Abstract
Aim:
Histamine plays an important role in morphine addiction and memory-dependent behavior. However, little is known about the effect of histamine on the impairment of memory after morphine withdrawal. This study was designed to investigate the effect of histamine on memory impairment induced by morphine withdrawal in histidine decarboxylase knockout (HDC-KO) and wild-type (WT) mice.
Methods:
WT and HDC-KO mice were given subcutaneous morphine or saline twice daily for 5 consecutive days. The mice received a cued or contextual fear conditioning session 7 days after the last injection. During subsequent days, mice received 4 cued or contextual extinction sessions (one session per day). Western blot was used to assess extracellular signal-regulated kinase (ERK) phosphorylation in the amygdala and hippocampus.
Results:
Morphine withdrawal did not affect the acquisition of cued or contextual fear responses. It impaired cued but not contextual fear extinction. The acquisition of cued and contextual fear responses was accelerated in HDC-KO mice. Histamine deficiency aggravated the impairment of cued fear extinction induced by morphine withdrawal, whereas histamine (icv, 5 μg/mouse) reversed this effect. Morphine withdrawal decreased ERK phosphorylation in the amygdala after cued fear extinction, especially in HDC-KO mice.
Conclusion:
These results suggest that morphine withdrawal specifically impairs cued fear extinction and histamine ameliorates this impairment. Its action might be mediated by the modulation of ERK phosphorylation in the amygdala. Histamine should be explored for possible roles in the prevention or treatment of morphine abuse and relapse.
Keywords: Fear extinction, Histamine, Morphine, Amygdala, Histidine decarboxylase gene knockout mice, Extracellular signal-regulated kinase
Introduction
Cumulative evidence indicates that chronic drug abuse is associated with impaired cognitive function1, 2, 3, which may contribute to negative long-term consequences in chronic drug users. Chronic exposure to opiates modulates memory processes in animal models. For example, chronic morphine treatment impairs residual working memory in rats, as well as the acquisition of spatial long-term memory in both radial maze and Y-maze behavioral tests4. Pu et al reported that morphine impairs the acquisition of spatial memory in the water maze with a regimen of equal daily doses or escalating doses, and this is restored after drug withdrawal5. Furthermore, chronic morphine inhibits cued fear extinction in rats6. These findings suggest that cognitive impairments contribute to drug misuse and drug relapse, and it is proposed that an improvement of cognitive impairment modulates morphine addiction.
The histaminergic system arises from the tuberomammillary nucleus of the posterior hypothalamus; it receives input mainly from the limbic system and projects efferents to all parts of the brain, including the amygdaloid-hippocampal formation7. Histamine is involved in regulating cognitive behavior. For example, it facilitates memory retrieval deficits induced by aging, hippocampal lesions, and scopolamine, as determined through passive and active avoidance tasks and an 8-arm radial maze test for rats8, 9, 10. α-Fluoromethylhistidine, a selective histidine decarboxylase (HDC) inhibitor, induces significant memory deficits in an active avoidance task and the 8-arm radial maze in rats11. Recently, it has been reported that histamine also participates in regulating drug abuse mechanisms: it attenuates the rewarding effect of morphine12. Histamine precursors, such as histidine and carnosine, inhibit the rewarding effect of morphine, but lesions of histaminergic neurons facilitate morphine addiction by modulating morphine-induced rewards13, 14. Therefore, histamine may help in developing treatments for opiate addiction by modulating cognitive changes that occur with chronic opiate use. Zarrindast et al recently reported that histamine interacts with opioidergic systems in learning and memory15. Histamine reverses the impairment of rat memory recall induced by pre-training administration of morphine, as evaluated by the passive avoidance response16, 17.
Classic fear conditioning is one of the most widely used paradigms to evaluate associative emotional learning. Animals are trained by pairing a cued or contextual conditioned stimulus (CS) with a foot-shock unconditioned stimulus (US). After repeated training, the previously neutral CS then produces a hypothetical state of fear that is expressed as freezing, which is one of the most prominent behavioral signs of fear in rats. Repeated or sustained presentation of the CS in the absence of the US results in a progressive decrease of the fear response, a phenomenon called fear extinction18, which is a form of new learning. Therefore, in the present study, we explored the effects of histamine on the impairment of fear extinction after chronic morphine treatment, as evaluated by the classic fear-conditioning model in histidine decarboxylase knockout (HDC-KO) mice that lack the enzyme for histamine synthesis. Furthermore, we analyzed by immunoblot the activation of ERK2, which is involved in the extinction training process19, 20, in the amygdala, medial prefrontal cortex, and hippocampus of mice following fear extinction.
Materials and methods
Animals
Male wild-type (WT) and HDC-KO mice, which were kindly provided by Professor Ohtsu (Tohoku University, Japan), were used. Both HDC-KO and WT mice had a pure C57BL/6 genetic background. To confirm the genotypes of the mice with respect to the HDC gene, tail biopsies were taken randomly from mice used in the experiment and analyzed by PCR, according to the procedure described in previous work21, 22. The animals were housed individually in a temperature and humidity-controlled environment with ad libitum access to food and water. Animals were maintained on a 12 h light/dark schedule. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals; every effort was made to minimize any pain or discomfort, and the minimum number of animals was used.
