al. Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol. 2004;202:486–95. doi: 10.1002/path.1531. [DOI] [PubMed] [Google Scholar]
- Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, et al. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol. 2003;29:490–8. doi: 10.1165/rcmb.2002-0262OC. [DOI] [PubMed] [Google Scholar]
- Mastruzzo C, Crimi N, Vancheri C. Role of oxidative stress in pulmonary fibrosis. Monaldi Arch Chest Dis. 2002;57:173–6. [PubMed] [Google Scholar]
- Day RM, Suzuki YJ. Cell proliferation, reactive oxygen and cellular glutathione. Dose Response. 2005;3:425–42. doi: 10.2203/dose-response.003.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–8. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- Larios JM, Budhiraja R, Fanburg BL, Thannickal VJ. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem. 2001;276:17437–41. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–9. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HW, Xie QM, Chen JQ, Deng YM, Tang HF. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76:29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–32. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–24. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman W, Beckett T, Goncz KK, Beatty BG, Weiss DJ. Dual Y chromosome painting and in situ cell-specific immunofluorescence staining in lung tissue: an improved method of identifying donor marrow cells in lung following bone marrow transplantation. Histochem Cell Biol. 2004;121:73–9. doi: 10.1007/s00418-003-0598-0. [DOI] [PubMed] [Google Scholar]
- Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- Dunsmore SE, Shapiro SD. The bone marrow leaves its scar: new concepts in pulmonary fibrosis. J Clin Invest. 2004;113:180–2. doi: 10.1172/JCI20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma A, Li YJ, Abe S, Usuki J, Matsuda K, Henmi S, et al. Interferon-{beta} inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-{beta} and thrombospondin. Am J Respir Cell Mol Biol. 2005;32:93–8. doi: 10.1165/rcmb.2003-0374OC. [DOI] [PubMed] [Google Scholar]
- Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–16. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R, Burdick M, Hong K, Lutz M, Murray L, Xue Y, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993;1155:357–71. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Riethmacher D, Birchmeier W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc Natl Acad Sci USA. 1995;92:2597–601. doi: 10.1073/pnas.92.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GA, Park M. Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene. 1994;9:2019–27. [PubMed] [Google Scholar]
- Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and SHP-2 mediates biological responses. J Cell Biol. 2000;149:1419–32. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–71. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E, Weidner KM, Birchmeier C. Expression of the met-receptor and its ligand, HGF-SF during mouse embryogenesis. EXS. 1993;65:381–94. [PubMed] [Google Scholar]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–10. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308:44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Roles of HGF as a pleiotropic factor in organ regeneration. EXS. 1993;65:225–49. [PubMed] [Google Scholar]
- Matsuda Y, Matsumoto K, Yamada A, Ichida T, Asakura H, Komoriya Y, et al. Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology. 1997;26:81–9. doi: 10.1053/jhep.1997.v26.pm0009214455. [DOI] [PubMed] [Google Scholar]
- Schaper W, Kubin T. Is hepatocyte growth factor a protein with cardioprotective activity in the ischemic heart. Circulation. 1997;95:2471–72. doi: 10.1161/01.cir.95.11.2471. [DOI] [PubMed] [Google Scholar]
- Yanagita K, Matsumoto K, Sekiguchi K, Ishibashi H, Niho Y, Nakamura T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem. 1993;268:21212–7. [PubMed] [Google Scholar]
- Igawa T, Matsumoto K, Kanda S, Saito Y, Nakamura T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol. 1993;265:F61–9. doi: 10.1152/ajprenal.1993.265.1.F61. [DOI] [PubMed] [Google Scholar]
