Abstract
Aim:
To determine whether Nrf2 signaling pathway activation could attenuate oxidative stress and neuronal damage following traumatic brain injury (TBI).
Methods:
Controlled cortical impact (CCI) injury was performed in Sprague-Dawley rats and Nrf2-knockout or control mice. Sulforaphane (SFN), a potent Nrf2 activator, was used to activate Nrf2. Oxidative stress, lesion volume, neuron degeneration, and neurologic dysfunction were determined using biochemical, histopathological and neuroethologic approaches. Protein and mRNA levels of Nrf2 and the antioxidant enzymes heme oxygenase 1 (HO-1) and NAD(P)H:quinine oxidoreductase 1 (NQO1) were assessed using Western blot analysis and RT-PCR.
Results:
Activation of Nrf2 by SFN( 5 mg/kg, ip) induced the nuclear translocation and activation of Nrf2, which resulted in an up-regulation of Nrf2-dependent antioxidant enzymes and a reduction of oxidative damage after TBI. In accordance with these biochemical changes, SFN also significantly reduced neuronal death, contusion volume, and neurological dysfunction after TBI. Furthermore, Nrf2-knockout mice showed more severe oxidative stress and neurologic deficits after TBI and did not benefit from the effects of SFN.
Conclusion:
Nrf2 plays a pivotal role in cell defenses against the oxidative stress of TBI. In addition, pharmacological activation of the Nrf2 signaling pathway by small molecule inducers such as SFN attenuated oxidative stress and neuronal damage following TBI.
Keywords: nuclear factor-erythroid 2-related factor 2, sulforaphane, traumatic brain injury, oxidative stress, heme oxygenase 1, NAD(P)H:quinine oxidoreductase 1, neurological dysfunction
Introduction
Increasing evidence suggests that reactive oxygen species (ROS)-induced oxidative stress may play a key role in the pathophysiology of secondary damage after traumatic brain injury (TBI)1, 2. The excessive production of ROS induced by trauma results from excitotoxicity and exhaustion of the endogenous antioxidant system (eg, superoxide dismutase, glutathione peroxidase, and catalase) and causes the peroxidation of lipids, protein oxidation, DNA cleavage, mitochondrial dysfunction and altered signal transduction3, 4, 5. Furthermore, increased oxidative damage in the form of elevated levels of protein nitration and lipid peroxidation was also observed in the cerebrospinal fluid in human TBI, which is associated with a poor outcome2, 6. Thus, the relationship between oxidative stress and TBI has generated considerable interest in the development of antioxidant therapies for neuroprotection. However, despite promising results in the treatment of TBI in animal models, evidence of successful antioxidant therapy in TBI patients is limited7. In addition, there are a number of drawbacks to the use of exogenous antioxidants for treatment, as many antioxidants do not efficiently cross the blood-brain barrier (BBB), are rather unstable in the body, have short therapeutic time windows, and should be given in a very narrow range of therapeutic dosages owing to their toxicity at high doses7, 8. Hence, alternative therapeutic approaches are needed to inhibit the detrimental effects of ROS, for instance, through the induction of endogenous enzymatic antioxidants.
Recent studies have demonstrated that nuclear factor erythroid 2-related factor 2 (Nrf2), a key transcription factor, plays an indispensable role in the induction of endogenous antioxidant enzymes against oxidative stress. Nrf2 belongs to the family of the cap “n” collar basic region-leucine zipper (CNC bZip) transcription factors. Under physiological conditions, Nrf2 is localized within the cytoplasm by binding to its negative regulator, Kelch-like ECH associating protein 1 (Keap1), which promotes Nrf2 ubiquitination by the Cul3-Rbk1 complex and subsequent degradation by the proteasome9. However, upon exposure to ROS, Nrf2 is liberated from the Keap1-Nrf2 complex and translocates from the cytoplasm to the nucleus, where it sequentially binds to the antioxidant response element (ARE), a regulatory enhancer region within gene promoters. This binding induces the production of many phase II detoxifying and antioxidant enzyme genes such as heme oxygenase 1 (HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO1), which protect cells against oxidative stress as well as a wide range of other toxins10, 11.
Recently, many lines of evidence have demonstrated that Nrf2 plays a critical role in the induction of endogenous antioxidant enzymes against oxidative damage in a variety of experimental models. Nrf2 protects cardiac fibroblasts and cardiomyocytes against oxidative stress by increasing detoxification pathways and antioxidant potentials12, 13. In addition, Nrf2 protects the lungs from oxidative injury caused by bleomycin and environmental oxidants including hyperoxia, diesel exhaust particles, and cigarette smoke14, 15, 16. Moreover, it has been reported that Nrf2 plays a critical role in cytoprotection and mitigates oxidative stress by activating antioxidant genes and preventing the pathogenesis of liver and gastrointestinal diseases such as liver fibrosis, gallstone development, drug-induced hepatotoxicity, acute gastric mucosal lesions, and inflammatory bowel diseases17, 18. Furthermore, the protective effects of the Nrf2-ARE axis against oxidative insults in the central nervous system (CNS) have also been reported. Activation of the Nrf2-ARE pathway is able to protect the brain from oxidative stress in both in vitro and in vivo models of neurodegenerative diseases, cerebral ischemia, and intracerebral hemorrhage19, 20, 21, 22, 23. Our previous studies have demonstrated that TBI can induce Nrf2-ARE pathway activation in the brain24, 25. However, the precise role of Nrf2 in limiting oxidative damage in TBI remains obscure.
The present study was designed to evaluate the antioxidative role of Nrf2 in experimental TBI. Controlled cortical impact (CCI) injury was performed in Sprague-Dawley rats and Nrf2-knockout or control mice. Sulforaphane (SFN), a potent Nrf2 activator found in cruciferous vegetables, was used to activate Nrf2. We determined (1) whether SFN could attenuate TBI-induced oxidative damage and (2) whether the antioxidative role of SFN is mediated by activating the Nrf2 signaling pathway. Our work may pave the way to assessing the therapeutic role of Nrf2 activators in patients with TBI.
