Abstract
Infection with Cytomegalovirus is associated with accelerated immunosenescence. Expansions of CMV-specific T cell responses have previously been demonstrated to affect the ability of T cells to respond to other infections. Most people above 60 years of age display M. tuberculosis-specific immunity because of vaccination, exposure, or both. T-cell responses can be assessed by measuring intracellular IFN-γ in vitro after tuberculin stimulation. Here we investigated tuberculin-specific CD4 T-cell responses in independently living healthy older people in the South of England using flow-cytometry. Individuals were investigated for tuberculin and CMV-specific T-cell immunity using in vitro antigen stimulation followed by intracellular staining for IFN-γ, TNF-α, IL2, as well as degranulation and CD154 upregulation. We also examined a control group of younger individuals (20–35 years of age). There was no significant difference between older and young people in regards to tuberculin responsiveness of CD4 T-cells; however, older people seemed to show more outliers. Increased responsiveness to tuberculin was significantly correlated to CMV responsiveness but not age. In older donors, the memory phenotype of tuberculin-induced T-cells was significantly skewed towards a more terminal differentiation phenotype in CMV-infected compared to uninfected individuals and the degree of skewing correlated quantitatively with the size of the CMV-specific CD4 T-cell response. This is a fundamental advance over previous reports of changes of the tuberculin-specific CD4 T-cell response with CMV serostatus. Our results show that how the immune system responds to CMV has a fundamental impact on the phenotype and function of the immune response to mycobacterial antigens in older life.
Keywords: Cytomegalovirus, Tuberculosis, Immunosenescence, T-cell response, Flow-cytometry
Highlights
-
•
We examine the CD4 T-cell response to tuberculosis antigens in older people.
-
•
The CD4 T-cell response to Cytomegalovirus is explored in parallel.
-
•
CMV infection changes the profile of the tuberculin-specific-response.
-
•
The size of the CMV T-cell response is linked to these changes in a quantitative way.
-
•
The way we respond to CMV (‘mode’) affects our T-cell immunity to other pathogens.
1. Introduction
Human Cytomegalovirus (HCMV) is the largest known herpes virus and infects up to 90% of the population worldwide. Although CMV establishes latent infections, which are asymptomatic in the immunocompetent host, the impact of CMV on the memory T cell compartment is substantial. CMV infection results in an accumulation of late differentiated T cells and an increased ratio of memory over naive T cells, with approximately 10% of total T cell responses dedicated to CMV antigens in seropositive people. (Almanzar et al., 2005; Chidrawar et al., 2009; Derhovanessian et al., 2011; Looney et al., 1999; Sylwester et al., 2005).
During ageing, both CD4 and CD8 naive T cells significantly decline in seropositive individuals (Chidrawar et al., 2009; Fagnoni et al., 2000; Moro-Garcia et al., 2012). Since the number of naive T cells is central to immune efficacy, CMV infection has been associated with premature T cell response decline (or immunosenescence) and reduced ability of the host to mount effective immune responses against other infections or vaccination.
Age-driven changes in T cell immunity associated with CMV infection have been linked to an increased risk of serious complications of influenza in elderly people (Moro-Garcia et al., 2012), in particular because CMV seropositive older people have reduced ability to mount effective cellular and antibody responses to influenza vaccination. In older age, CD4 T cells lose the expression of the costimulatory receptor CD28, hence become impaired in their ability to provide help to B cells and stimulate effective antibody responses (Saurwein-Teissl et al., 2002). Furthermore, terminally differentiated T cells accumulating in CMV infection lose proliferative and antiviral activity (Appay et al., 2002). Recent studies confirm a negative correlation between CMV titer and responses to vaccine influenza virus in older people. In particular, late differentiated CD4 T cells are associated with poorer vaccine responses (Derhovanessian et al., 2013; Moro-Garcia et al., 2012).
CMV seropositivity can also compromise the ability of older people to respond to simultaneous Epstein–Barr virus (Khan et al., 2004), further demonstrating that the accumulation of CMV-specific CD8 T cells is detrimental to responses against heterologous viruses.