- Panos RJ, Patel R, Bak PM. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am J Respir Cell Mol Biol. 1996;15:574–81. doi: 10.1165/ajrcmb.15.5.8918364. [DOI] [PubMed] [Google Scholar]
- Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. Circulation. 1997;95:2552–58. doi: 10.1161/01.cir.95.11.2552. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Matsumoto K, Inoue T, Nose T, Murayama S, Teshima T, et al. Induction of hepatocyte growth factor in the liver, kidney and lung following total body irradiation in rat. Cytokine. 1996;8:927–32. doi: 10.1006/cyto.1996.0124. [DOI] [PubMed] [Google Scholar]
- Maeda J, Ueki N, Hada T, Higashino K. Elevated serum hepatocyte growth factor/scatter factor levels in inflammatory lung disease. Am J Respir Crit Care Med. 1995;152:1587–91. doi: 10.1164/ajrccm.152.5.7582299. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Kawaida K, Takao S, Shimazu H, Noji S, Matsumoto K, et al. Rapid and marked induction of hepatocyte growth factor during liver regeneration after ischemic or crush injury. Hepatology. 1992;16:1485–92. doi: 10.1002/hep.1840160626. [DOI] [PubMed] [Google Scholar]
- Yanagita K, Nagaike M, Ishibashi H, Niho Y, Matsumoto K, Nakamura T. Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem Biophys Res Commun. 1992;182:802–9. doi: 10.1016/0006-291x(92)91803-x. [DOI] [PubMed] [Google Scholar]
- Adamson Y, Bakowska J. Relationship of keratinocyte growth factor an hepatocyte growth factor levels in rat lung lavage fluid to epithelial cell regneration after bleomycin. Am J Pathol. 1999;155:949–54. doi: 10.1016/S0002-9440(10)65194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Sato S, Dai W, Yamanaka N. The protective effect of hepatocyte growth-promoting factor (pHGF) against hydrogen peroxide-induced acute lung injury in rats. Med Electron Microsc. 2001;34:92–102. doi: 10.1007/s007950170003. [DOI] [PubMed] [Google Scholar]
- Stern JB, Fierobe L, Paugam C, Rolland C, Dehoux M, Petiet A, et al. Keratinocyte growth factor and hepatocyte growth factor in bronchoalveolar lavage fluid in acute respiratory distress syndrome patients. Crit Care Med. 2000;28:2326–33. doi: 10.1097/00003246-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Sakamaki Y, Matsumoto K, Mizuno S, Miyoshi S, Matsuda H, Nakamura T. Hepatocyte growth factor stimulates proliferation of respiratory epithelial cells during postpneumonectomy compensatory lung growth in mice. Am J Respir Cell Mol Biol. 2002;26:525–33. doi: 10.1165/ajrcmb.26.5.4714. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Kubo H, Yamada M, Kobayashi S, Suzuki T, Mizuno S, et al. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem Biophys Res Commun. 2004;324:276–80. doi: 10.1016/j.bbrc.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Yamada T, Hisanaga M, Nakajima Y, Mizuno S, Matsumoto K, Nakamura T, et al. Enhanced expression of hepatocyte growth factor by pulmonary ischemia-reperfusion injury in the rat. Am J Respir Crit Care Med. 2000;162:707–15. doi: 10.1164/ajrccm.162.2.9908064. [DOI] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. Hepatocyte growth factor gene therapy and angiotensin II blockade synergistically attenuate renal interstitial fibrosis in mice. J Am Soc Nephrol. 2002;13:2464–77. doi: 10.1097/01.asn.0000031827.16102.c1. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, Shokei K, et al. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102:246–52. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- Harrison P, Bradley L, Bomford A. Mechanism of regulation of HGF/SF gene expression in fibroblasts by TGF-beta1. Biochem Biophys Res Commun. 2000;271:203–11. doi: 10.1006/bbrc.2000.2612. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Tajima H, Okazaki H, Nakamura T. Negative regulation of hepatocyte growth factor gene expression in human lung fibroblasts and leukemic cells by transforming growth factor-beta 1 and glucocorticoids. J Biol Chem. 1992;267:24917–20. [PubMed] [Google Scholar]