Materials and methods
Animals
Sprague-Dawley male rats weighing 200–250 g were purchased from the Animal Center of Zhejiang University School of Medicine (Hangzhou, China). Breeding pairs of Nrf2-deficient ICR mice were obtained from the Animal Center of Nanjing University School of Medicine (Nanjing, China). Homozygous wild-type Nrf2+/+ mice and Nrf2−/−-deficient mice were generated from inbred heterozygous Nrf2+/− mice26. Genotypes of Nrf2−/− and Nrf2+/+ mice were confirmed by polymerase chain reaction (PCR) amplification of genomic DNA isolated from the blood. PCR amplification was performed using three different primers, 5′-TGGACGGGACTATTGAAGGCTG-3′ (sense for both genotypes), 5′-CGCCTTTTCAGTAGATGGAGG-3′ (antisense for wild-type) and 5′-GCGGATTGACCGTAATGGGATAGG-3′ (antisense for LacZ). Animals were housed in air-filtered, temperature-controlled units with free access to food and water. All experimental protocols were approved by the Animal Care Committee of Zhejiang University School of Medicine, and all experiments were done in conformity with the Guiding Principles for Research Involving Animals of Zhejiang University School of Medicine.
TBI model
Animals were anesthetized using pentobarbital sodium [50 mg/kg intraperitoneally (ip)]. The method of producing CCI injury has been described previously27. Briefly, the rat head was mounted in a stereotaxic frame by ear bars and an incisor bar. Following a midline incision and retraction of the skin, a 6-mm-diameter craniotomy was made approximately midway between the bregma and the lambda on the right side, with the medial edge of the craniotomy 1 mm lateral to the midline. The skull disk was then removed without disturbing the dura. CCI was performed perpendicular to the brain surface using The Benchmark CCI Stereotaxic Impactor (Benchmark Deluxe™; MyNeurolab, St Louis, MO) with the following parameters: diameter of the impact tip, 5 mm; impact velocity, 4 m/s; impact duration, 120 ms; and displacement of the brain, 2 mm. Core body temperature was maintained at 36.0–36.5 °C during surgery using a rectal thermometer coupled to a heating pad. The mouse CCI model was performed using the same CCI impactor with the following parameters: diameter of the impact tip, 3 mm; impact velocity, 4 m/s; impact duration, 100 ms; and displacement of the brain, 1 mm. After injury, the bone flap was immediately replaced and sealed, and the scalp was sutured closed. Sham-operated animals received identical surgical procedures except for the CCI. All animals were allowed to recover from anesthesia completely after surgery in a heated chamber before being sent back to the home cages.
Experimental design
Animals were randomly assigned to three experimental groups: (a) sham group: animals were subjected to sham surgery; (b) SFN-treated TBI group: animals were subjected to TBI, and SFN (LKT Laboratories, St Paul, MN) at 5 mg/kg in 1% DMSO that was administered ip 15 min later; (c) TBI-vehicle group: animals were subjected to TBI and received equal volumes of 1% DMSO following the same schedule. The dosage and time-point of SFN used here to treat TBI was selected based on previous studies showing that this dosage at this time-point of SFN effectively reduces infarct volume following focal cerebral ischemia and protects the blood brain barrier after brain injury22, 28.
Evaluation of neurological deficits
To examine the effects of the SFN on the neurological deficits of animals after TBI, the neurological functions of each experimented group (n=8) were evaluated at 7 d after injury by an experimenter blinded to the treatment status of the groups using the Modified Neurological Severity Score (mNSS), which included motor, sensory, reflex, and balance tests29.
Tissue processing and histology
For histological and immunohistochemical analysis of the lesioned brains, rats were killed 1 d or 7 d after injury (n=8 per group at each time point). Animals were anesthetized with an overdose of pentobarbital (200 mg/kg, ip) and perfused transcardially, first with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde. The tissues were processed for Fluoro Jade B (FJB) staining (d 1) and for toluidine blue staining (d 7) after injury. Brains were removed and post-fixed in 4% paraformaldehyde overnight. Then, the tissues were cryoprotected using 30% sucrose. Each brain was sectioned coronally (20-μm-thick slices at an interval of 500 μm from −1.4 mm to −7.5 mm with respect to the bregma, covering the cortical lesion according to the rat brain atlas of Paxinos and Watson). Every 12th section per brain was used for FJB staining or toluidine blue staining. The tissue sections were stored at −80 °C until subsequent analysis.
Calculation of cortical lesion volume
Lesion volume was measured as previously described30. The sections were stained with 1% toluidine blue (Sigma-Aldrich, St Louis, MO). Images of all sections were photographed with a stereomicroscope using brightfield illumination (Olympus SZX7) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The volume of the ipsilateral and contralateral hemisphere was calculated using the following formula ∑(An+An+1)×d/2, where A is the corresponding hemispheric area and d is the distance between sections. Hemispheric tissue loss was expressed as a percentage calculated by [(contralateral hemispheric volume-ipsilateral hemispheric volume)/(contralateral hemispheric volume)×100%], as previously reported30.
FJB histochemistry
To assess the extent of neural injury, we used FJB, a polyanionic fluorescein derivative that sensitively and specifically binds to degenerating and dying neurons31. Twenty-micrometer coronal sections were cut on a cryostat as described above and stained with FJB (Chemicon, Temecula, CA) using a published protocol31. In brief, sections were first immersed in a solution containing 1% sodium hydroxide in 80% alcohol for 5 min, followed by 2 min in 70% alcohol and 2 min in distilled water. The slides were then incubated in a solution of 0.06% potassium permanganate for 10 min, rinsed in distilled water for 2 min and incubated in a 0.0004% solution of FJB made in 0.1% acetic acid for 30 min. After being rinsed, slides were air dried, cleared in xylene for a minute and coverslipped in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). All sections were observed and photographed under a fluorescence microscope (Olympus BX-51) with blue (450–490 nm) excitation light. ImageJ software was used for cell counting. FJB staining was quantified on FJB-stained sections between Bregma level −1.4 mm and −7.5 mm. Twelve 20-μm sections per animal were selected and stained with FJB. The region of interest was defined and delineated under a 4×objective on each section as the FJB-positive cells in the contusion margin along the cortex. Using a 20×objective, six randomly selected, nonoverlapping regions with an area of 1.43 mm2 were examined for FJB staining. The total number of FJB-positive cells was expressed as the mean number per field of view. Analysis was conducted by two experimenters who were blind to the condition of the animals.
