Abstract
Pirfenidone is currently the only approved therapy for idiopathic pulmonary fibrosis, following studies demonstrating that treatment reduces the decline in lung function and improves progression-free survival. Although generally well tolerated, a minority of patients discontinue therapy due to gastrointestinal and skin-related adverse events (AEs). This review summarizes recommendations based on existing guidelines, research evidence, and consensus opinions of expert authors, with the aim of providing practicing physicians with the specific clinical information needed to educate the patient and better manage pirfenidone-related AEs with continued pirfenidone treatment. The main recommendations to help prevent and/or mitigate gastrointestinal and skin-related AEs include taking pirfenidone during (or after) a meal, avoiding sun exposure, wearing protective clothing, and applying a broad-spectrum sunscreen with high ultraviolet (UV) A and UVB protection. These measures can help optimize AE management, which is key to maintaining patients on an optimal treatment dose.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-014-0112-1) contains supplementary material, which is available to authorized users.
Keywords: Adverse event management, Expert opinion, Gastrointestinal events, Idiopathic pulmonary fibrosis, Photosensitivity, Pirfenidone, Respiratory, Safety, Skin-related events
Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare, progressive, irreversible and ultimately fatal chronic lung disease. Studies in Europe suggest that prevalence ranges from 1 to 23 cases per 100,000 persons [1–6]. Prevalence and incidence rates increase with age, are higher among males and appear to be on the increase in recent years [7]. Prognosis is poor, with a median survival time of 2–5 years [8–12].
Pirfenidone is currently the only approved drug for the treatment of adult patients with mild to moderate IPF [13]. It is licensed for use in the European Union and Canada (under the trade name Esbriet®, InterMune UK Ltd., London, UK) and in Japan, India, South Korea, and China. Data from Phase III, randomized, double-blind, placebo-controlled trials demonstrate that pirfenidone reduces the decline in lung function and improves progression-free survival time [14, 15]. In these studies, treatment with pirfenidone was safe and generally well tolerated; the most commonly reported treatment-emergent adverse events (AEs) were gastrointestinal (GI) and skin-related. These were generally mild to moderate in severity and rarely resulted in treatment discontinuation.
While pirfenidone is generally well tolerated, a minority of patients discontinue treatment due to AEs. In light of the vital importance of treatment compliance in patients with IPF, a clinical advisory board comprising experts in pulmonology (E. Bendstrup, U. Costabel, V. Cottin, J. J. J. Egan, M. Kreuter, T. Maher, M. Molina-Molina, C. Vancheri), gastroenterology (P. Dewint, P.M. Hellström, A. Sarafidis), and dermatology (J. Ferguson, R. Groves, K. Nordlind), and supported by a grant from InterMune International AG, was convened in Basel, Switzerland, to discuss the prevention and management of pirfenidone-related GI and skin-related AEs.
This review summarizes the recommendations that have been generated based on existing guidelines, a review of current research evidence, and consensus opinions of this international group of experts. The recommendations may be adapted for use in different countries or regions according to the availability of treatment modalities and treating physicians’ judgment. The experts’ meeting aimed to provide practicing physicians with specific clinical information needed to best educate the patient and manage AEs with continued pirfenidone treatment. These recommendations will be revised regularly following systematic review of new research evidence as it becomes available.
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Overview of Pirfenidone
Pirfenidone is an orally active, synthetic small molecule that inhibits the synthesis of transforming growth factor-β and tumor necrosis factor-α in preclinical models, both of which are cytokines known to play an active role in fibrosis and inflammation [16–21]. Following oral administration with food to improve tolerability, pirfenidone is slowly absorbed and reaches peak concentrations after approximately 4 h [22]. The mean apparent terminal elimination half-life is approximately 2.4 h [23].
Efficacy Profile
The clinical efficacy and safety of pirfenidone has been evaluated in four randomized, double-blind, placebo-controlled trials, including a Phase II Japanese study, a Phase III Japanese study, and two multinational Phase III trials [the CAPACITY studies PIPF004 (Clinicaltrials.gov #NCT00287716) and PIPF006 (Clinicaltrials.gov #NCT00287729)] conducted in Europe, North America and Australia [14, 15, 24]. Data from the three Phase III, randomized, double-blind, placebo-controlled studies demonstrate that pirfenidone reduces the decline in lung function and improves progression-free survival [14, 15]. The two Phase III CAPACITY studies of pirfenidone in patients with IPF were designed similarly in order to enable pooling of data [25]. Analyses of pooled data from both trials enabled precise estimates of the magnitude of the treatment effect [25]. Results from the pooled analysis of categorical FVC change showed that pirfenidone reduced the proportion of patients experiencing at least a 10% decline by 30% compared with placebo [15]. Additionally, numerically fewer overall deaths occurred in the pirfenidone versus placebo group [19 (6%) vs. 29 (8%); P = 0.141]. Significant and clinically meaningful treatment effects were observed across multiple outcomes in the CAPACITY studies (Fig. 1a) [26]. At Week 72, pirfenidone also reduced the proportion of patients with a 50 m or greater decrement in 6-min walk distance (31% relative reduction vs. placebo) and reduced the risk of death or disease progression (26% reduction vs. placebo, P = 0.025) [15].