- Matsumoto K, Morishita R, Moriguchi A, Tomita N, Yo Y, Nishii T, et al. Prevention of renal damage by angiotensin II blockade, accompanied by increased renal hepatocyte growth factor in experimental hypertensive rats. Hypertension. 1999;34:279–84. doi: 10.1161/01.hyp.34.2.279. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Morishita R, Tomita N, Moriguchi A, Komai N, Aoki M, et al. Improvement of endothelial dysfunction by angiotensin II blockade accompanied by induction of vascular hepatocyte growth factor system in diabetic spontaneously hypertensive rats. Heart Vessels. 2003;18:18–25. doi: 10.1007/s003800300003. [DOI] [PubMed] [Google Scholar]
- Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, et al. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. Am J Respir Crit Care Med. 1997;156:1937–44. doi: 10.1164/ajrccm.156.6.9611057. [DOI] [PubMed] [Google Scholar]
- Dohi M, Hasegawa T, Yamamoto K, Marshall BC. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am J Respir Crit Care Med. 2000;162:2302–7. doi: 10.1164/ajrccm.162.6.9908097. [DOI] [PubMed] [Google Scholar]
- Ebina M, Shimizukawa M, Narumi K, Miki M, Koinuma D, Watabe M, et al. Towards an effective gene therapy for idiopathic pulmonary fibrosis. Chest. 2002;121:32S–33S. doi: 10.1378/chest.121.3_suppl.32s-a. [DOI] [PubMed] [Google Scholar]
- Gazdhar A, Fachinger P, van Leer C, Pierog J, Gugger M, Friis R, et al. Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L529–36. doi: 10.1152/ajplung.00082.2006. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Ebina M, Orson FM, Nakamura A, Kubota K, Koinuma D, et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Mol Ther. 2005;12:58–67. doi: 10.1016/j.ymthe.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Umeda Y, Marui T, Matsuno Y, Shirahashi K, Iwata H, Takagi H, et al. Skeletal muscle targeting in vivo electroporation-mediated HGF gene therapy of bleomycin-induced pulmonary fibrosis in mice. Lab Invest. 2004;84:836–44. doi: 10.1038/labinvest.3700098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi CH, Liu IL, Lo WY, Liaw BS, Wang YS, Chi KH. Hepatocyte growth factor gene therapy prevents radiation-induced liver damage. World J Gastroenterol. 2005;11:1496–502. doi: 10.3748/wjg.v11.i10.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M, Matsumoto K, et al. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension. 2001;37:581–6. doi: 10.1161/01.hyp.37.2.581. [DOI] [PubMed] [Google Scholar]
- Day RM, Soon L, Breckenridge D, Bridges B, Patel BK, Wang LM, et al. Mitogenic synergy through multilevel convergence of hepatocyte growth factor and interleukin-4 signaling pathways. Oncogene. 2002;21:2201–11. doi: 10.1038/sj.onc.1205289. [DOI] [PubMed] [Google Scholar]
- Day RM, Cioce V, Breckenridge D, Castagnino P, Bottaro DP. Differential signaling by alternative HGF isoforms through c-Met: activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene. 1999;18:3399–406. doi: 10.1038/sj.onc.1202683. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, et al. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–12. [PMC free article] [PubMed] [Google Scholar]
- Shiratori M, Michalopoulos G, Shinozuka H, Singh G, Ogasawara H, Katyal SL. Hepatocyte growth factor stimulates DNA synthesis in alveolar epithelial type II cells in vitro. Am J Respir Cell Mol Biol. 1995;12:171–80. doi: 10.1165/ajrcmb.12.2.7532419. [DOI] [PubMed] [Google Scholar]
- Singh-Kaw P, Zarnegar R, Siegfried JM. Stimulatory effects of hepatocyte growth factor on normal and neoplastic human bronchial epithelial cells. Am J Physiol. 1995;268:L1012–20. doi: 10.1152/ajplung.1995.268.6.L1012. [DOI] [PubMed] [Google Scholar]
- Taipale J, Keski-Oja J. Hepatocyte growth factor releases epithelial and endothelial cells from growth arrest induced by transforming growth factor-β1. J Biol Chem. 1996;271:4342–48. doi: 10.1074/jbc.271.8.4342. [DOI] [PubMed] [Google Scholar]
- Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24:1749–66. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- Fan S, Ma YX, Gao M, Yuan RQ, Meng Q, Goldberg ID, et al. The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair. Mol Cell Biol. 2001;21:4968–84. doi: 10.1128/MCB.21.15.4968-4984.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Sugita K, Inukai T, Goi K, Kagami K, Kawasaki K, et al. Hepatocyte growth factor protects small airway epithelial cells from apoptosis induced by tumor necrosis factor-alpha or oxidative stress. Pediatr Res. 2004;56:336–44. doi: 10.1203/01.PDR.0000134255.58638.59. [DOI] [PubMed] [Google Scholar]