Biochemical assays in rats and mice
An ELISA was performed to determine the expression of oxidative stress proteins in the brain after TBI. Three groups of rats (sham=8, vehicle=8, SFN-treated=8) and four groups of mice (vehicle Nrf2+/+=8, vehicle Nrf2−/−=8, SFN-treated Nrf2+/+= 8, SFN-treated Nrf2−/−=8) were sacrificed by decapitation at 24 h after TBI. We chose 24 h as a timepoint because in a previous study, oxidative damage increased markedly at 24 h after TBI32. In order to standardize the sampling of cortical tissue, the same sample location and equivalent sample size were obtained under microscopic control. The ipsilateral peri-core parietal cortex from injured rats and the same area in the sham-operated rats were dissected at 24 h after TBI as previously described28. This same sampling procedure was also performed in the four groups of mice. All samples were frozen immediately in liquid nitrogen and stored at −80 °C for subsequent oxidative stress analyses.
Measurement of Protein Carbonyls
Protein carbonyls is an indicator of oxidative stress and a key marker of protein oxidation33. The level of protein carbonyls was determined using the OxiSelect™ Protein Carbonyl ELISA Kit (Cell Biolabs, San Diego, CA) according to the manufacturer's instructions. The protein carbonyl contents were determined by colorimetric analysis at 450 nm using a predetermined protein carbonyl standard curve.
Measurement of lipid peroxidation
The extent of lipid peroxidation in brain homogenates was determined by measuring HNE-bound proteins, which are considered one of the major reactive products and a specific marker of lipid peroxidation33. The level of HNE-bound protein adducts was also quantified using The Oxiselect HNE–His Adduct ELISA Kit (Cell Biolabs, San Diego, CA). The HNE protein adduct content in brain homogenates was determined by comparing its absorbance at 450 nm with a standard curve that is prepared from predetermined HNE-BSA standards.
Measurement of 8-OHdG
8-hydroxy-2'-deoxyguanosine (8-OHdG), which is considered a marker of oxidative injury to DNA, was evaluated using an oxidative DNA ELISA kit (Cell Biolabs, San Diego, CA) following the kit instructions. The 8-OHdG content in brain samples was determined by comparing its absorbance at 450 nm with a predetermined 8-OHdG standard curve. Each sample was assayed in duplicate.
Western blot analysis
Twenty-four hours after TBI, rats (n=8 per group) were decapitated, the brains were quickly removed, and the ipsilateral peri-core parietal cortex (injured) and the equivalent area in the sham-operated rats were rapidly dissected and frozen in dry ice. Protein extraction for Nrf2, HO-1, and NQO1 was performed as follows. The tissue was homogenized in an ice-cold hypotonic lysis buffer consisting of 10 mmol/L HEPES, pH 7.9; 10 mmol/L KCl; 1.5 mmol/L MgCl2; 0.1 mmol/L EDTA; 0.1 mmol/L EGTA; 1 mmol/L DTT; and a 1:1000 protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO) for 15 min. After adding NP-40, the samples were centrifuged at 10 000 rpm at 4 °C for 3 min. We then collected the supernatant as cytoplasmic protein for HO-1, NQO1. The pellets were resuspended in ice-cold nuclear extract buffer (20 mmol/L HEPES, pH 7.9; 420 mmol/L NaCl; 1.5 mmol/L MgCl2; 1 mmol/L EDTA; 1 mmol/L EGTA; and 1 mmol/L DTT containing proteinase inhibitors) for 15 min. Then, the pellets were centrifuged at 12 000 rpm and 4 °C for 10 min, and the supernatant was collected and added with PMSF to the final concentration of 1 mmol/L as the nuclear protein for Nrf2.
Protein concentration was assayed using the BCA Protein Assay reagent kit (Novagen, Madison, WI), and equal amounts (50 μg) of protein per sample were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to a nitrocellulose membrane. The nonspecific binding of antibodies was blocked using TBS with 5% nonfat milk and with 0.1% Tween-20 for 2 h at room temperature. Membranes were then incubated overnight with the primary antibody at the appropriate dilution (rabbit anti-Nrf2, 1:200, Santa Cruz Biotechnology; rabbit anti-HO-1, 1:2000, Epitomics, Burlingame, USA; rabbit anti-NQO1 antibody, 1:200, Santa Cruz Biotechnology). Protein loading was normalized by Western blotting to histone 1 (H1, 1:1000, Santa Cruz Biotechnology) or anti-β-actin (1:2000; Santa Cruz Biotechnology). Subsequently, the membranes were incubated for 1 h at room temperature with the corresponding horseradish peroxidase-conjugated secondary antibodies (1:3000, goat anti-rabbit; 1:5000, goat anti-mouse, both from Chemicon, Temecula, CA). Finally, immunoreactive bands were detected by chemiluminescence using the Amersham ECL Plus Western Blotting Detection kit (GE Healthcare Bio-Sciences, Piscataway, NJ), according to the manufacturer's instructions. Nrf2 expression levels were normalized to H1, while HO-1 and NQO1 expression levels were normalized to β-actin.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
The mRNA expression levels of HO-1 and NQO1 were determined by RT-PCR. At 24 h after TBI, rats (n=8 per group) were reanesthetized and the brains were removed and frozen in liquid nitrogen. Total RNA from the injured cortex was extracted using TriPure Reagent (Roche Diagnostics Corp, Indianapolis, IN) following the manufacturer's protocol. The cDNA synthesis from the isolated RNA was performed using a reverse transcriptional system. Primer sequences and PCR conditions are described below: HO-1, 5′-ATCGTGCTCGCATGAACACT-3′ and 5′-CCAACACTGCATTTACATGGC-3′ NQO1, 5′-ACTCGGAGAACTTTCAGTACC-3′ and 5′-TTGGAGCAAAGTAGAGTGGT-3′ and β-actin, 5′-AGTGTGACGTTGACATCCGTA-3′ and 5′-GCCAGAGCAGTAATCTCCTTCT-3′. PCR amplification was performed with a program of 3 min incubation at 94 °C followed by 30 reaction cycles (denaturation at 94 °C for 30 s, annealing at 55 °C for 50 s, and extension at 72 °C for 1 min) and by a final extension at 72 °C for 10 min. PCR products were analyzed by agarose gel electrophoresis in 2 % NuSieve agarose gels (FMC, Rockland, ME) and visualized by ethidium bromide staining. The intensity of the bands was quantified using Glyko Bandscan software (Glyko, Novato, CA). HO-1/β-actin and NQO1/β-actin product ratios were calculated and considered as the index of HO-1 and NQO1 mRNA expression, respectively. The experiments were performed in triplicate to verify the results.