Fig. 1.
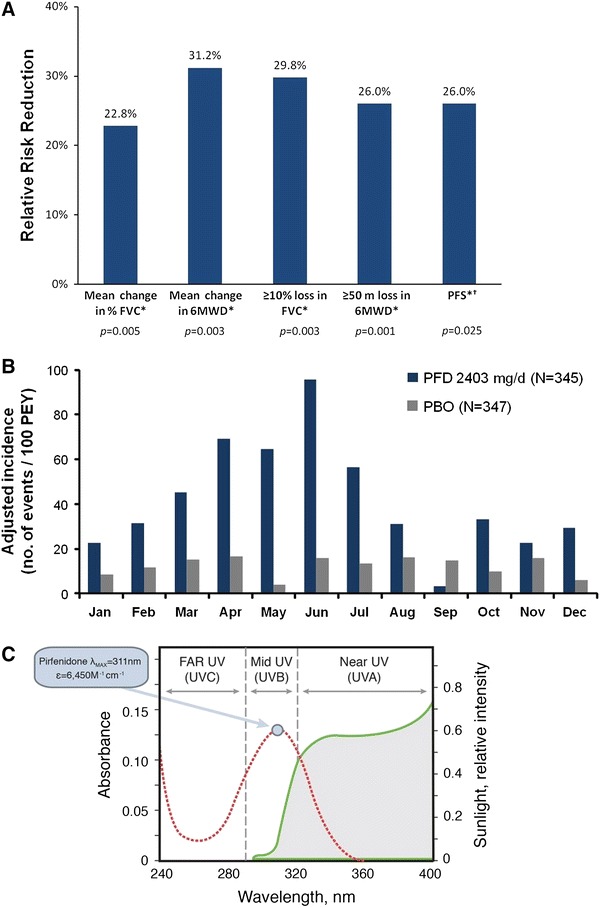
Clinical and preclinical pirfenidone data. a Consistent magnitude of treatment effect with pirfenidone across multiple clinically meaningful outcomes in patients with IPF [15]. Bars represent the magnitude of treatment effect, reported as relative difference between the pirfenidone and placebo groups. *From the pooled analysis of the CAPACITY studies at 72 weeks. †Progression-free survival was defined as time to confirmed ≥10% decline in percentage predicted FVC, ≥15% decline in percentage predicted DLco or death. Deaths were not adjudicated and studies were not powered to evaluate mortality. b The incidence of pirfenidone-related skin rashes in patients with IPF was higher in the early summer months of the CAPACITY studies 004 and 006. Adapted from European Medicine Agency. Pirfenidone CHMP assessment report [38]. c Pirfenidone absorbs light in the ultraviolet spectrum. Adapted from Seto et al. [30]. 6MWD 6-min walk distance, DLco carbon monoxide diffusing capacity, ε molar absorptivity coefficient, FVC forced vital capacity, IPF idiopathic pulmonary fibrosis, λ max wavelength of the most intense ultraviolet absorption, PBO placebo, PEY patient-exposed years, PFD pirfenidone, PFS progression-free survival, UV ultraviolet
Safety Profile
The clinical safety profile of pirfenidone in patients with IPF has been demonstrated in large randomized, double-blind, placebo-controlled trials [14, 15, 24], as well as in an ongoing long-term open-label extension trial [RECAP (Clinicaltrials.gov #NCT00662038)]. Pirfenidone’s safety profile has been evaluated in a well-defined cohort of 789 patients exposed to treatment for up to 8 years (long-term safety pooling) [27]. In the CAPACITY studies, treatment with pirfenidone was generally well tolerated. Treatment discontinuation due to AEs was more common among pirfenidone 2,403 mg/day patients (15%) compared with placebo patients (9%); relative risk of 1.85 (95% CI 1.28–2.67, P = 0.001) [15, 28].
Based on observations from the Japanese trials [14, 24], proactive protocol-defined strategies including dose modification guidelines were implemented in the CAPACITY studies to minimize the incidence and severity of selected AEs (such as GI and skin-related AEs) and encourage patients to continue treatment. The most common AE leading to treatment discontinuation in both groups in the CAPACITY studies was IPF (3% in each group). Other AEs leading to treatment discontinuation in at least 1% of pirfenidone-treated patients were rash and nausea.
The relatively low incidence of treatment discontinuation due to GI and skin-related AEs in the CAPACITY studies, compared with the Japanese studies, supports the use of protocol-defined strategies for proactively managing these events. However, GI and skin-related AEs remained the most commonly reported AEs in the CAPACITY studies. Gastrointestinal events of nausea, diarrhea, dyspepsia and vomiting were reported in 36%, 29%, 19% and 14% of pirfenidone-treated patients versus 17%, 19%, 7%, and 4% of placebo-treated patients, respectively [15] (InterMune data on file). Skin-related AEs of rash and photosensitivity were reported in 32% and 12% of pirfenidone-treated patients versus 12% and 2% of placebo-treated patients, respectively [15]. These events were generally mild to moderate in intensity, reversible, and with minimal clinical consequence (Tables 1, 2). Gastrointestinal and skin-related AEs led to treatment discontinuation in six (1.7%) and eight (2.3%) pirfenidone-treated patients, respectively. The long-term safety pooling including 789 patients showed that GI and skin-related AEs have a tendency to occur early in the course of treatment and decrease over time [27].