On the other hand, we and others have recently described that polyfunctionality of both CD4 and CD8 T cells elicited by CMV infection is not affected by age (Lachmann et al., 2012; Lelic et al., 2012) so, although memory phenotype is clearly affected by CMV infection, functional results are controversial.
Among the most relevant antigen-specific responses, the CD4 T cell response to Mycobacterium tuberculosis (M.tb) is one of the most tested, as the majority of people have either been exposed to M.tb or have received Bacille Calmette–Guerin (BCG) vaccination and have a positive reaction to tuberculin. Tuberculin purified protein derivative (PPD) is commonly used as recall antigen for testing in vivo systemic immunity to tuberculin in inexpensive skin tests (tuberculin test or Mantoux test)(Huebner, 1993).
An in vitro assessment of systemic PPD and CMV responses can be achieved by testing the IFN-γ production after antigen restimulation of blood derived T cells. The number of IFN-γ+ T cells reactivated in vitro with selected CMV antigens has generally been found to be higher in older seropositive compared to younger subjects (Ouyang et al., 2004; Vescovini et al., 2007), further suggesting that ageing is associated with a dysfunctional expansion of CMV effector responses that reduce the available repertoire of T cells for other antigens.
IFN-γ produced by antigen-specific CD4 Th1 cells is critical for the control of M.tb infection (Kaufmann, 2005) and several studies have demonstrated that both recently transmitted and reactivated tuberculosis rates increase with the impairment of antigen-specific CD4 T cell responses in ageing (Cruz-Hervert et al., 2012; Friedman et al., 2008; Horsburgh et al., 2010).
Also, just like observed in CMV (Fletcher et al., 2005), chronic M.Tb stimulation is thought to drive M.Tb-specific CD4 T cells to end-stage differentiation, replicative exhaustion and progressive degeneration (Day et al., 2011; Reiley et al., 2010).
In this study, we set out to explore CMV and tuberculin-specific CD4 T-cell responses in healthy young and older people in the South of England and questioned how responses to tuberculin may be affected by age and CMV infection but, more importantly, by the size of the CMV-specific immune response in older age. Interestingly in this context, Akbar et al. (Akbar et al., 2013) previously reported that IFN-γ responses following in vitro restimulation with tuberculin are comparable across different age groups, and Fletcher et al. previously demonstrated that in CMV infected (CMV+) older people, tuberculin-specific CD4 T-cell are more differentiated (measured by the presence of CD28−CD27− CD4 T-cells) than in CMV − older people. However, we were interested in particular if, beyond simple CMV infection status, the CD4 T-cell CMV-specific response size had an effect on the size and/or functionality of tuberculin-specific CD4 T cell response.
Our results confirm that in older people tuberculin-induced T-cells show a more terminal differentiation phenotype in CMV-infected compared to uninfected individuals; however, our results significantly extend previous findings to show that it is the actual size of the CMV-specific response rather than its mere presence that affects both phenotype and function of the immune response to mycobacterial antigens in older life.