Statistical analysis
SPSS software 13.0 (SPSS, Inc, Chicago, IL) was used for the statistical analysis. All data in this study were expressed as means±SEM. A one-way analysis of variance (ANOVA) followed by the appropriate post hoc analysis was used to determine individual group differences between SFN-treated, vehicle-treated, and sham-control groups. Student's t-test was used to analyze the differences between the vehicle-treated and SFN-treated groups within a single genotype as well as between genotypes. A value of P<0.05 was considered statistically significant.
Results
SFN treatment improves neurologic function after TBI in rats
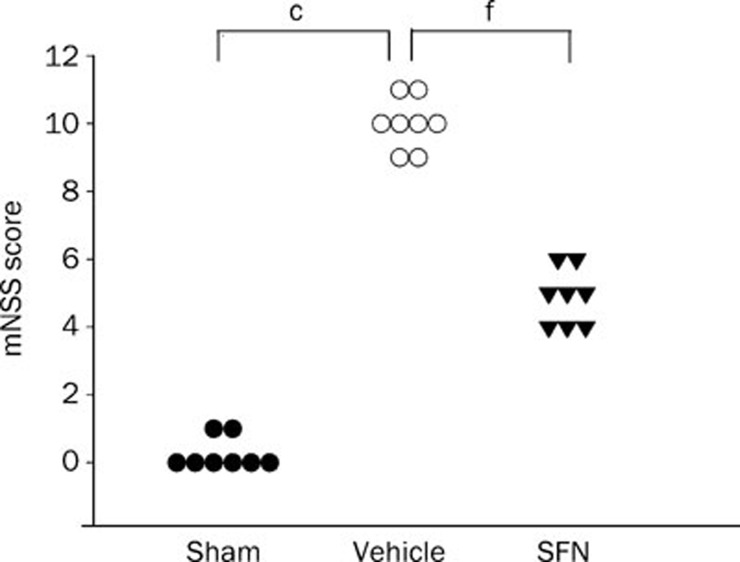
As shown in Figure 1, CCI resulted in neurologic functional deficits as measured by mNSS. The mNSS scores were significantly higher in the vehicle-treated TBI group than in the sham operation group (P<0.01). Compared with the vehicle-treated group, the SFN-treatment resulted in a significantly greater reduction of neurologic deficits 7 d after TBI (P<0.01).
Figure 1.
Effect of SFN on neurologic function after TBI in rats. Animals received SFN (5 mg/kg ip) or DMSO treatment 15 min after TBI. mNSS was measured 7 d after TBI. SFN treatment resulted in a significantly greater reduction of neurologic deficits relative to the vehicle treatment group. Data are expressed as means±SEM. n=8 rats per group. cP<0.01 vs sham operation group. fP<0.01 vs vehicle group, analyzed by one-way ANOVA.
SFN treatment reduces brain contusion volume and cortical neuronal death
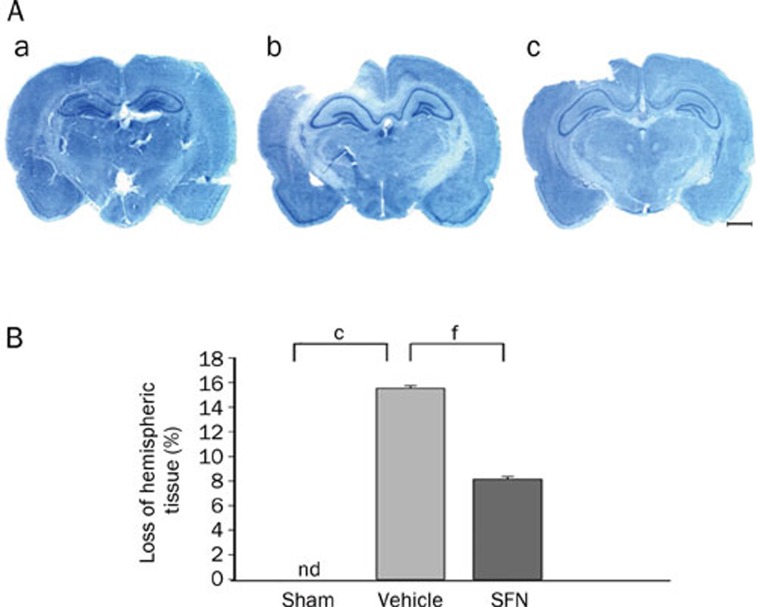
CCI induced a significant loss of cortical tissue in the ipsilateral parietal cortex at 7 d after injury, as reflected by the gross reduction in toluidine blue staining intensity. However, the contralateral cortex tissue remained normal. Treatment with SFN significantly reduced contusion volume compared with the vehicle control (P<0.01) (Figure 2).
Figure 2.
Effect of SFN on cortical contusion volume as evaluated by toluidine blue staining 7 d after TBI. (A) Representative photographs of 20-μm-thick coronal slices (-3.8 mm caudal from bregma) with toluidine blue staining. (a) sham operation group. (b) vehicle-treated TBI group. (c) SFN-treated TBI group. There is a considerable lesion in the injured hemisphere of the vehicle-treated TBI rat, and the lesion in the SFN-treated rat is much smaller. Scale bar, 2.5 mm. (B) Loss of hemispheric tissue. CCI induced the significant loss of hemispheric tissue, which was reduced in the SFN-treated group. Data are expressed as means±SEM. n=8 rats per group. cP< 0.01 vs sham operation group. fP<0.01 vs vehicle group, analyzed by one-way ANOVA.