- Moumen A, Ieraci A, Patane S, Sole C, Comella JX, Dono R, et al. Met signals hepatocyte survival by preventing Fas-triggered FLIP degradation in a PI3k-Akt-dependent manner. Hepatology. 2007;45:1210–7. doi: 10.1002/hep.21604. [DOI] [PubMed] [Google Scholar]
- Liu Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol. 1999;277:F624–33. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]
- Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–31. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou Y, Kim HP, Song R, Zarnegar R, Ryter SW, et al. Hepatocyte growth factor protects against hypoxia/reoxygenation-induced apoptosis in endothelial cells. J Biol Chem. 2004;279:5237–43. doi: 10.1074/jbc.M309271200. [DOI] [PubMed] [Google Scholar]
- Makiuchi A, Yamaura K, Mizuno S, Matsumoto K, Nakamura T, Amano J, et al. Hepatocyte growth factor prevents pulmonary ischemia-reperfusion injury in mice. J Heart Lung Transplant. 2007;26:935–43. doi: 10.1016/j.healun.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Wang R, Alam G, Zagariya A, Gidea C, Pinillos H, Lalude O, et al. Apoptosis of lung epithelial cells in response to TNF-alpha requires angiotensin II generation de novo. J Cell Physiol. 2000;185:253–9. doi: 10.1002/1097-4652(200011)185:2<253::AID-JCP10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Wang R, Zagariya A, Ang E, Ibarra-Sunga O, Uhal BD. Fas-induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. Am J Physiol. 1999;277:L1245–50. doi: 10.1152/ajplung.1999.277.6.L1245. [DOI] [PubMed] [Google Scholar]
- Day RM, Thiel G, Lum J, Chevere RD, Yang Y, Stevens J, et al. Hepatocyte growth factor regulates angiotensin converting enzyme expression. J Biol Chem. 2004;279:8792–801. doi: 10.1074/jbc.M311140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Suzuki YJ, Griffin AJ, Day RM. Hepatocyte growth factor regulates cyclooxygenase-2 expression via beta-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L778–86. doi: 10.1152/ajplung.00410.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AE, Greenhough A, Roberts HR, Hicks DJ, Patsos HA, Williams AC, et al. HGF/Met signalling promotes PGE(2) biogenesis via regulation of COX-2 and 15-PGDH expression in colorectal cancer cells. Carcinogenesis. 2009;30:1796–804. doi: 10.1093/carcin/bgp183. [DOI] [PubMed] [Google Scholar]
- Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293:L417–28. doi: 10.1152/ajplung.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Deheuninck J, Reveneau S, Foveau B, Ji Z, Villenet C, et al. HGF/SF regulates expression of apoptotic genes in MCF-10A human mammary epithelial cells. Ann N Y Acad Sci. 2006;1090:188–202. doi: 10.1196/annals.1378.021. [DOI] [PubMed] [Google Scholar]
- Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–45. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla MN, Rose JL, Ray R, Lathrop KL, Ray A, Ray P. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol. 2009;40:643–53. doi: 10.1165/rcmb.2008-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas-Read S, Shaul PW, Yuhanna IS, Willis BC. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L212–21. doi: 10.1152/ajplung.00475.2006. [DOI] [PubMed] [Google Scholar]
- Makondo K, Kamikawa A, Ahmed M, Terao A, Saito M, Kimura K. Geldanamycin enhances hepatocyte growth factor stimulation of eNOS phosphorylation in endothelial cells. Eur J Pharmacol. 2008;582:110–5. doi: 10.1016/j.ejphar.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Makondo K, Kimura K, Kitamura N, Kitamura T, Yamaji D, Jung BD, et al. Hepatocyte growth factor activates endothelial nitric oxide synthase by Ca(2+)- and phosphoinositide 3-kinase/Akt-dependent phosphorylation in aortic endothelial cells. Biochem J. 2003;374:63–9. doi: 10.1042/BJ20030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–2. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]