Table 1.
Gastrointestinal adverse events in the CAPACITY studies
| Nausea | Diarrhea | Dyspepsia | Vomiting | |||||
|---|---|---|---|---|---|---|---|---|
| Pirfenidone 2,403 mg/day (N = 345) | Placebo (N = 347) | Pirfenidone 2,403 mg/day (N = 345) | Placebo (N = 347) | Pirfenidone 2,403 mg/day (N = 345) | Placebo (N = 347) | Pirfenidone 2,403 mg/day (N = 345) | Placebo (N = 347) | |
| Grade 3 or 4 TEAEs, n (%) | 6 (1.7) | 2 (0.6) | 2 (0.6) | 0 (0.0) | 1 (0.3) | 2 (0.6) | 1 (0.3) | 0 (0.0) |
| TE SAE, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Deaths, n | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hospitalizations, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discontinuation, n (%) | 5 (1.4) | 0 (0.0) | 0 (0.0) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Dose modification, n (%) | 25 (7.2) | 7 (2.0) | 18 (5.2) | 4 (1.2) | 8 (2.3) | 0 (0.0) | 14 (4.1) | 3 (0.9) |
| Events, n | 195 | 77 | 153 | 85 | 77 | 29 | 62 | 17 |
| Median duration, days | 46 | 7 | 7 | 5 | 168 | 4 | 2 | 3 |
| Resolved, n (%) | 149 (76) | 65 (84) | 130 (85) | 73 (52) | 40 (52) | 23 (79) | 59 (95) | 17 (100) |
TEAE treatment-emergent adverse event, TE SAE treatment-emergent severe adverse event, Grade 3 TEAE severe or medically significant but not immediately life-threatening events; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care Activities of Daily Living, Grade 4 TEAE life-threatening consequences; urgent intervention indicated. InterMune data on file
Table 2.
Skin-related adverse events in the CAPACITY studies
| Rash | Photosensitivity reaction | |||
|---|---|---|---|---|
| Pirfenidone 2,403 mg/day (N = 345) | Placebo (N = 347) | Pirfenidone 2,403 mg/day (N = 345) | Placebo (N = 347) | |
| Grade 3 or 4 TEAEs, n (%) | 2 (0.6) | 0 (0.0) | 3 (0.9) | 1 (0.3) |
| TE SAEs, n (%) | 1 (0.3)a | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Deaths (n) | 0 | 0 | 0 | 0 |
| Hospitalization, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discontinuation, n (%) | 5 (1.4) | 0 (0.0) | 3 (0.9) | 1 (0.3) |
| Dose modification, n (%) | 42 (12.2) | 5 (1.5) | 19 (5.5) | 1 (0.3) |
| Events (n) | 159 | 52 | 60 | 8 |
| Median duration (days) | 38 | 31 | 88 | 60 |
| Resolved, n (%) | 132 (83) | 46 (88) | 47 (78) | 6 (75) |
No formal definition of rash versus photosensitivity reaction was employed; the distinction was made by the physician based on his/her clinical observation
TEAE treatment-emergent adverse event, TE SAE treatment-emergent severe adverse event, Grade 3 TEAE severe or medically significant but not immediately life-threatening events; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care Activities of Daily Living, Grade 4 TEAE life-threatening consequences; urgent intervention indicated. InterMune data on file
aGrade 3 erythema with desquamation
Elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) of more than three times the upper limit of normal occurred more frequently in the pooled pirfenidone group than in the pooled placebo group [14/345 (4%) vs. 2/347 (<1%), respectively] in the CAPACITY studies [15]. Rarely, these have been associated with concomitant elevations in bilirubin. Liver function tests (ALT, AST, and bilirubin) should be conducted prior to the initiation of treatment with pirfenidone, and subsequently at monthly intervals for the first 6 months and then every 3 months thereafter [23].
Gastrointestinal Adverse Events
Gastrointestinal AEs occurred at higher frequencies in patients treated with pirfenidone 2,403 mg/day compared with placebo in the CAPACITY studies (Table 1). Importantly, there were no GI-related treatment-emergent serious AEs or hospitalizations among patients treated with pirfenidone. Overall, five (1.4%) and one (0.3%) pirfenidone patients discontinued therapy due to nausea and vomiting, respectively, and two (0.6%) discontinued due to diarrhea. Gastrointestinal AEs were mostly transient in nature, although dyspepsia was present for a median duration of 168 days [15]. While GI-related AEs were generally mild to moderate and transient, the frequency of such events suggests the need for proactive management.
Animal studies have shown that pirfenidone reduces the rate of gastric emptying and small intestinal transit [29]. These effects in rats were ameliorated by co-administration of prokinetic agents. Concomitant administration of mosapride at 3 and 10 mg/kg resulted in significant amelioration in the decrease in gastric emptying rate induced by 100 mg/kg pirfenidone, while 1 mg/kg mosapride or greater ameliorated the decreased small intestinal transit rate [29]. Furthermore, GI effects in animals were observed following subcutaneous injection, indicating that systemic exposure is required for pirfenidone’s gastric motility effects (InterMune data on file). The effect of food on the pharmacokinetics of pirfenidone has previously been examined in healthy adult subjects. It was demonstrated that co-administration with food decreased the peak concentration of pirfenidone (area under the curve was unchanged), with data also suggesting that food intake reduces the risk of GI-related AEs, thereby improving overall tolerability [22].