Chronic morphine treatment
Morphine (10 g/L, Shenyang Pharmaceutical Factory, Shenyang, China) was dissolved in sterile saline that was used as the control solution. Animals were chronically treated by subcutaneous injection of morphine twice per day at 12 h intervals for 5 days. The dose ranged from 10 mg/kg on day 1 to 50 mg/kg on day 5. Control mice were treated similarly, except that only saline was used.
Spontaneous somatic signs of morphine withdrawal
At 24 h and 7 days after the last saline or morphine injection, mice were individually placed in transparent plexiglass cylinders (23 cm diameter, 50 cm high) for 15 min, and somatic signs of morphine withdrawal were quantified by direct observation. Mouse behavior was scored by an experimenter blinded to the treatment condition. Jumping, paw tremor, rearing, chewing and wet dog shake behaviors were recorded as the number of events occurring during the total test time; ptosis events were recorded as the number of 3-min intervals in which they occurred (maximum score=5).
Intracerebroventricular injection of histamine
After chronic morphine treatment, mice were anesthetized with sodium pentobarbital (35 mg/kg, ip), and fixed on a stereotaxic apparatus (Narishige, SR-5, Tokyo, Japan). A guide cannula (0.5 mm outer diameter) made of stainless steel tubing was implanted into the right lateral ventricle. At least 7 days were allowed for recovery from the surgery. Histamine or saline was injected intracerebroventricularly (icv, 5 μg in 0.2 mL saline) at a perfusion rate of 0.2 mL/min, 10 min before the extinction session in HDC-KO mice.
Behavioral procedures
Apparatus
Cued fear conditioning and extinction training were conducted in two different observation boxes, A and B. Box A (25.4 cm×20.3 cm×35.7 cm) was located in a brightly lit and isolated room. Illumination was provided by an LED house light mounted on the box ceiling, and 65 dB background noise was supplied by a ventilation fan in the cabinet. Three of the walls in box A were constructed of stainless steel and the other wall was made of transparent opaque plexiglass. The floor of the box consisted of 31 stainless steel rods (3 mm in diameter), spaced 0.5 cm center to center, that were connected to a shock source for the delivery of foot-shocks (US). An acoustic CS (2800 Hz, 85 dB) was presented through a speaker mounted on the box ceiling. Stimulus presentations were controlled by a custom-written computer program. The chamber was washed with 75% ethanol between each mouse before habituation, fear conditioning, and extinction training. Box B was the same as box A, except that three walls of box B were constructed of black opaque plexiglass and the other wall was of transparent opaque plexiglass. The floor of box B was covered with a smooth black plastic sheet. For contextual fear conditioning and extinction training, a behavioral apparatus identical to that used in context A was utilized.
Experiment 1
In experiment 1, we tested the effects of morphine withdrawal on the acquisition and extinction of the cued fear response. The behavioral procedure involved three phases: habituation, fear conditioning, and extinction training. During the habituation phase, from day 4 to day 6 after the last morphine or saline injection, the mice were handled (2–3 min per mouse per day) to habituate them to the experimenter. Mice were taken from their home cages and transported to both box A and box B for 10 min each, on each of 3 consecutive days, without the presentation of stimuli, to habituate them to both backgrounds. Twenty-four hours after the last habitation session (7 days after the last morphine or saline injection), mice were transported from their home cages to context A to receive 5 tone-foot-shock conditioning trials (tone: 2800 Hz, 85 dB, 30 s duration; shock: 0.5 mA, 1 s duration; CS-US co-terminating), beginning 2 min after being placed in the chamber. The average inter-trial interval was 60 s (range, 30–90 s). Thirty seconds after the final shock, the mice were returned to their home cages. Twenty-four hours after the fear conditioning session (days 8–11 after the last morphine or saline injection), mice received four extinction training sessions (one session per day). During each extinction session, mice received 5 tone alone presentations (2800 Hz, 85 dB, 30 s duration), 2 min after placement in context B. The average inter-trial interval was 60 s (range, 30–90 s). Thirty seconds after the final tone presentation, mice were immediately placed in their home cages. The percentage of time spent freezing was used to measure the conditioned response during the fear conditioning and extinction training phases. Freezing is the absence of all movements except those related to respiration. The total time spent freezing during each tone presentation was scored by a person who was blind with regard to the experimental condition of each animal. We also recorded the time spent freezing during the first 29 s of tone presentation in the conditioning session and each extinction session.