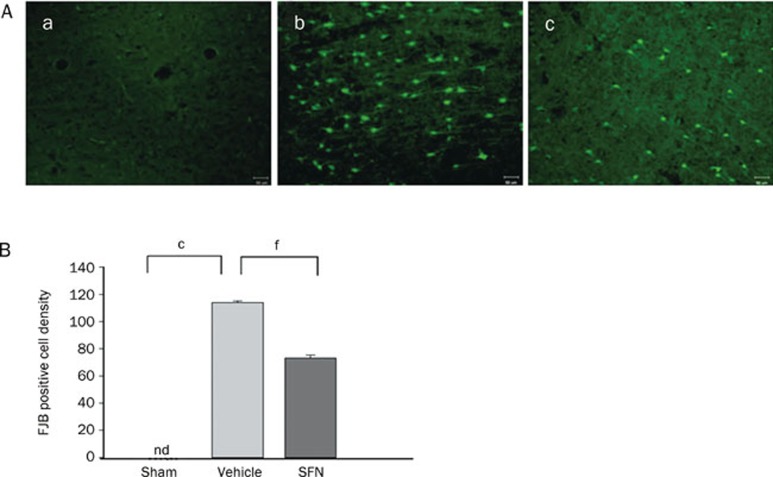
Differences in FJB staining, a sensitive and specific marker of degenerating and dying neurons, have also been observed in our study. In the sham operation group, there were no FJB-positive cells detected. At 24 h after CCI, FJB-positive cells with neuronal morphology were evident in the cortical contusion margin in the ipsilateral but not in the contralateral hemisphere, which indicated that CCI resulted in significant cortical neuronal damage in the ipsilateral cortex but not in the contralateral cortex. SFN treatment significantly reduced (by 31%) the number of FJB-positive cells compared with the vehicle treatment (P<0.01) (Figure 3).
Figure 3.
Effect of SFN treatment on the changes in the degenerated neuron density in injured cerebral cortex as evaluated by FJB staining at 24 h after TBI in rats. (A) Representative images of FJB-positive cells in sham (a), vehicle (b), and SFN (c) treated rats. There was a marked decrease in the number of FJB-positive cells after SFN treatment. Scare bar, 50 μm. (B) Bar graphs of mean densities of FJB-positive cells in the three groups showing a significant decrease in the number of FJB-positive cells in the SFN-treated group. The total number of FJB-positive cells was expressed as the mean number per field of view (1.43 mm2). Data are expressed as means±SEM. n=8 rats per group. cP<0.01 vs sham operation group. fP<0.01 vs vehicle group, analyzed by one-way ANOVA.
SFN reduces oxidative damage after TBI
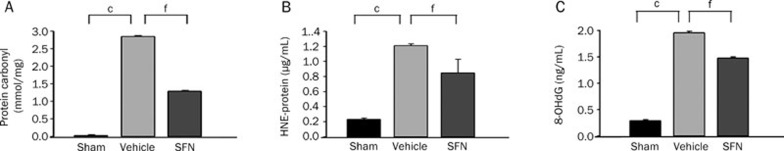
ROS-induced damage to lipids, proteins and DNA plays an important role in TBI pathogenesis. To determine whether SFN affects oxidative stress, we treated the rats with SFN and tested the level of oxidation-mediated changes in proteins (protein carbonyls), lipids (4-HNE), and DNA (8-OHdG) in the injured cortex 24 h after TBI by ELISA. Basal levels of protein carbonyls, 4-HNE, and 8-OHdG protein levels were low in the cortex of sham-operated animals. After injury, protein carbonyls, 4-HNE, and 8-OHdG protein levels in the ipsilateral cortex of vehicle-treated TBI rats were significantly increased compared with sham rats, which indicates that TBI induced marked oxidative damage surrounding the injury site. Treatment with SFN significantly reduced the injury-induced increase of all three oxidative stress markers in cortical tissue (Figure 4).
Figure 4.
Effect of SFN treatment on the changes in oxidative stress in the injured cortex as assessed by ELISA 24 h after TBI in rats. Bar graphs of protein carbonyls (A), 4-HNE (B), and 8-OHdG (C) protein concentrations in sham control, vehicle and SFN-treated injured rats in ipsilateral cortices 24 h after TBI. CCI significantly elevated protein carbonyls, 4-HNE, and 8-OHdG protein concentrations and SFN treatment significantly reduced the injury-induced increases compared with vehicle-treated injured rats. Data are expressed as means±SEM. n=8 rats per group. cP<0.01 vs sham operation group. fP<0.01 vs vehicle group, analyzed by one-way ANOVA.
SFN induces nuclear Nrf2 activation and upregulates Nrf2-dependent antioxidative and detoxifying enzymes after TBI
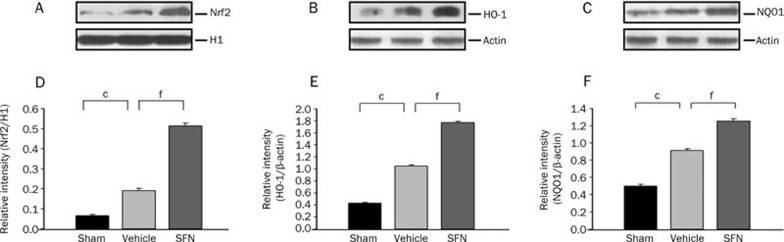
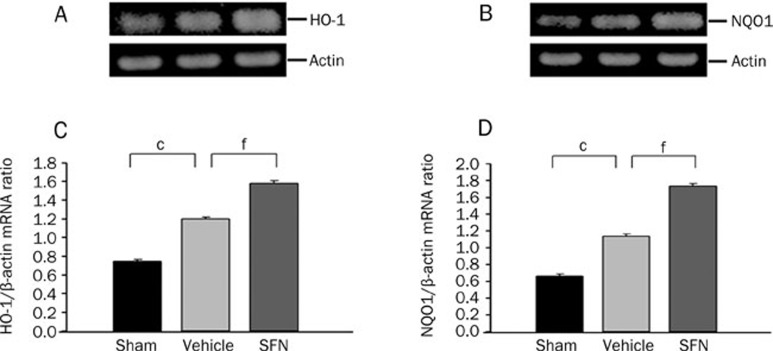
To test whether SFN was capable of inducing nuclear translocation and activation of Nrf2 after TBI, we measured Nrf2 protein in the nuclear extracts prepared from the injured ipsilateral cortex. Western blotting showed that the protein levels of nuclear Nrf2 were upregulated at 24 h after TBI, which was consistent with our previous study and suggests that Nrf2 might be activated by oxidative stress after TBI. Additionally, the protein expression of Nrf2 in the nuclei increased significantly after treatment with SFN when compared with the vehicle control. We also tested the expression of Nrf2-dependent antioxidative and detoxifying enzymes after SFN treatment under TBI. We found that both the mRNA and the protein levels of Nrf2-regulated genes encoding important antioxidative enzymes, including HO-1 and NQO1, were upregulated in the ipsilateral cortex in the vehicle control, but were significantly more upregulated in the SFN-treated animals (Figure 5, 6).