2. Results
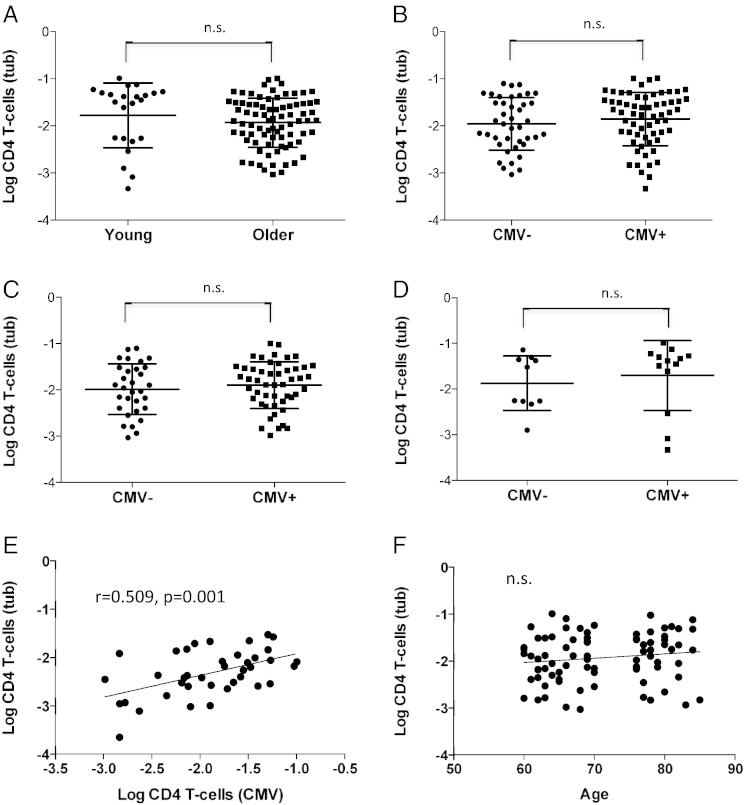
As a starting point to this investigation we observed several extremely high tuberculin-inducible CD4 T-cell responses in healthy older people (of the order of several percent of CD4 T-cells), which we had never seen in young or middle-aged people. This raised the obvious question whether there is an increase of tuberculin-specific T-cells in older age with expansions similar to those seen in CMV infection. A systematic comparison between a group of young and older individuals originally recruited to study CMV-specific immunity revealed, however, that while there were indeed some extremely big responses in the older group, there was no significant overall difference (mean or median) and some young people had exceptionally large responses as well (Fig. 1A). This was true when response size was based on each individual activation marker (degranulation, CD40L upregulation, IL-2, TNF-α, or IFN-γ production) or on all markers at the same time, i.e. counting cells as activated if at least one of the markers is upregulated. Since CMV infection is thought to drive inflammation in older life, we wondered if there was a difference in tuberculin-specific CD4 T-cell response sizes between infected (CMV+) and uninfected (CMV−) individuals, across both age groups and in each group alone (Fig. 1B–D). However, none of the differences were statistically significant.
Fig. 1.
Tuberculin-specific CD4 T-cell responses by age group or CMV serostatus.
Tuberculin-specific CD4 T-cells were analyzed by flow-cytometry in young and older individuals after ex-vivo antigen stimulation. Activated T cells were identified by intracellular cytokine staining. Whereas young donors had marginally higher median responses to tuberculin than older people (A), there were no significant differences in regards to CMV serostatus, neither in the whole group (B), nor in the older (C) and young (D) subgroups, indicating that neither age nor CMV-infection increases immunity to mycobacterial antigen.However, the size of the CMV-specific CD4 T-cell response had a positive effect on the size of the tuberculin-specific CD4 T-cell response (E). No such effect was seen with age (F).
It was of note though that among the older people very high CMV-specific CD4 T-cell responses (induced by stimulation with virus lysate) occurred in the same people that displayed very large tuberculin-specific responses. Indeed, there was a significant albeit weak correlation between these responses among the whole group (0.289, 0.037, n = 49) but a much stronger correlation in the older people (p = 0.509, p = 0.001, n = 39) (Fig. 1E). There were only ten cases among the young with both responses, but they were not well correlated (0.055, p = 0.881, n = 10). Neither the CMV-specific (not shown) nor the tuberculin-specific response (Fig. 1F) correlated significantly with age.