Experiment 2
Experiment 2 was designed to test the effects of chronic morphine administration on the acquisition and extinction of the contextual fear response. The behavioral procedure also involved three phases: habituation, fear conditioning, and extinction training. During the habituation phase, from days 4 to 6 after the last morphine or saline injection, mice were taken from their home cages and transported to box A for 10 min each, without stimuli being presented to habituate them to the observation chamber. The following day (day 7 after the last morphine or saline injection), mice were taken from their home cages and transported to the observation chamber. The mice received 10 foot-shock trials (0.5 mA, 1 s duration) without tone presentation, beginning 2 min after being placed in the chambers. The average inter-trial interval was 60 s (range, 30–90 s). Thirty seconds after the final shock, the mice were returned to their home cages. Percent freezing time of each time block during which no foot-shock was presented was scored by a person who was blind with regard to the experimental condition of each animal. Twenty-four hours after the fear conditioning session (days 8–11 after the last morphine or saline injection), mice underwent four extinction training sessions (one session per day). During each extinction session, mice were placed in box A for 5 min without stimulus presentation. The total time spent freezing in each 5 min extinction training session was scored.
Tissue sample preparation
At the end of the last session of cued fear extinction training, the mice were decapitated, and the brains were extracted and placed on an ice-cold stainless steel plate. The hippocampus and amygdala were isolated on the basis of a stereotaxic atlas23. Brain samples were homogenized, suspended in protease inhibitor solution (complete Mini TM EDTA-free protease inhibitor cocktail tablets in H2O; Roche Diagnostics) containing phosphatase inhibitors (phosphatase inhibitor cocktails I and II; Sigma), and sonicated. The protein concentrations of all samples were determined using a bicinchoninic acid assay (Pierce). Sample buffer was added immediately and samples were aliquoted and stored at −80 °C. The protein concentrations of samples were determined in a single assay together with brain samples from 8-10 naive mice.
Western blot assays
The samples were treated as previously described24. Loading buffer (16% glycerol, 20% β-mercaptoethanol and 0.05% bromophenol blue) was added to each sample (3:1, sample/loading buffer) before boiling for 3 min. Samples were cooled and subjected to SDS-polyacrylamide gel electrophoresis (10% acrylamide/0.27% N,N′-methylenebisacrylamide resolving gel) for 4 h at 150 V. For each episode of electrophoresis, increasing amounts of protein pooled from all samples were electrophoresed to produce a standard curve. Proteins were transferred electrophoretically to PVDF transfer membranes (Millipore) at 0.3 A for 2 h. Blots were blocked in TBST (20 mmol/L Tris/HCl, 150 mmol/L NaCl, 0.1% Tween-20, at pH 7.6) containing 5% fat-free milk powder, for 1 h at room temperature. Blots were incubated with rabbit polyclonal anti-phospho-ERK1/2 (Thr202/Tyr204; 1:1000; Cell Signaling) diluted in 5% fat-free milk powder in TBST overnight at 4 °C. After washing, blots were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (1:2,000 in TBST containing 5% fat-free milk powder; DAKO) and developed using the enhanced chemoluminescence method (ECL+plus, Amersham). After stripping (in 2% SDS, 50 mmol/L DTT, 50 mmol/L Tris/HCl at pH 7.0 for 30 min at 70 °C), the blots previously probed with anti-phospho-ERKs were incubated with a polyclonal anti-ERK1/2 antibody (1:1000; Cell Signaling). Densitometric analysis of bands was performed using Quantity One software (BioRad).
Statistical analysis
Percent freezing values in the fear conditioning session and extinction training session were analyzed using three-factor (genotype×group×trial) ANOVA with the Bonferroni test as a post-test. The results of spontaneous withdrawal signs and Western blot were analyzed for significance by two-way ANOVA with the Bonferroni test as a post-test. All data are presented as mean±SEM. The significance level was set at P<0.05.
Results
Spontaneous somatic signs following morphine withdrawal
Spontaneous somatic signs were evaluated 24 h and 7 days after morphine withdrawal (Table 1). After 24 h of morphine withdrawal, two-way ANOVA analysis revealed significant effects of genotype (F1, 17=19.67, P<0.01) and treatment (F1, 17=13.80, P<0.01). Even if WT mice showed more rearing and chewing than HDC-KO mice (P<0.05), morphine withdrawal induced somatic signs in both HDC-KO and WT mice. Furthermore, HDC-KO mice made fewer jumps than WT mice (P<0.01). However, the somatic withdrawal signs were no longer evident at 7 days of withdrawal. No significant effect on the somatic withdrawal signs was detected between the different genotypes or treatment groups, which suggests that the somatic withdrawal behavior had disappeared at 7 days of withdrawal.
Table 1. Somatic signs of spontaneous morphine withdrawal in wild-type and histidine decarboxylase gene knockout mice.