Figure 5.
Western blot analysis for the nuclear Nrf2, HO-1, and NQO1 protein levels 24 h after TBI and SFN treatment. (A−C) Representative photographs of the Western blot show Nr2, HO-1, and NQO1 protein levels in sham, vehicle and SFN-treatment animals, respectively. (D−F) The graphs show that Nrf2, HO-1, and NQO1 protein levels were increased after TBI and more significantly induced by treatment with SFN. Bars represent means±SEM, presented as a ratio of the H1 or β-actin value. n=8 rats per group. cP<0.01 vs sham operation group. fP<0.01 vs vehicle group, analyzed by one-way ANOVA.
Figure 6.
RT-PCR analysis for HO-1 and NQO1 mRNA levels at 24 h after TBI and SFN treatment. (A, B) Representative RT-PCR shows HO-1 and NQO1 mRNA levels in sham, vehicle and SFN-treated animals, respectively. (C, D) Quantification of HO-1 and NQO1 mRNA levels. The graphs show that significantly increased HO-1 and NQO1 mRNA levels were induced after TBI and more significantly induced by treatment with SFN. Bars represent means±SEM, presented as a ratio of the β-actin value. n=8 rats per group. cP<0.01 vs sham operation group, fP<0.01 vs vehicle group, analyzed by one-way ANOVA.
Nrf2-knockout mice exhibit exacerbated neurologic functional deficits and oxidative damage and do not respond to SFN after TBI
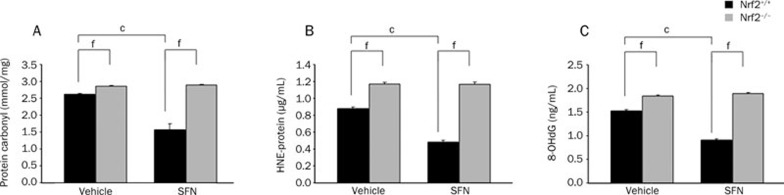
To further verify the special role of Nrf2 in oxidative stress after TBI, we analyzed markers of oxidative stress and neurologic function in both Nrf2-knockout mice and wild-type mice subjected to TBI in the presence or absence of SFN. As expected, Nrf2−/− mice had more oxidative markers such as protein carbonyls, 4-HNE, and 8-OHdG in the injured area and more severe neurologic dysfunction after TBI compared with their wild-type Nrf2+/+ counterparts. Furthermore, the pharmacologic activation of Nrf2 with SFN significantly reduced the oxidative damage and neurologic deficient produced by TBI in Nrf2+/+ mice but not in mice lacking the Nrf2 gene, confirming both the neuroprotective action and the Nrf2 dependence of SFN in vivo (Figure 7, 8).
Figure 7.
The mNSS score in SFN-treated Nrf2+/+ and Nrf2−/− mice 7 d after TBI. mNSS was significantly increased after TBI and was higher in Nrf2−/− mice than in Nrf2+/+ mice. SFN treatment decreased mNSS in Nrf2+/+ mice but not in Nrf2−/− mice. Data are expressed as means±SEM. n=8 mice per group. cP<0.01 vs genotype-matched vehicle group; fP<0.01 vs treatment-matched or vehicle-matched Nrf2+/+ mice, analyzed by Student's t-test.
Figure 8.
Protein expression levels of oxidative stress in SFN-treated Nrf2+/+ and Nrf2−/− mice 24 h after TBI. The markers of oxidative stress, protein carbonyls (A), 4-HNE (B), and 8-OHdG (C), were significantly increased after TBI, and were higher in Nrf2−/− mice than in Nrf2+/+ mice. SFN treatment decreased oxidative damage in Nrf2+/+ mice but not in Nrf2−/− mice. Data are expressed as means±SEM. n=8 mice per group; cP<0.01 vs genotype-matched vehicle group, fP<0.01 vs treatment-matched or vehicle-matched Nrf2+/+ mice, analyzed by Student's t-test.
Discussion
In the present study, we used pharmacologic and genetic strategies to shed light on the role of Nrf2 in antioxidant defense in TBI. First, in a rat CCI model, we demonstrated that the pharmacological activation of Nrf2 by SFN treatment protected the brain against TBI-induced oxidative damage. SFN induced the nuclear translocation and activation of Nrf2, which consequently resulted in the upregulation of a battery of Nrf2-dependent antioxidative and detoxifying enzymes, as well as a reduction in oxidative damage around the damaged area after TBI. In accordance with these biochemical changes, the neurologic and histologic evaluation revealed that SFN also significantly reduced neuronal death, contusion volume, and neurological dysfunction after TBI. Second, in Nrf2-knockout mice, we showed that the loss of Nrf2 in vivo exacerbates oxidative damage after TBI and abrogates the protective effects of SFN, consistent with a specific role for Nrf2. Collectively, these results highlight the important role of Nrf2 in counteracting TBI-induced oxidative stress.