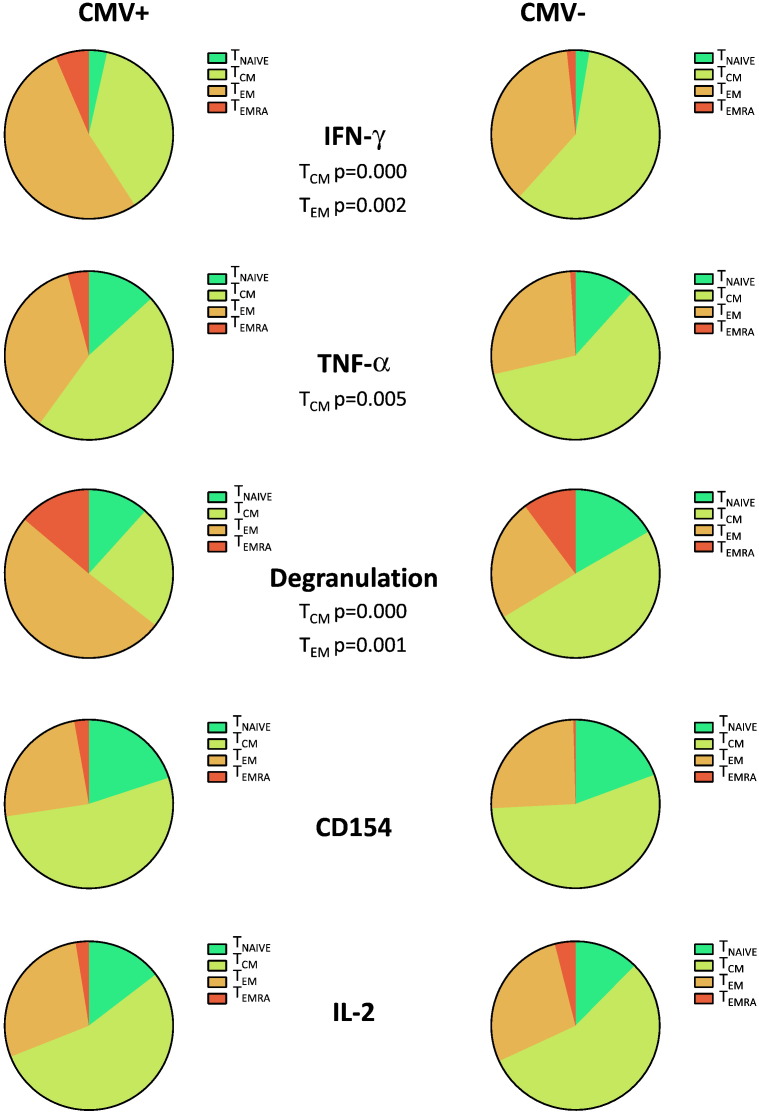
As CMV is known to drive the whole T-cell compartment towards a more terminal differentiation stage (Fletcher et al., 2005), we wondered if phenotype and functionality of the tuberculin-specific response in older people would be affected by CMV-status, phenotype or functional profile of the CMV-specific T-cell response. When comparing CMV+ and CMV− individuals we found that the memory phenotype distribution of tuberculin-induced CD4 T-cells displaying degranulation, TNF-α and IFN-γ production was more skewed toward more differentiated memory compartments. For all three markers, a significantly smaller proportion of these cells was in the TCM compartment in CMV+ compared to CMV− people (Fig. 2); for IFN-γ and TNF-α the proportion of the TEM compartment was significantly larger in CMV+ older people.
Fig. 2.
Tuberculin-induced CD4 T-cells in older individuals display a more advanced differentiation phenotype in the presence than in the absence of CMV infection.
The phenotypic distribution of tuberculin-induced CD4 T-cells in older donors was analyzed by flow cytometry. Pies were normalized to represent the proportion of each of four memory subsets in terms of the whole response defined by the selected functions. Pie charts in each row allow comparison of the effect of CMV infection. It is obvious that in CMV+ individuals, effector functions such as degranulation, IFN-γ or TNF-α production originate from cells that are on average more terminally differentiated than in CMV− individuals. Because the functions overlap, a comparison between pie charts within the same columns is not useful.
We next explored if the phenotype distribution of tuberculin-specific CD4 T-cells was affected by the size of the CMV-specific CD4 T-cell response and, for comparison, by the size of the tuberculin-specific response. In order to do so, we selected IFN-γ and TNF-α responses to analyze the distribution of tuberculin-specific CD4 T-cells identifiable by these markers across the subsets defined by CD27 and CD45RA. These T-cell memory compartments are frequently referred to as ‘naive’ (TNAIVE = CD45RA+/CD27+), ‘central memory’ (TCM = CD45RA−CD27+), ‘effector memory’ (TEM = CD45RA−CD27−), and ‘revertant’ (TEMRA = CD45RA+CD27−). Some authors use definitions of these subsets based on other markers resulting in similar albeit not identical distributions (Appay et al., 2008). Interestingly, the tuberculin-specific TCM subset (both with respect to IFN-γ and TNF-α responses) seemed to decrease whereas the TEM and TEMRA compartments seemed to increase as a function of CMV-specific CD4 T-cell response size. However, only the correlations between the CMV-specific CD4 T-cell response size and tuberculin-specific IFN-γ+ producing CD4 TCM and TEMRA compartments were significant (r = − 0.451, p = 0.004 and r = 0.336, p = 0.0346 respectively) (Table 1). All other correlations shown in Table 1 have to be described as non-significant trends.