Skin Reactions
Skin-related AEs in the context of pirfenidone therapy can manifest as an erythematous (with/without edema) or as a phototoxic burn-like skin rash, occurring on sun-exposed body areas. This differs from an allergic type of eruption, which will usually affect all parts of the skin, including areas not exposed to sunlight. In the CAPACITY studies, the most commonly observed skin-related AEs were photosensitivity reactions (observed in 12.2% of pirfenidone-treated patients compared with 1.7% of placebo-treated patients) and rash (occurring in 32.2% of pirfenidone-treated compared with 11.5% of placebo-treated patients). Events were mild to moderate in severity (Table 2). Photosensitivity reaction led to discontinuation in three (0.9%) pirfenidone-treated patients and one (0.3%) placebo-treated patient. Rash led to discontinuation in five (1.4%) pirfenidone-treated patients and no placebo-treated patients [15]. An example of photosensitivity on the exposed skin of a pirfenidone-treated patient is shown in Fig. 2a.
Fig. 2.
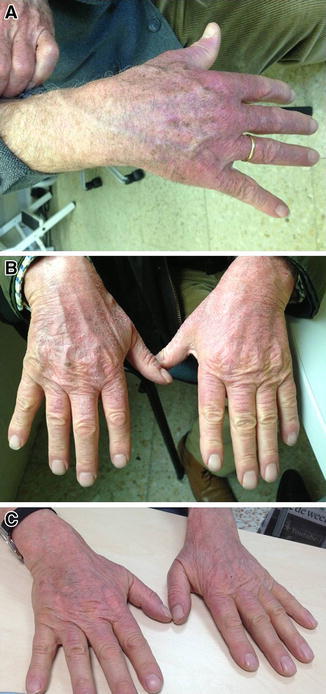
Photosensitivity in pirfenidone-treated patients. a Patient 1: mild photosensitivity after 8 h driving. The patient had been receiving treatment with pirfenidone for 7 days. The patient presented with erythema localized to the more photoexposed areas of the hands. He had previously followed advice regarding photoprotection. On the day in question, he used a broad-spectrum SPF50+ sunscreen in the morning before sun exposure, but did not repeat the application. b Patient 2: moderate photosensitivity after 14 days pirfenidone treatment. The patient presented with erythema and peeling, mainly on the hands and face. Patient had omitted to apply sunscreen protection. c Patient 2: the same patient improved following 7 days of topical treatment (silver sulfadiazine), sunscreen protection, and pirfenidone dose reduction. The patients and treating physicians provided permission for the use of these images. SPF sun protection factor
The seasonal variation in frequency of skin reactions in the CAPACITY studies suggests an association with high-intensity sunlight exposure (Fig. 1b). Laboratory studies have suggested that the underlying mechanism of pirfenidone in photosensitivity reactions is likely to be phototoxic and related to the drug’s ability to absorb both ultraviolet (UV) B and UVA (Fig. 1c) [30]. Absorption of UV light by pirfenidone in skin tissue could result in skin lesions due to the generation of reactive oxygen species and lipid peroxidation, as both occur in vitro at physiologically relevant concentrations [30]. Although no wavelength dependency studies have been conducted in humans, preclinical guinea pig findings support the hypothesis that pirfenidone-induced skin reactions are phototoxic, and therefore proportional to light exposure and drug concentration [31]. These animal studies also demonstrate that the use of a sunscreen with a high protection factor significantly reduces the severity of skin reactions. Whether the wavelength dependency extends into the visible region in man is yet unknown.
Recommendations for the Prevention and Management of Pirfenidone-Related AEs
Treatment management should be deployed in centers with appropriate expertise and experience in the management of IPF. The recommended daily dose of pirfenidone in Europe for adult patients with IPF is three 267 mg capsules three times a day with food (preferably at the end rather than the beginning of the meal) for a total of 2,403 mg/day [23]. Upon initiating treatment, the dose is titrated over a 14-day period to the recommended daily dose of nine capsules (2,403 mg) per day: first week, one capsule (267 mg) three times a day; second week, two capsules (534 mg) three times a day; third week onwards, full dose of three capsules (801 mg) three times a day [23]. It is recommended that pirfenidone is taken with food, typically during or after a meal in order to prevent drug-related AEs [23]. Generally, a stepwise clinical approach as described in Fig. 3 can be adopted to manage pirfenidone-related GI and skin-related AEs.
Fig. 3.