Recently, the Nrf2 signaling pathway, which has been considered an endogenous antioxidant mechanism in the cellular defense against oxidative or electrophilic stress, has generating increasing interest. The ability to induce phase 2 detoxifying genes and antioxidant enzymes at the transcriptional level could have significant advantages over more conventional approaches. Numerous studies have reported that Nrf2 plays a critical role in counteracting oxidative stress in many organs, such as the heart, lung, liver, kidney and gastrointestinal tract12, 13, 14, 15, 16, 17, 18. Recently, Nrf2 has also been considered a crucial regulator of oxidative stress in several CNS diseases. Nrf2-induced antioxidant enzymes are able to protect primary astrocytes from ROS-induced apoptosis, and decreased levels result in increased susceptibility to oxidative stress34. In addition, astrocytes overexpressing Nrf2 are able to rescue neurons from oxidative stress35. Furthermore, activation of the Nrf2 pathway by a pharmacologic inducer is able to protect neurons from oxidative stress in animal models of neurodegeneration, cerebral ischemia and intracerebral hemorrhage19, 20, 21, 22, 23. However, the protective effect of Nrf2 on TBI is less known. Our and others' previous studies have shown that the Nrf2-ARE pathway was activated in the brain and played an important role in limiting inflammation and apoptosis after TBI24, 25, 36, 37. Zhao and Dash et al have shown that SFN, a potential Nrf2 inducer, attenuates blood-brain barrier permeability, reduces cerebral edema and improves cognitive function after TBI28, 38, 39. However, the antioxidative role of Nrf2 in inhibiting free radical release and neuronal death around the damaged area after TBI remains unknown. In addition, there was no direct evidence that an exogenous Nrf2-inducer such as SFN was capable of inducing the nuclear translocation and activation of Nrf2 after TBI. The above questions have been addressed in our current research.
Using a rat CCI model, we found that the CCI episode in the rat parietal cortex induced marked oxidative damage surrounding the injury site, as demonstrated by elevated levels of oxidative stress markers, such as protein carbonyls, 4-HNE, and 8-OHdG. Consistent with these biochemical changes, histologic and neurologic deficits were also exacerbated after TBI, as evidenced by neuronal death, contusion volume and neurological dysfunction. These data are in agreement with the result of a previous study, which showed that oxidative stress plays a major role in neuronal death and functional disability after TBI1, 2. Moreover, we found that the systemic administration of SFN, a potent Nrf2 activator, attenuates TBI-induced oxidative stress and confers histologic and neurologic protection. Consistent with these data, SFN was previously shown to protect the brain from oxidative damage produced by intracerebral hemorrhage23. However, questions remain as to how SFN can suppress oxidative damage after TBI.
SFN, a naturally occurring isothiocyanate present in abundance in cruciferous vegetables, is well known for its chemopreventive and antioxidant properties. Although the molecular targets of this molecule are not completely characterized, the best known effect of SFN is to induce Nrf2-dependent gene expression28. An in vitro study has demonstrated that SFN can disrupt the Nrf2/Keap1 interaction, leading to Nrf2 stabilization and nuclear localization and the expression of ARE-containing phase II genes, which play a major role in the detoxification of ROS produced by xenobiotics10, 11. However, there is no direct evidence regarding whether the antioxidative effect of SFN on TBI is mediated by the Nrf2 signaling pathway and whether SFN is capable of inducing nuclear translocation and activation of Nrf2 under the TBI experimental condition. Therefore, the activity of SFN on the Nrf2 signaling pathway was further investigated.
In the present study, we found that the expression of nuclear Nrf2 is modestly upregulated after TBI, as indicated by western blot analysis. This finding is consistent with our previous study and suggests that the Nrf2 pathway can be activated by oxidative stress after TBI24, 25. However, this Nrf2 activation is not enough to prevent TBI-induced oxidative damage and neuronal death, which is confirmed by our present result. While using SFN treatment after TBI, we showed that the level of nuclear Nrf2 is significantly increased compared with that of the vehicle-treatment group. Meanwhile, the mRNA and protein expressions of downstream genes that boost cell detoxification processes and antioxidant potential, such an HO-1 and NQO1, are coordinately upregulated. Increasing evidence demonstrates the protective role of these Nrf2-regulated gene products in CNS disease. HO-1, also known as heat shock protein 32, has received special attention because of its protective effects against oxidative stress-induced brain damage by degrading toxic heme into free iron, carbon monoxide and biliverdin40, 41. The latter could be further reduced by biliverdin reductase to bilirubin, which serves as a potent radical scavenger and protects cells against oxidative stress42. Additionally, gene-knockout mice have also confirmed that HO-1 plays a crucial role in the endogenous defense against oxidative stress because cells from HO-1−/− mice have an increased susceptibility to oxidative insults43. NQO1 is another well-known Nrf2 induced gene that posses antioxidative and detoxifying activity. NQO1 is a cytosolic flavoprotein that catalyzes the two-electron reduction of quinones, preventing their participation in redox cycling and the subsequent generation of ROS as well as having broad-spectrum antioxidant properties44. Recently, ample evidence suggests that NQO1 serves as a neuroprotective guard to sequester ROS and prevent cellular injury in brain ischemia and various neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis45, 46, 47, 48. In summary, our study provides direct evidence that the Nrf2 signaling pathway can be activated by SFN under the TBI experimental condition, an activity that may play an important role in preventing oxidative stress-induced brain damage.
However, it is unclear whether a causal relation exists between the Nrf2-ARE pathway activation by SFN and its antioxidative effect in TBI. To further verify the special role of Nrf2 in oxidative stress after TBI, we used the TBI model of Nrf2 knockout mice. Our study revealed that the disruption of Nrf2 in mice caused higher sensitivity to oxidative stress after experimental TBI. Nrf2−/− mice showed higher levels of oxidative markers such as protein carbonyls, 4-HNE, and 8-OHdG production in the injured area and more severe neurologic dysfunction after TBI compared with their wild-type Nrf2+/+ counterparts. Furthermore, pharmacological activation of Nrf2 with SFN significantly reduced oxidative damage and neurologic deficiencies produced by TBI in Nrf2+/+ mice but not in mice lacking the Nrf2 gene, confirming both the neuroprotective action and the Nrf2 dependence of SFN in vivo. Thus, these findings combined with the above results further verify that Nrf2 plays a crucial role in protecting the brain from oxidative damage caused by TBI and that the enhancement of Nrf2 activity might be a viable strategy to prime the antioxidant capacity of the brain, thereby attenuating oxidative injury caused by TBI.