| 24 h | 7 d | |||||||
|---|---|---|---|---|---|---|---|---|
| Signs | WT | KO | WT | KO | ||||
| Saline | Morphine | Saline | Morphine | Saline | Morphine | Saline | Morphine | |
| Wet-dog shaking | 1.28±0.07 | 7.04±3.58c | 1.327±0.036 | 10.57±3.58c | 0.93±0.02 | 1.03±0.13 | 1.23±0.41 | 1.70±0.47 |
| Rearing | 2.03±0.85 | 8.71±3.82c | 1.372±0.027e | 8.43±3.06c | 1.61±0.03 | 1.68±0.06 | 1.61±0.63 | 1.75±0.08 |
| Chewing | 1.37±0.05 | 5.56±2.61c | 0.937±0.013e | 6.28±4.27c | 1.55±0.48 | 2.01±0.20 | 1.25±0.53 | 1.79±0.09 |
| Paw tremor | 0 | 9.00±6.40c | 0 | 5.86±5.32c | 0 | 0.25±0.004 | 0 | 0.24±0.01 |
| Jumping | 0 | 14.48±11.19c | 0 | 5.46±3.88ci | 0 | 0 | 0 | 0 |
| Ptosis (N/5 min) | 0 | 0.85±0.02c | 0 | 0.83±0.01c | 0 | 0 | 0 | 0 |
Each value represents mean±SEM from 8-10 mice. cP<0.01 vs saline-treated with the same genotype; eP<0.05 vs WT mice with saline treatment; iP<0.01 vs WT mice with morphine treatment. WT: wild-type mice; KO: histidine decarboxylase gene knockout mice.
Experiment 1: Acquisition and Extinction of Cued Fear Response
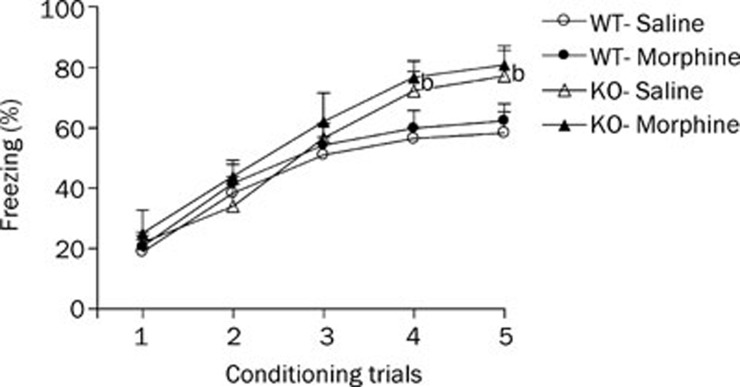
Acquisition of the cued fear response is illustrated in Figure 1. All mice displayed a progressive increase in the duration of the freezing response. Freezing responses increased rapidly across the first three trials, then slowed down and reached a plateau. This finding suggested that both HDC-KO and WT mice could acquire fear memory after 5 CS-US training sessions. A three-factor ANOVA performed on these data yielded a significant effect of genotype (F1, 38=6.62, P<0.05), treatment (F1, 38=9.60, P<0.01) and trial (F4, 169=84.83, P<0.01). Post-hoc tests showed that the percentage freezing in the morphine treatment group tended to be higher than those in the saline treatment groups of HDC-KO or WT mice, but this trend did not reach significance. These results suggest that morphine withdrawal does not affect the acquisition of cued fear memory in HDC-KO and WT mice. In addition, the levels of percentage freezing increased significantly in HDC-KO mice, which suggests that a long-term histamine deficiency accelerated acquisition of the cued fear response.
Figure 1.
Effect of morphine withdrawal on acquisition of cued fear memory in WT and HDC-KO mice. Each group (n=8−11) received fear conditioning on day 7 after the last morphine injection. bP<0.05 vs saline-treated controls with the same genotype during the same conditioning trial. KO: HDC-KO.
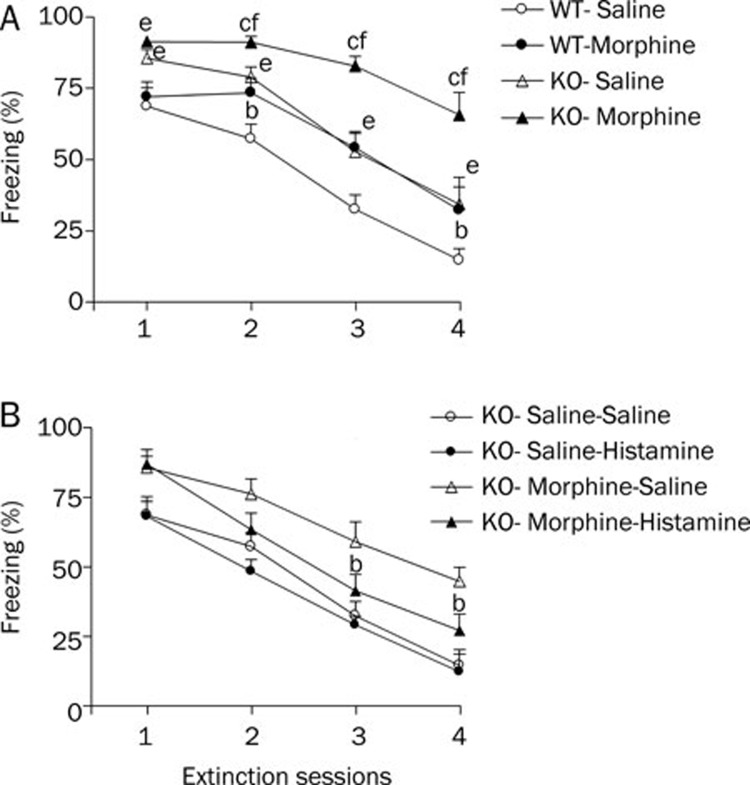
During the extinction phase (Figure 2), all groups presented a progressive decrease in the conditioned fear response across sessions, which suggests that the extinction training decreased the cued fear response. During extinction training, the percentage freezing showed significant effects of genotype (F1, 38=36.91, P<0.01), treatment (F1, 38=37.17, P<0.01) and session (F3, 152=34.21, P<0.01). Post-hoc comparisons indicated that morphine-treated groups showed a significant increase in the levels of percentage freezing response as compared to the corresponding saline groups from extinction sessions 2 to 4; this was true for both WT (all