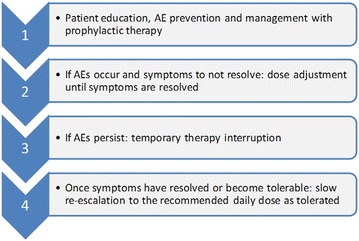
Stepwise approach to the prevention and management of pirfenidone-related AEs. AE adverse event
The following sections report recommendations on additional measures that may be adopted in daily practice for the prevention and management of GI and skin-related AEs, based on the summary of product characteristics (SmPC) and complemented by our opinion and observations from clinical experience. It should be noted that decisions regarding treatment, particularly re-introduction following discontinuation due to AEs, should always be made in conjunction with the patient, who should be made fully aware of potential drug side-effects. Both parties should consider the balance between quality of life and efficacy benefits of continued treatment in order make informed decisions on disease management.
Gastrointestinal Adverse Events
In addition to current recommendations to take pirfenidone during or after a meal [23], we suggest further practical considerations in patients experiencing tolerability challenges from GI AEs, such as taking each of the three capsules separately throughout the meal, rather than simultaneously. If the meal consists of several courses, each capsule could be taken between courses. In support for this recommendation, preclinical studies have shown that splitting pirfenidone doses partially alleviates pirfenidone’s inhibitory effect on gastric motility in animal models (InterMune data on file). This is further supported by a real-world study in which patients were encouraged to take pirfenidone at the start, middle, and end of a meal to help minimize side-effects [32].
To further improve pirfenidone’s tolerability, we suggest that a longer initial dosing titration scheme—for example, starting with one capsule three times a day and titrating up to the full dose (three capsules three times a day) over a period of 4 weeks instead of 2 weeks—may be employed. This approach is currently under investigation in a clinical setting (Clincialtrials.gov #NCT01933334) [33].
Dose Reduction
If pirfenidone-related AEs are not tolerable, dose reductions may be helpful, as the incidence of GI and skin-related AEs with pirfenidone treatment appear to be dose dependent as shown in animal models [34]. Depending on the nature and severity of the AE, we suggest that the pirfenidone dose may be reduced to 1 or 2 capsules (267–534 mg), 2–3 times a day with food, with re-escalation to the recommended daily dose as tolerated. In support of this suggestion, preclinical studies have shown that pirfenidone reduces gastric emptying and small intestinal transit rates in a dose-dependent manner [29].
If dose reduction is required, we suggest to first reduce the dose corresponding to the time of the day when AEs are the most pronounced. For example, if nausea is experienced in the morning, delaying or reducing the morning dose to one or two capsules, may help mitigate GI symptoms. Dietary and cultural habits should be taken into account when advising patients.
Temporary Dose Interruption and Re-challenging
If AEs still persist despite dose reduction, temporary treatment discontinuation until symptoms become tolerable (typically 1–2 weeks) should be considered. Once symptoms have resolved or become tolerable, pirfenidone may be re-introduced. In some treatment centers, a slower re-escalation scheme (i.e., longer than the usual 2 weeks) after temporary treatment interruption has been employed.
Prokinetic Medication
Use of prokinetic agents such as domperidone, mosapride, and metoclopramide may help mitigate treatment-related GI AEs [35]. Indeed, animal studies have shown that prokinetic agents such as mosapride reduce pirfenidone’s inhibitory effects on gastric emptying and intestinal transit rates [29]. Metoclopramide (for a maximum of 5 days) and domperidone (no restriction on length of use) are both dosed at 10 mg three times a day. Use of proton pump inhibitors may also be helpful for managing GI side-effects in pirfenidone-treated patients with IPF [36].
Skin-Related Adverse Events
Preclinical studies have shown that pirfenidone-induced photosensitivity reactions reflect the drug’s pharmacokinetic profile and are proportional to sunlight exposure, decreasing with the use of higher sun protection factor (SPF) sunscreens. Therefore, sun exposure should be avoided for a few hours following pirfenidone intake, to allow the drug’s plasma concentration to decrease. Animal studies have suggested that the extent of phototoxicity is linked to pirfenidone’s plasma concentration (InterMune data on file). The standard approach for preventing and managing skin AEs as recommended in the SmPC [23] includes avoiding exposure to direct sunlight (including sunlamps), use of sunscreen active against both UVA and UVB, use of protective clothing, and avoidance of other medicinal products known to cause photosensitivity. We expand on this guidance by suggesting: behavioral avoidance of indirect (in addition to direct) sunlight, as well as intense artificial light sources (such as energy-saving fluorescent lamps); wearing of thick-weave clothing, use of a broad-brimmed hat, and, when necessary, gloves.
To further illustrate the above guidance of the SmPC, Table 3 details our expert recommendations to prevent skin photosensitivity reactions, highlighting the importance of behavioral changes and protection through appropriate clothing and thorough sunscreen application.
Table 3.