In conclusion, our results suggest that Nrf2 plays a pivotal role in cell defenses against oxidative stress in TBI. In addition, pharmacologic activation of the Nrf2 signaling pathway by small-molecule inducers such an SFN might be a practical preventative treatment for TBI patients.
Author contribution
Jian-min ZhANG designed the research; Yuan HONG and Wei YAN performed the resarch; Sheng CHEN contributed new analytical tools and reagents; Chong-ran SUN analyzed the data; Yuan HONG and Wei YAN wrote the paper.
Acknowledgments
This work was funded by the Research Fund for the Doctoral Program of Higher Education of China (Grant No 0090101120118) and Zhejiang Provincial Natural Science Foundation in China (Grant No Y2090098).
References
- Clausen F, Lundqvist H, Ermark S, Lewén A, Ebendal T, Hillered L. Oxygen free radical dependent activation of extracellular signal-regulated kinase mediates apoptosis-like cell death after traumatic brain injury. J Neurotrauma. 2004;21:1168–82. doi: 10.1089/neu.2004.21.1168. [DOI] [PubMed] [Google Scholar]
- Darwish RS, Amiridze N, Aarabi B. Nitrotyrosine as an oxidative stress marker: evidence for involvement in neurologic outcome in human traumatic brain injury. J Trauma. 2007;63:439–42. doi: 10.1097/TA.0b013e318069178a. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J Neurosci Res. 2007;85:2216–23. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. 2002;51:571–8. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Rigg JL, Elovic EP, Greenwald BD. A review of the effectiveness of antioxidant therapy to reduce neuronal damage in acute traumatic brain injury. J Head Trauma Rehabil. 2005;20:389–91. doi: 10.1097/00001199-200507000-00010. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–84. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- Sekhar KR, Yan XX, Freeman ML. Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene. 2002;21:6829–34. doi: 10.1038/sj.onc.1205905. [DOI] [PubMed] [Google Scholar]
- Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, et al. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem. 1998;273:7382–9. doi: 10.1074/jbc.273.13.7382. [DOI] [PubMed] [Google Scholar]
- Ku BM, Joo Y, Mun J, Roh GS, Kang SS, Cho GJ, et al. Heme oxygenase protects hippocampal neurons from ethanol-induced neurotoxicity. Neurosci Lett. 2006;405:168–71. doi: 10.1016/j.neulet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–36. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Purdom-Dickinson SE, Lin Y, Dedek M, Morrissy S, Johnson J, Chen QM. Induction of antioxidant and detoxification response by oxidants in cardiomyocytes: evidence from gene expression profiling and activation of Nrf2 transcription factor. J Mol Cell Cardiol. 2007;42:159–76. doi: 10.1016/j.yjmcc.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–59. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, et al. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol. 2008;128:366–373. doi: 10.1016/j.clim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Manautou JE. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol Pathol. 2007;35:459–73. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- Ueda K, Ueyama T, Yoshida K, Kimura H, Ito T, Shimizu Y, et al. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol. 2008;295:G460–9. doi: 10.1152/ajpgi.00204.2007. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–9. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106:2933–8. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–35. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–12. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–6. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- Yan W, Wang HD, Hu ZG, Wang QF, Yin HX. Activation of Nrf2-ARE pathway in brain after traumatic brain injury. Neurosci Lett. 2008;431:150–4. doi: 10.1016/j.neulet.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Yan W, Wang HD, Feng XM, Ding YS, Jin W, Tang K. The expression of NF-E2-related factor 2 in the rat brain after traumatic brain injury. J Trauma. 2009;66:1431–5. doi: 10.1097/TA.0b013e318180f5c7. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Brody DL, Mac Donald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657–73. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–8. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–8. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Zhang C, Raghupathi R, Saatman KE, Smith DH, Stutzmann JM, Wahl F, et al. Riluzole attenuates cortical lesion size, but not hippocampal neuronal loss, following traumatic brain injury in the rat. J Neurosci Res. 1998;52:342–9. doi: 10.1002/(SICI)1097-4547(19980501)52:3<342::AID-JNR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma. 2007;24:1119–31. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–60. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–38. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lucius R, Sievers J. Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996;743:56–62. doi: 10.1016/s0006-8993(96)01029-3. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Xu L, Wang X, Zhao X, et al. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008;2008:725174. doi: 10.1155/2008/725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y, et al. Role of Nrf2 in protection against traumatic brain injury in mice. J Neurotrauma. 2009;26:131–9. doi: 10.1089/neu.2008.0655. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett. 2009;460:103–7. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C. Heme oxygenase and its products in the nervous system. Antioxid Redox Signal. 2004;6:878–87. doi: 10.1089/ars.2004.6.878. [DOI] [PubMed] [Google Scholar]
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–13. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol. 1995;49:127–40. doi: 10.1016/s0006-2952(94)00333-5. [DOI] [PubMed] [Google Scholar]
- Laxton AW, Sun MC, Shen H, Murphy TH, Honey CR. The antioxidant enzyme quinone reductase is up-regulated in vivo following cerebral ischemia. Neuroreport. 2001;12:1045–8. doi: 10.1097/00001756-200104170-00036. [DOI] [PubMed] [Google Scholar]
- SantaCruz KS, Yazlovitskaya E, Collins J, Johnson J, DeCarli C. Regional NAD(P)H:quinone oxidoreductase activity in Alzheimer's disease. Neurobiol Aging. 2004;25:63–9. doi: 10.1016/s0197-4580(03)00117-9. [DOI] [PubMed] [Google Scholar]
- van Muiswinkel FL, de Vos RA, Bol JG, Andringa G, Jansen Steur EN, Ross D, et al. Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol Aging. 2004;25:1253–62. doi: 10.1016/j.neurobiolaging.2003.12.010. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Schreibelt G, Bö L, Montagne L, Drukarch B, van Muiswinkel FL, et al. NAD(P)H:quinone oxidoreductase 1 expression in multiple sclerosis lesions. Free Radic Biol Med. 2006;41:311–7. doi: 10.1016/j.freeradbiomed.2006.04.013. [DOI] [PubMed] [Google Scholar]