P<0.05) and HDC-KO mice (all P<0.01). In the saline-treated group, HDC-KO mice displayed a significant increase in the level of freezing as compared to the WT mice from extinction sessions 2 to 4 (P<0.05), but the fear response score of HDC-KO mice (85.6%±2.6%) was higher than that in WT mice (68.7%±6.6%) before the first fear extinction training. The decreasing slope of the fear response in both HDC-KO and WT mice was similar. An injection of histamine (icv, 5 μg/mouse) 10 min before the extinction sessions did not accelerate the cued fear extinction in HDC-KO mice (Figure 2B, F1, 16=0.53, P>0.05). However, in the morphine-treated group, HDC-KO mice displayed a greater increase in the level of freezing from extinction sessions 1 to 4 (P<0.01, Figure 2), and the decreasing slope of the fear response in HDC-KO mice was significantly more gradual than in WT mice. Furthermore, histamine (icv, 5 μg/mouse) reversed the increase in the level of freezing (Figure 2B, F1, 17=5.08, P<0.05). These results indicate that chronic morphine treatment impairs the between-session extinction of the cued fear response, whereas histamine reverses the effect of morphine withdrawal.
Figure 2.
A: Effect of morphine withdrawal on extinction of cued fear memory in WT and HDC-KO mice. Each group (n=8−11) received four fear extinction sessions on days 8–11 after the last morphine injection. bP<0.05, cP<0.01 vs saline-treated mice with the same genotype during the same extinction session; eP<0.05, fP<0.01 vs the group receiving the same treatment, compared between genotypes. KO: HDC-KO. B: Effect of injection of histamine (icv, 5 μg/mouse) 10 min before extinction sessions on extinction of cued fear memory in HDC-KO mice (n=8−9). bP<0.05 vs saline (icv)-treated morphine withdrawal group during the same extinction session.
Experiment 2: Acquisition and Extinction of Contextual Fear Response
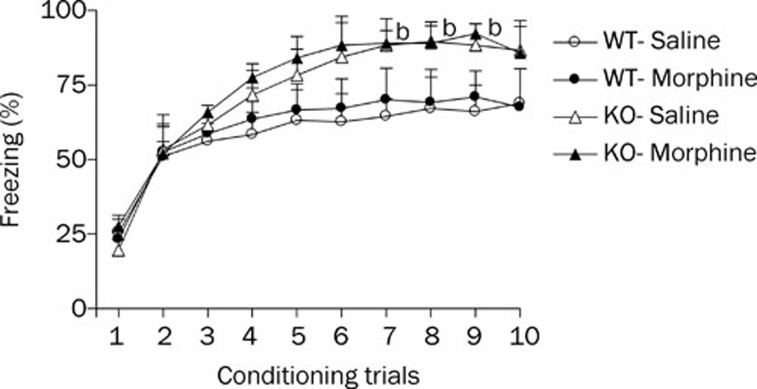
Conditioned fear responses increased progressively across the first 4 trials and then reached a plateau (Figure 3). A three-factor ANOVA of percentage freezing revealed significant effects of genotype (F1, 36=5.24, P<0.05) and trial (F9, 336=34.24, P<0.01) but no significant treatment effect (F1, 36=0.98, P>0.05), indicating that the morphine- and saline-treated groups showed equivalent fear learning. Post-hoc tests showed that percentage freezing in the morphine treatment group did not differ significantly between the HDC-KO and WT mice. These results suggest that morphine withdrawal does not affect the acquisition of contextual fear memory. However, in the saline-treated groups, the percentage freezing in HDC-KO mice was significantly higher than that in WT mice from training trials 7 to 9. Thus, these results demonstrate that a long-term deficiency of histamine potentiates acquisition of the contextual fear response.
Figure 3.
Effect of morphine withdrawal on acquisition of contextual fear memory in WT and HDC-KO mice. Each group (n=8−10) received a fear conditioning session on day 7 after the last morphine injection. bP<0.05 vs WT mice that received the same saline treatment. KO: HDC-KO.