Table 1.
Correlations between the phenotypic distribution of IFN-γ+ or TNF-α+ tuberculin-specific CD4 T-cells and the size of the CMV-specific CD4 T cells.
| TNF-α |
IFN-γ |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TNAIVE | TCM | TEM | TEMRA | TNAIVE | TCM | TEM | TEMRA | ||
| Age | Pearson correlation | 0.149 | 0.076 | − 0.147 | − 0.016 | 0.219 | 0.140 | − 0.187 | − 0.080 |
| Significance (2-tailed) | 0.366 | 0.644 | 0.371 | 0.924 | 0.179 | 0.394 | 0.255 | 0.629 | |
| N | 39 | 39 | 39 | 39 | 39 | 39 | 39 | 39 | |
| CD4 CMV (log) | Pearson correlation | − 0.064 | − 0.297 | 0.236 | 0.222 | 0.098 | − 0.451⁎⁎ | 0.226 | 0.336⁎ |
| Significance (2-tailed) | 0.699 | 0.066 | 0.147 | 0.175 | 0.553 | 0.004 | 0.166 | 0.0364 | |
| N | 39 | 39 | 39 | 39 | 39 | 39 | 39 | 39 | |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
While phenotype is not necessarily correlated with T-cell effector potential, polyfunctionality is thought to correlate with response efficiency (protection) at least in some situations. In regards to tuberculosis, it is doubtful though if the most polyfunctional subsets are correlated with protection; it appears rather that certain functional subsets are more frequently found in individuals who control TB infection.
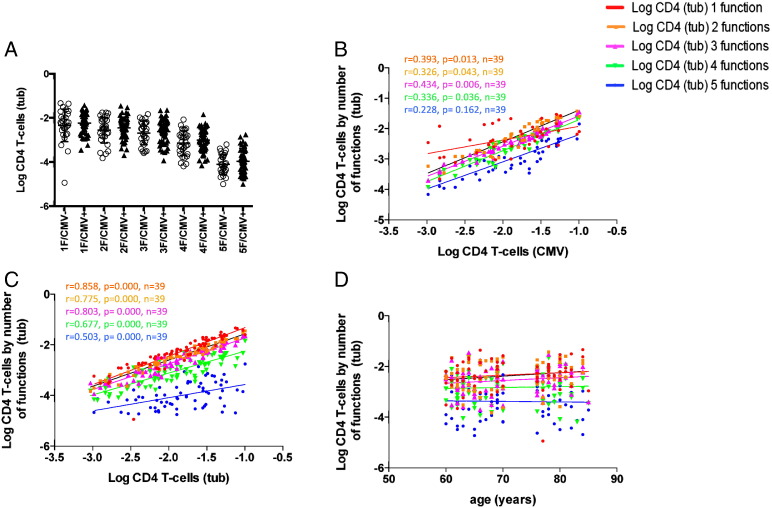
Subsets with exactly one, two, three, four, or, five parallel ‘functions’, where ‘function’ refers to each tested activation markers (IFN-γ, TNF-α, IL2, as well as degranulation and CD154 upregulation) are non-overlapping and can be expressed as percentage of all CD4 T-cells. This is a somewhat crude approach but has been useful when analyzing polyfunctionality of CMV-specific T-cell responses in the past (Lachmann et al., 2012). Fig. 3A shows that there was no significant difference between CMV+ and CMV− individuals in regards to these subsets. However, when plotted against the size of the CMV-specific CD4 T-cell response, it became obvious that all of these functional subsets tend to increase as a function of the size of that response (Fig. 3B). They correlated even better with the size of the tuberculin-specific CD4 T-cell response (Fig. 3C), but not with age (Fig. 3D).