Summary of recommendations for the prevention and management of pirfenidone-related AEs
| General approaches to preventing and managing pirfenidone-related AEs |
| Pirfenidone administration and dose modifications |
| Pirfenidone capsules (267 mg) should be taken individually, over the course of a meal: fine tuning should take into account cultural dietary habits |
| If nausea is experienced in the morning, the morning dose may be delayed or reduced |
| A maximum of three capsules (801 mg) should be taken with the main meal of the day, while fewer [1 or 2 capsules (267–534 mg)] may be taken with lighter meals |
| Upon treatment initiation, the up-titration scheme can be extended to 4 weeks until the daily recommended dose is reached |
| Temporary treatment interruptions should be considered if symptoms do not resolve following dose reduction |
| Following dose interruption, pirfenidone could be re-introduced with a slower re-escalation scheme to the full dose |
| All treatment-related decisions should be made following discussion with the patient and with the aim of balancing quality of life and efficacy benefits |
| Additional measures to manage GI AEs |
| Prokinetic agents may help mitigate GI AEs |
| Additional measures to prevention and manage skin AEs |
| Prevention of photosensitivity reactions |
| Change behavior to avoid sun exposure: |
| • Seek to avoid sun exposure as much as possible, especially at mid-day, in the late afternoon, and during seasonal high UV periods. Remember that the sun’s UVA component can penetrate clouds, clothing, and car windows |
| • Avoid sun exposure for a few hours after the meal at which pirfenidone was administered |
| Protect skin from the sun with appropriate clothing: use of wide-brimmed hats, sunglasses, long-sleeve shirts, and trousers is recommended, as are gloves for driving and outdoor activities |
| Protect skin from the sun with sunscreen: frequent and thorough application of a broad-spectrum SPF50 sunscreen with both UVA and UVB protection is mandatory |
| Management of skin rashes |
| In case of rashes, pirfenidone dose should be reduced. If rashes still persist after 7 days, pirfenidone therapy should be discontinued for 15 days and may be slowly re-introduced once symptoms have resolved |
| If rashes are due to an allergic reaction, pirfenidone therapy should be permanently discontinued |
| In cases of severe photosensitivity reactions, pirfenidone should be discontinued and replaced with prednisone 25 mg/day for 7–10 days. After disappearance of the skin reaction, pirfenidone may be re-introduced following a very slow re-escalation |
| Education |
| Patient (and spousal) education should be undertaken at several levels (physician, nurse, and pharmacist) and should be supported by existing educational materials (e.g., patient information leaflet) |
| Physicians play a central role in patient education and in providing best advice by considering the patient’s clinical history, potential outdoor occupations/hobbies/sport, or possible concomitant treatment with other photosensitizing drugs |
AE adverse event, GI gastrointestinal, SPF sun protection factor, UV ultraviolet
Dose Reduction
In cases of mild to moderate photosensitivity reaction or rash (i.e., when the vast majority of burn is superficial—painful erythema and scaling may be present on exposed areas, but with no edema, exudation, or blistering) that does not spontaneously resolve, the dose may be reduced to one capsule (267 mg) three times a day for 7 days, or until symptom resolution. Symptoms are considered to be resolved once the redness and scaling have disappeared.
Temporary Dose Interruption and Re-challenging
If a rash persists for more than 7 days despite pirfenidone dose reduction, therapy should be discontinued for approximately 15 days. Upon resolution, pirfenidone may be re-introduced and re-escalated to the recommended daily dose, or as tolerated, in the same manner as the initial regime, or using the slower re-escalation regime previously described (i.e., extended over 4 weeks as opposed to 2 weeks).
Patients experiencing a severe photosensitivity event (erythema and edema with exudation, erosions, blistering, cracked or scarred skin, possible dehydration) of sun-exposed skin (i.e., a non-allergic reaction), should be instructed to seek medical advice on dose adjustments and temporary discontinuation, until the skin reaction normalizes. In our collective clinical experience, use of topical treatments for burns (e.g., silver sulfadiazine or potent steroids) immediately after the appearance of skin lesions may help attenuate reactions and improve associated symptoms. An example of a patient with moderate sensitivity treated with topical emollients and a reduction in pirfenidone dose is shown in Fig. 2b, c.
After the skin reaction has cleared, pirfenidone may be re-introduced with the slower stepwise re-escalation scheme. If exposure to sunlight at mid-day is unavoidable, the pirfenidone dose at lunchtime could be reduced.
It should be noted that a significant number of skin-related AEs experienced with pirfenidone are associated with unprotected sun exposure. Rashes that appear allergic in nature (urticarial or occurring on parts of the body that are not exposed to sunlight) are uncommon. If such a rash occurs, however, pirfenidone should be permanently discontinued, and the use of anti-histamines and oral prednisone should be considered. Correct diagnosis of an allergic rash is very important, as these patients should not be re-challenged with pirfenidone.
In cases of photosensitivity, the decision about re-challenging following treatment interruption must be made according to clinical judgment based on the type and severity of the reaction; dermatological advice can be helpful. Dose interruption can be avoided if enough emphasis is placed on prevention methods, as described in Table 3. Patients should be encouraged to take photographs of any skin changes to aid diagnostic evaluation, particularly if uncertainty exists as to whether a rash has a phototoxic- or an allergic-type mechanism.
Improving Patient Education
Patient education is a central part of the management of skin AEs and should include communication with a patient’s spouse or carer. Accordingly, physicians, nurses, and pharmacists play an important role in the dissemination of patient educational materials (such as the patient information leaflet [37]) and in emphasizing preventive measures (e.g., behavioral, clothing, and sun protective barrier use). Ideally, physicians and nurses should dedicate adequate time to educate patients with respect to preventive measures and behavioral changes.