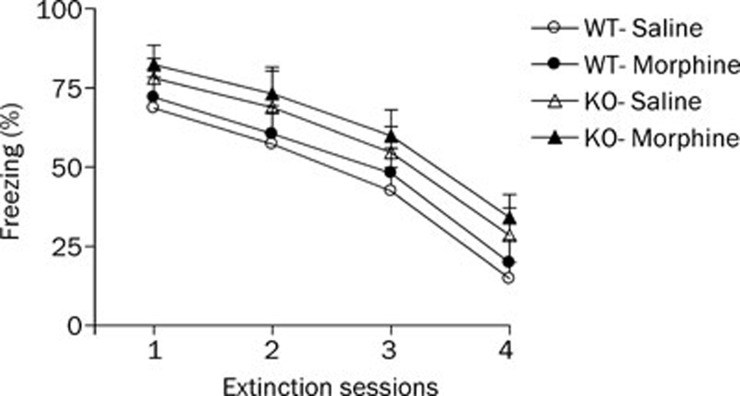
During the extinction training phase (Figure 4), three-factor ANOVA revealed a significant effect of extinction session (F3, 162=7.53, P<0.05) but no significant treatment (F1, 36=0.18, P>0.05) or genotype effect (F1, 36=0.27, P>0.05). Post-hoc comparisons indicated that the morphine-treated groups did not differ significantly from the saline-treated groups in the levels of percentage freezing in WT and HDC-KO mice. However, HDC-KO mice did not display a significant increase in the level of freezing when compared with the WT mice in the saline-treated and morphine-treated groups.
Figure 4.
Effect of morphine withdrawal on extinction of contextual fear memory in WT and HDC-KO mice. Each group (n=8−10) received four fear extinction sessions on days 8–11 after the last morphine injection. KO: HDC-KO.
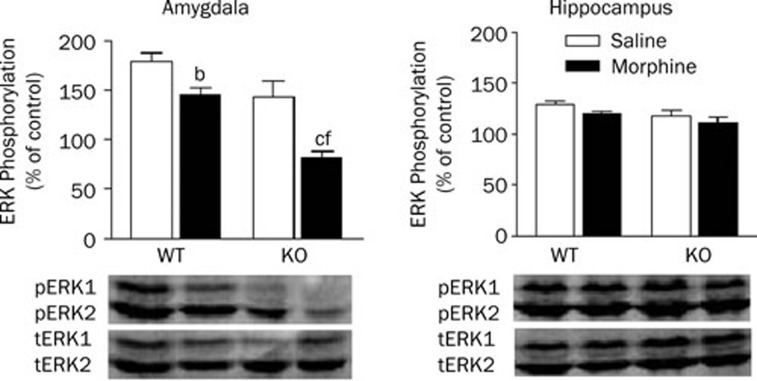
Effect of morphine withdrawal on ERK phosphorylation in the amygdala and hippocampus
The results above demonstrate that morphine withdrawal markedly inhibits extinction of cued fear memory, and chronic histamine deficiency aggravates the effects of morphine withdrawal. To identify the activation of ERK protein in the amygdala and hippocampus during the extinction of cued fear extinction, levels of pERK/ERK were measured after the last extinction training session (Figure 5). In the amygdala, a two-way ANOVA revealed significant effects of genotype (F1, 20=25.39, P<0.01) and treatment (F1,20=3.84, P<0.05). Post-hoc analysis showed that morphine withdrawal significantly inhibited ERK phosphorylation in the amygdala of WT and HDC-KO mice (P<0.05). Furthermore, the percentage of ERK phosphorylation in HDC-KO mice (81.64±6.67%) was lower than that in WT mice (145.67±7.65%) in both the morphine withdrawal group (P<0.01) and the saline group (P<0.01). In the hippocampus, however, two-way ANOVA showed no significant effects of genotype or treatment, and there was no interaction between the two factors.
Figure 5.
Effect of morphine withdrawal on ERK phosphorylation in the amygdala and hippocampus of WT and HDC-KO mice during cued fear extinction. bP<0.05, cP<0.01 vs saline-treated mice with the same genotype; fP<0.01 vs the group receiving the same treatment, as compared between genotypes. KO: HDC-KO.
Discussion
In the present study, we found that morphine withdrawal inhibited extinction of the cued fear response, which suggests that morphine withdrawal impairs fear extinction. Compared with WT mice, HDC-KO mice showed higher levels of fear response in the acquisition and extinction of both cued and contextual fear memory, which suggests that a long-term deficiency of histamine facilitates fear conditioning. Furthermore, chronic histamine deficiency in HDC-KO mice aggravated the inhibitory effect on cued fear extinction induced by morphine withdrawal. These findings strongly suggest that brain histamine is involved in morphine addiction by modulating cognitive activity after withdrawal, and a chronic deficiency of histamine can enhance the action of morphine.
The abrupt interruption of 5 days of morphine administration was sufficient to induce somatic signs of spontaneous morphine withdrawal in HDC-KO and WT mice. Compared with saline-treated animals, mice undergoing morphine withdrawal showed apparent withdrawal behavior, such as wet-dog shakes, rearing, chewing, paw tremor, jumping, and ptosis, when animals were examined at 24 h of withdrawal. HDC-KO mice jumped fewer times than WT mice. Furthermore, the somatic signs gradually decreased with withdrawal time and disappeared 7 days after morphine withdrawal in both HDC-KO and WT mice. Mice were then subjected to a cued or contextual fear conditioning and extinction paradigm 7 d after morphine withdrawal.