Fig. 3.
Polyfunctionality of tuberculin-specific CD4 T-cell subsets correlates with the size of CMV and tuberculin-specific CD4 T-cell response but not age.
Older donors were analyzed by flow cytometry for polyfunctional responses following ex-vivo stimulation with tuberculin or CMV lysate. Polyfunctionality was defined as the parallel presence of several activation markers in antigen activated T-cells. CD154+/TNF-α+/IFN-γ+/IL-2+/degranulation+ CD4 T-cells were the most polyfunctional cells in the study and correspond to cells with 5 parallel functions in the diagrams. Rather than displaying 31 functional subsets, cells were grouped by the number of parallel functions from 1 to 5. There is no significant difference of tuberculin-specific CD4 T-cell responses between CMV− and CMV+ individuals grouped by the number of parallel functions (A). There were significant correlations between the percentage of subsets with 2, 3, and 4 parallel functions and both the sizes of the CMV and tuberculin-specific CD4 T-cell responses (B and C respectively) but not age (D). The Pearson correlation coefficient (r) and corresponding p-values for significant associations are indicated.
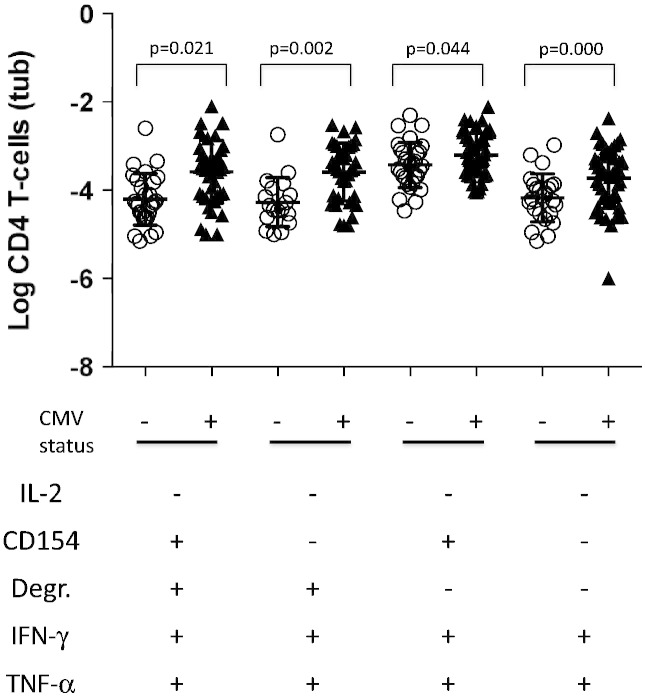
In order to understand if more subtle functional changes were associated with CMV serostatus, we finally analyzed the distribution of 31 non-overlapping Boolean subsets defined by the 5 activation markers (Lachmann et al., 2012; Roederer et al., 2011). Not all subsets were detected in all participants but some were clearly very dominant. The most dominant subset displayed only one of the tested functions, CD154+, followed by the subset displaying only CD154+ and TNF-α +. The third most dominant subset displayed CD154+, IL-2+, and TNF-α + but no other functions. These dominant subsets did not discriminate between CMV+ and CMV− individuals. However, four subsets seemed to divide the two groups with a p-value below 0.05; they had in common that they were IFN-γ+/TNF-α+/IL-2−. However, since this was a comparison with 31 statistical endpoints, the significance level dropped to p = 0.05/31 = 0.0016 by way of Bonferoni multiple end-point correction. Two subsets still remained significantly different between the groups; they were both CD154− (Fig. 4). This reflected the phenotype analysis (Fig. 2) showing that tuberculin-specific CD4 T-cells displaying IL-2 or CD154 upregulation had no significantly different memory subset distribution in CMV+ and CMV− individuals.
Fig. 4.
Individual functional subsets of tuberculin-induced CD4 T-cells differentiate between CMV+ and CMV− individuals.