As previously described, the importance of generous and frequent re-application of a high SPF and high UVA (extending into the visible region) type of sunscreen should be emphasized. The use of existing supporting patient educational materials [37] and simple educational tools is highly recommended. Some centers have dedicated nurses and/or specific home-care programs that play an important role in this area. Patients should be made aware that although dense cloud, thick clothing, and glass may offer some protection against UV exposure, UVA rays may penetrate such barriers, which is important with respect to driving. Prescribing or recommending a broad-spectrum sunscreen at the same time as pirfenidone may emphasize the importance of sun protection.
In terms of AE management, the physician plays a central role by communicating preventive measures and existing educational tools to the patient. During patient assessment, the physician has the opportunity to educate and provide the best advice to the patient by considering clinical history (skin phototype and previous history of photosensitive skin disease such as lupus erythematosus, polymorphic light eruption, etc.), possible outdoor occupations (e.g., laborer, farmer) or hobbies/sport (e.g., golfing, sailing), concomitant treatment with other photosensitizing drugs (e.g., tetracycline antibiotics), and the patient’s mental health state (which can affect motivation and adherence). For patients who present with photosensitivity despite significant photoprotection, it is important to consider the possibility of vitamin D deficiency. A vitamin D plasma assay should be considered with administration of oral vitamin D as appropriate.
The outcomes of efficient patient communication are highlighted in a real-world pirfenidone study of 40 patients with IPF [32]. During the first 6 months of this study six patients (15%) discontinued treatment due to AEs. In the latter 10 months, however, there were no discontinuations. The authors of this study [32] attribute this to a number of factors, including a monthly specialist nurse review (for the first 3 months of treatment) to assess and reinforce AE avoidance measures. Patients were also given a contact number and encouraged to speak with a specialist nurse if they experienced any AEs; this ensured that appropriate measures such as dose reduction, temporary discontinuation, and/or additional treatment of side-effects could be enforced without delay in order to rapidly alleviate AEs [32]. This real-world study suggests that simple patient education and communication measures can substantially increase treatment adherence and compliance, which enables patients with IPF to benefit from the decreased disease progression observed with continued pirfenidone treatment.
Conclusion
Data from clinical trials show the clinically meaningful benefit and favorable safety profile of pirfenidone. While dose reduction can improve AE tolerability by mitigating AE occurrence/severity, better management of AEs will lead to optimized care and allow patients to benefit from the potential effectiveness of pirfenidone. To prevent and manage GI and skin-related AEs more effectively, further research is required to understand the effect of pirfenidone on gastric motility and photosensitivity reactions. Our opinions regarding the prevention and management of pirfenidone-related AEs in clinical practice are summarized in Table 3. These should be regarded as a complement to the available and published information on pirfenidone.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This manuscript is based on discussions between authors at a clinical advisory board which was supported by a grant from InterMune International AG, Basel, Switzerland. The authors thank Dominique Spirig, formerly of Fishawack Communications GmbH, Basel, Switzerland, Neil Burton of Fishawack Communications GmbH, and Róisín O’Connor from inScience Communications, Springer Healthcare Ltd., London, UK, for medical writing and editorial assistance, which was funded by InterMune International AG. The authors also thank Christophe Giot (InterMune International AG, Basel, Switzerland) for his contribution to the discussion of pirfenidone clinical trial data, and Lin Pan and Scott Seiwert (InterMune Ltd., Brisbane, USA) for their contribution to the discussion of pirfenidone preclinical data. All named authors met the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interest
UC has received consultancy fees, speaker fees and research grants from InterMune Inc. and Boehringer Ingelheim, consultancy fees from Gilead, Roche, Bayer Centocor, and speaker fees from Zambon. EB has received research grants and speaker fees from InterMune Inc, and is an investigator in the PASSPORT and PANORAMA (EudraCT: #2012-000564-14) studies. VC has participated as a consultant, speaker and investigator for Actelion, Boehringer Ingelheim, Gilead, InterMune Inc. and Roche. PD, JJE, JF, RG and PMH have no conflicts of interest to disclose. MK has received an unrestricted academic grant from InterMune Inc., and consulting and speaker’s fees from InterMune Inc. and Boehringer Ingelheim. TMM is in receipt of unrestricted academic grants from GlaxoSmithKline and Novartis. In the last 3 years, TMM (or his institution) has received advisory board or consultancy fees from Boehringer Ingelheim, GlaxoSmithKline, InterMune Inc, Novartis, Lanthio Pharma, Takeda, Sanofi-Aventis and UCB. He has received speaker’s fees from UCB, Boehringer Ingelheim, InterMune Inc. and AstraZeneca and participated as an investigator in industry sponsored clinical trials run by Boehringer Ingelheim, GlaxoSmithKline, InterMune Inc., Novartis, Roche and Celgene; his institute has received educational grant support from InterMune Inc. MMM has participated as a consultant and speaker for InterMune Inc., Actelion and Esteve-Teijin, and has participated in clinical trials sponsored by Sanofi, Roche, Boehringer Ingelheim and InterMune Inc. KN and AS have no conflicts of interest to disclose. CV has received research grants and consulting and speaker fees from InterMune Inc., and is an investigator in the PASSPORT and PANORAMA studies.