Although previous studies have shown that acute microinjection of morphine into the amygdala or nucleus accumbens impairs the acquisition and expression of fear memory25, 26, little is known about the effect of chronic morphine on fear memory. We found that morphine withdrawal in mice did not affect the acquisition of contextual or cued fear responses, but inhibited the extinction of the cued fear response. Our results are consistent with the previous report by Gu et al, which found that chronic morphine in rats inhibits extinction of the fear response but has no appreciable effect on the acquisition process6. These results at least suggest that morphine withdrawal inhibits cued fear extinction in a variety of animals and ameliorating the impairment of cued fear extinction induced by morphine withdrawal may inhibit morphine relapse. In addition, we found that a long-term deficiency of histamine in HDC-KO mice accelerated the acquisition of contextual and cued fear responses, which suggests that an inhibitor of histaminergic neuronal activity may enhance fear memory. Notably, the cued fear response is enhanced in both histamine H1 and H2 receptor knockout mice27. Moreover, HDC-KO mice have improved contextual and cued fear responses, which are reversed by intracerebroventricular injection of histamine immediately after training21. Interestingly, the inhibitory effect of morphine withdrawal on cued fear extinction was enhanced in HDC-KO mice, but histamine reversed the effect. These data at least in part suggest that histamine inhibits morphine relapse by modulating negative fear memory after withdrawal.
In addition, we were interested to find that morphine withdrawal affected only extinction of the cued and not the contextual fear response. The amygdala and hippocampus in general are considered to be important sites of neural circuits related to fear extinction. The amygdala is essential for the cued fear response, while the hippocampus is necessary for the contextual fear response28. Several findings have demonstrated that repeated morphine treatment changes a number of receptors and signal pathways in the amygdala but not in the hippocampus. Chronic morphine treatment increases the expression of NMDAR1 and NMDAR2A mRNA levels, only in the amyg-dal29, 30. Therefore it is likely that chronic morphine treatment induces adaptive changes in brain structures in different regions. Furthermore, we measured ERK phosphorylation in the amygdala and hippocampus after cued fear extinction. Morphine withdrawal decreased ERK phosphorylation in the amygdala after cued fear extinction in both HDC-KO and WT mice, which suggests that specific chemical changes, such as ERK phosphorylation, occur in the brain after morphine treatment and its subsequent withdrawal and that these changes are involved in the extinction of the fear response. Previous findings demonstrated that ERK is activated by phosphorylation in the amygdala during extinction of the cued fear response and that inhibition of ERK phosphorylation by intra-amygdala infusion of U0126, a MEK inhibitor, completely blocks extinction of this response31, 32. Therefore, it seems that morphine withdrawal impairs cued fear extinction by inhibiting ERK phosphorylation in the amygdala. However, we found that neither morphine withdrawal nor histamine deficiency affected ERK phosphorylation in the hippocampus after cued fear extinction. This finding indicates that the hippocampus is not essential for cued fear memory. Therefore, it is likely that ERK phosphorylation in the amygdala and hippocampus participates in different types of memory formation and that morphine withdrawal may differentially activate ERK signaling in different brain regions to modulate cued or contextual fear extinction.
Moreover, we were surprised to find that long-term histamine deficiency produced no appreciable effect on cued or contextual fear extinction, but it potentiated the inhibition of cued fear extinction induced by morphine withdrawal, which indicates that inhibiting histaminergic activity might aggravate the amygdala-dependent memory impairment following administration of morphine. Although we lack additional data to explain the phenomenon, we found that histamine deficiency also decreased the ERK phosphorylation induced by morphine withdrawal in the amygdala after cued fear extinction, which may suggest that histamine inhibits the impairment of cued fear extinction by modulating ERK signaling.
In summary, the present study demonstrated that morphine withdrawal selectively impairs cued fear extinction, which is one of the reasons for morphine abuse and relapse. Second, histamine reduces the impairment of fear extinction by morphine withdrawal and inhibits morphine relapse. Its action might modulate cognitive impairment after withdrawal. Therefore, histamine and related compounds may be of value in treating and preventing morphine abuse and relapse.
Author contribution
Ying-xia GONG and Hui-juan WANG performed research, analyzed data and wrote the manuscript; Wen-ting SHOU, Bo FENG and Wei-ping ZHANG provided ideas and reagents; Hiroshi OHTSU provided the mice; Zhong CHEN designed research.
Acknowledgments
This project was supported by grants from the National Natural Science Foundation of China (No 30725047 and 30801392), the Zhejiang Scientific Foundation (No 2006C23025), the New Century Excellent Talents Program, Ministry of Education, China (No NCET-06-0511), and the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents. We are very grateful to Dr Iain C BRUCE for reading the manuscript.