Older donors were analyzed by flow cytometry for tuberculin-specific CD4 T-cell responses following in vitro antigen stimulation. Activated T cells were identified by intracellular cytokine staining. Based on 5 individual functions, IFN-γ, TNF-α, IL2 production, as well as degranulation and CD154 upregulation, 31 non-overlapping functional Boolean subsets were generated. Of these, 4 were significantly different in CMV+ and CMV− individuals given a significance level of p = 0.05. However, following multiple end-point correction (Bonferoni) differences with respect to only 2 subsets remained significant. These were IFN-γ+, TNF-α + but IL-2− and CD154−, with or without degranulation. This indicates that being CMV-positive selectively increases some terminally differentiated effector subsets.
3. Discussion
Tuberculin-inducible T-cell responses in the skin result from TB infection, exposure, or vaccination and are often used to assess T-cell immunity to M. tuberculosis. We and others have previously shown that such responses can also be effectively tested using in vitro activation with tuberculin followed by intracellular staining of cytokines (Streitz et al., 2007; Tesfa et al., 2004). In the present study this response was analyzed in parallel to the CMV-specific response in 80 healthy individuals. In keeping with reports by others, systematic data analysis did not confirm our initial observation that older people have higher tuberculin-specific T-cell responses than younger people (Akbar et al., 2013). Interestingly though comparisons between different age groups in regards to CD4 T-cell responses to CMV-antigens have provided equivocal data (Fletcher et al., 2005; Lachmann et al., 2012; Ouyang et al., 2004; Wills et al., 2011). In the present study, no correlation between age and the size of the CMV-specific CD4 T-cell response was seen. It is likely that the size of the CMV-specific T-cell response (and probably responses to other infectious antigens as well) is related to the time since first exposure or duration of infection and there is general agreement that chronological age is only a weak correlate of the former (Lachmann et al., 2012). As a result, different studies might find different results depending on the background of the population studied, in particular the percentage of CMV-infected people at a younger age. None of the donors recruited in this study had a known history of TB infection and, for most, their TB exposure would have resulted from a combination of vaccination and exposure to active TB cases, which would have provided a booster to the vaccination response. It is not possible to speculate how the way of exposure would have affected the response in any specific case.
It was striking, however, that the largest tuberculin-specific responses were found in the same people who had the largest CMV-specific responses. This is the first report to show that these responses are highly correlated in older people, but less so in younger people (where not many individuals displayed both responses, possibly due to the small group size). Some striking differences between CMV-infected and uninfected people in regards to general lymphocyte compartment composition (Chidrawar et al., 2009) suggest that CMV infection is indeed a driver of profound immune system changes (which does not rule out that there may be other such drivers in CMV-uninfected people as well). In extension of these results, the sizes of CMV-specific CD8 and CD4 T-cell responses have previously been shown to quantitatively correlate with the distribution of the CMV-specific and also the whole T-cell compartment into memory T-cell subsets defined by the expression of CD27 and CD45RA. Larger CMV-specific responses go together with a more terminal differentiation phenotype of all T-cells (fewer naive and more cells of advanced differentiation phenotype, particularly in central and effector memory compartments) (Lachmann et al., 2012). This truly important observation suggests that comparisons of inflammatory parameters across groups defined by CMV serostatus alone often give less clear results. The huge variability of the T-cell response size in CMV-infected individuals is simply not well reflected by a positive or negative CMV infection status. A correlation between CMV-antibody levels (OD-values) and the size of the T-cell response has not been reported in the literature and was not seen in the present study either (not shown). As a result, CMV-serostatus or antibody levels do not reflect very well what we would like to call the CMV-related immune ‘response mode’.