Compliance with ethics guidelines
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors. The patients and treating physicians provided permission for the use of the images in Fig. 2.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57:338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakatsani A, Papakosta D, Rapti A, Antoniou KM, Dimadi M, Markopoulou A, et al. Epidemiology of interstitial lung diseases in Greece. Respir Med. 2009;103:1122–1129. doi: 10.1016/j.rmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, et al. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax. 2011;66:462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 5.Thomeer MJ, Costabe U, Rizzato G, Poletti V, Demedts M. Comparison of registries of interstitial lung diseases in three European countries. Eur Respir J Suppl. 2001;32:114s–118s. [PubMed] [Google Scholar]
- 6.von Plessen C, Grinde O, Gulsvik A. Incidence and prevalence of cryptogenic fibrosing alveolitis in a Norwegian community. Respir Med. 2003;97:428–435. doi: 10.1053/rmed.2002.1466. [DOI] [PubMed] [Google Scholar]
- 7.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev [Research Support, Non-U.S. Gov’t Review] 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 13.Antoniou KM, Margaritopoulos GA, Siafakas NM. Pharmacological treatment of idiopathic pulmonary fibrosis: from the past to the future. Eur Respir Rev. 2013;22:281–291. doi: 10.1183/09059180.00002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 15.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 16.Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB. Effects of three anti-TNF-alpha drugs: etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro. Int Immunopharmacol. 2008;8:679–687. doi: 10.1016/j.intimp.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Drew P, Gaugler AC, Cheng Y, Visner GA. Pirfenidone inhibits lung allograft fibrosis through l-arginine-arginase pathway. Am J Transplant. 2005;5:1256–1263. doi: 10.1111/j.1600-6143.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 18.Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol. 2002;446:167–176. doi: 10.1016/S0014-2999(02)01757-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama S, Mukae H, Sakamoto N, et al. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008;82:210–217. doi: 10.1016/j.lfs.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–408. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino CM, Bhavnani SM, Ambrose PG, Forrest A, Loutit JS. Effect of food and antacids on the pharmacokinetics of pirfenidone in older healthy adults. Pulm Pharmacol Ther. 2009;22:279–285. doi: 10.1016/j.pupt.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 23.European Medicine Agency. Esbriet® (pirfenidone) Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002154/WC500103049.pdf (last accessed Feb 2014).
- 24.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 25.du Bois RM, Nathan SD, Richeldi L, Schwarz MI, Noble PW. Idiopathic pulmonary fibrosis: lung function is a clinically meaningful endpoint for phase III trials. Am J Respir Crit Care Med. 2012;186:712–715. doi: 10.1164/rccm.201206-1010PP. [DOI] [PubMed] [Google Scholar]
- 26.Cottin V. Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes. Eur Respir Rev. 2012;21:161–167. doi: 10.1183/09059180.00001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valeyre D, Albera C, Bradford W, et al. The long-term safety of pirfenidone (PFD) in patients with idiopathic pulmonary fibrosis (IPF). Eur Respir J. 2013;42(Suppl 57)(563s):P3159.
- 28.Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS ONE. 2012;7:e47024. doi: 10.1371/journal.pone.0047024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh T, Koyabu K, Morimoto A, et al. Ameliorative effects of mosapride or Rikkunshi-to on the suppression of gastrointestinal motility by pirfenidone in rats. Jpn Pharmacol Ther. 2012;40:405–411. [Google Scholar]
- 30.Seto Y, Inoue R, Kato M, Yamada S, Onoue S. Photosafety assessments on pirfenidone: photochemical, photobiological, and pharmacokinetic characterization. J Photochem Photobiol B. 2013;120:44–51. doi: 10.1016/j.jphotobiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Pharmaceuticals and Medical Devices Agency Japan. Pirfenidone report. http://www.pmda.go.jp/english/service/pdf/drugs/pirespa_oct2008_e.pdf (last accessed Feb 2014).
- 32.Chaudhuri N, Duck A, Frank R, Holme J, Leonard C. Real world experiences: pirfenidone is well tolerated in patients with idiopathic pulmonary fibrosis. Respir Med. 2014;108:224–226. doi: 10.1016/j.rmed.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Clinical trials.gov. Safety and tolerability of pirfenidone in patients with systemic sclerosis-related interstitial lung disease (SSc-ILD) (LOTUSS). http://clinicaltrials.gov/ct2/show/NCT01933334?term=lotuss&rank=1 (last accessed Feb 2014).
- 34.Cho ME, Kopp JB. Pirfenidone: an anti-fibrotic therapy for progressive kidney disease. Expert Opin Investig Drugs. 2010;19:275–283. doi: 10.1517/13543780903501539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito M. Post-marketing surveillance of pirfenidone for idiopathic pulmonary fibrosis in Japan: Interim analysis of 973 patients. Eur Respir J. 2012;40:563s. [Google Scholar]
- 36.Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Electronic Medicines Compendium. Patient information leaflet: pirfenidone. http://www.medicines.org.uk/emc/medicine/26916/PIL/Esbriet+267+mg+hard+capsules/ (last accessed Feb 2014).
- 38.European Medicine Agency. Pirfenidone CHMP assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002154/WC500103073.pdf (last accessed Feb 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