References
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–7. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW.The neuropsychology of chronic drug abuseIn MA Ron & TW Robbins (Eds), Disorders of brain and mind. Cambridge: Cambridge University Press; 2003
- Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–66. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Spain JW., and, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y maze choice escape. Psychopharmacology. 1991;105:101–6. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–21. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Li P, Hu B, Ouyang X, Fu J, Gao J, et al. Chronic morphine selectively impairs cued fear extinction in rats: implications for anxiety disorders associated with opiate use. Neuropsychopharmacology. 2008;33:666–73. doi: 10.1038/sj.npp.1301441. [DOI] [PubMed] [Google Scholar]
- Wada H, Inagaki N, Yamatodani A, Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity. Trends Neurosci. 1991;14:415–8. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kamei C. Facilitating effects of histamine on spatial memory deficit induced by scopolamine in rats. Acta Pharmacol Sin. 2000;21:814–8. [PubMed] [Google Scholar]
- Chen Z. Effect of histamine H3-receptor antagonist clobenpropit on spatial memory of radial maze performance in rats. Acta Pharmacol Sin. 2000;21:1905–10. [PubMed] [Google Scholar]
- Huang YW, Chen Z, Hu WW, Zhang LS, Wu W, Ying LY, et al. Facilitating effect of histamine on spatial memory deficits induced by dizocilpine as evaluated by 8-arm radial maze in SD rats. Acta Pharmacol Sin. 2003;24:1270–6. [PubMed] [Google Scholar]
- Chen Z, Sugimoto Y, Kamei C. Effects of intracerebroventricular injection of alpha-fluoromethylhistidine on radial maze performance in rats. Pharmacol Biochem Behav. 1999;64:513–8. doi: 10.1016/s0091-3057(99)00128-8. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takamori K, Misawa M, Onodera K. Effects of the histaminergic system on the morphine-induced conditioned place preference in mice. Brain Res. 1995;675:195–202. doi: 10.1016/0006-8993(95)00064-w. [DOI] [PubMed] [Google Scholar]
- Gong YX, Wang HJ, Zhu YP, Zhang WP, Dai HB, Zhang SH, et al. Carnosine ameliorates morphine-induced conditioned place preference in rats. Neurosci Lett. 2007;422:34–8. doi: 10.1016/j.neulet.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Gong YX, Lv M, Zhu YP, Zhu YY, Wei EQ, Shi H, et al. Endogenous histamine inhibits the development of morphine-induced conditioned place preference. Acta Pharmacol Sin. 2007;28:10–8. doi: 10.1111/j.1745-7254.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Eidi M, Eidi A, Oryan S. Effects of histamine and opioid systems on memory retention of passive avoidance learning in rats. Eur J Pharmacol. 2002;452:193–7. doi: 10.1016/s0014-2999(02)02269-0. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Khalilzadeh A, Rezayat M, Sahebgharani M, Djahanguiri B. Influence of intracerebroventricular (icv) administration of histaminergic drugs on morphine state-dependent memory of passive avoidance in mice. Pharmacology. 2005;74:106–12. doi: 10.1159/000085590. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Khalilzadeh A, Malekmohammadi N, Fazli-Tabaei S. Influence of morphine- or apomorphine-induced sensitization on histamine state-dependent learning in the step-down passive avoidance test. Behav Brain Res. 2006;171:50–155. doi: 10.1016/j.bbr.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–84. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–84. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang S, Zhu Y, Fu Q, Zhu Y, Gong Y, et al. Improved learning and memory of contextual fear conditioning and hippocampal CA1 long-term potentiation in histidine decarboxylase knock-out mice. Hippocampus. 2007;17:634–41. doi: 10.1002/hipo.20305. [DOI] [PubMed] [Google Scholar]
- Shen Y, He P, Fan YY, Zhang JX, Yan HJ, Hu WW, et al. Carnosine protects against permanent cerebral ischemia in histidine decarboxy-lase knockout mice by reducing glutamate excitotoxicity. Free Radic Biol Med. 2010;48:727–35. doi: 10.1016/j.freeradbiomed.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.The mouse brain in stereotaxic coordinatesSan Diego: Academic Press; 2001
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–32. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good AJ, Westbrook RF. Effects of a microinjection of morphine into the amygdala on the acquisition and expression of conditioned fear and hypoalgesia in rats. Behav Neurosci. 1995;109:631–41. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, Good AJ, Kiernan MJ. Microinjection of morphine into the nucleus accumbens impairs contextual learning in rats. Behav Neurosci. 1997;111:996–1013. doi: 10.1037//0735-7044.111.5.996. [DOI] [PubMed] [Google Scholar]
- Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res. 2007;57:306–33. doi: 10.1016/j.neures.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Sepehrizadeh Z, Sahebgharani M, Ahmadi S, Shapourabadi MB, Bozchlou SH, Zarrindast MR. Morphine-induced behavioral sensitization increased the mRNA expression of NMDA receptor subunits in the rat amygdala. Pharmacology. 2008;81:333–4. doi: 10.1159/000122959. [DOI] [PubMed] [Google Scholar]
- Turchan J, Maj M, Przewlocka B. The effect of drugs of abuse on NMDAR1 receptor expression in the rat limbic system. Drug Alcohol Depend. 2003;72:193–6. doi: 10.1016/s0376-8716(03)00193-5. [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–9. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]