While no strict correlations exist between T-cell subset phenotype and function, there is good evidence that the most polyfunctional CMV-specific T-cell subsets are in the TCM compartment, whereas subsets producing IL-2 are more likely to be in the TNAIVE compartment (Lachmann et al., 2012). Subsets focused on just one or two functions, in particular effector functions such as degranulation, TNF-α, or IFN-γ production are more likely to be found in TEM and TEMRA compartments. Our analysis of the present study indicates that tuberculin-specific CD4 T-cells that are degranulating and producing TNF-α or IFN-γ are more differentiated in the presence than in the absence of CMV infection and that certain functional subsets displaying effector markers (degranulation, TNF-α, IFN-γ) but not IL-2 or CD40L are significantly increased in CMV-infected older people. These subsets are quite small in absolute terms; however, their difference illustrates the polarizing effect of CMV on T-cell immunity more generally. The fact that these differences were observed when comparing groups simply by CMV serostatus, in light of the discussion above, suggests that these are strong effects; it is important to note, though, that the degree of phenotype skewing is a function of response size, suggested by the present data and as previously shown (Lachmann et al., 2012). Larger responses are thus more likely to affect the T-cell response to other pathogens through a bystander effect that yet needs to be defined but may be related to cytokine production.
In summary, we conclude that the size of the CMV-specific T-cell response is probably an indicator of a T-cell ‘response mode’ that is likely to be related to ‘inflammaging’ and potentially survival at older age (Derhovanessian et al., 2009). That CMV-specific responses grow bigger in some people than others is likely a multifactorial effect including disposition as well as environmental factors. Whether the changes of the tuberculin-specific response seen in the face of large CMV-specific responses positively or negatively affect immunity to mycobacterial antigens such as, for example, the response to reactivation in latently tuberculosis-infected people is not known but will be worth looking into.
Finally, we believe that CMV-specific response size should also be considered in studies examining the effect of CMV carriage on the results of influenza vaccination (den Elzen et al., 2011). A recent study (Derhovanessian et al., 2013) has shown that , among CMV-seropositives, a poorer vaccine take was associated with more advanced differentiation of CD4 T-cells in peripheral blood, which, according to previously published data (Almanzar et al., 2005; Chidrawar et al., 2009; Derhovanessian et al., 2011; Looney et al., 1999), would reflect a larger CMV-specific CD4 T-cell response.
4. Materials and methods
4.1. Donors
Blood was taken from 131 healthy older volunteers (60 to 85 years old) recruited from general practices and from 55 young people (20 to 35 years old) recruited from university students/staff (over 90% were white Caucasian, the remainder black African or Asian). The protocol was approved by the UK National Research Ethics Service (NRES) and appropriate written informed consent was obtained. Routine serology (CMV-IgG) was obtained from the Brighton and Sussex University Hospital (BSUH) virology laboratory. Tuberculin-specific CD4 T-cells were above detection threshold (defined as 1/10,000) in 103 participants, of whom 62 were CMV+ (including n = 10 young and n = 31 older people) and 42 were CMV− (including n = 13 young and n = 41 older people). ‘Young’ people were between 20 and 35 years old; older people were 60–85 years old.
Exclusion criteria included immunodeficiency (including HIV-infection), organ transplantation, use of immunosuppressive/immunomodulating drugs within the last year, cancer or cancer treatment within the last 5 years, insulin dependent diabetes, moderate/advanced renal failure, liver disease, endocrine disorders (other than corrected thyroid dysfunction), autoimmune disease, dementia/mental incompetence (MMSE < 28), alcohol/other drug abuse, acute infection/illness in the last 4 weeks, and raised body temperature. Among older participants, 42% were taking antihypertensive drugs and/or beta blockers; 14% were taking cholesterol-lowering drugs).
4.2. Reagents
SEB (Source, Town, Country) and PPD (Purified Protein Derivative ‘human tuberculin’ SSI, Copenhagen, Denmark proteins) were used in PBMC assays at a final concentration of 5 μg/ml and 10 μg/ml respectively. Custom peptide-pools (“PepMix”, JPT Peptide Technologies, Berlin) for each CMV protein (Table 1) were dissolved in DMSO (Sigma-Aldrich), aliquoted for short-term storage at − 20 °C and used in stimulations at a final concentration of 1 μg/ml per peptide for each pool.
4.3. PBMC activation assays
Peripheral blood mononuclear cells (PBMCs) were isolated from heparin blood by density gradient centrifugation (Ficoll-Hypaque, PLUS Healthcare, Buckinghamshire, UK). PBMCs were washed twice with PBS, resuspended in complete media and stimulated in sterile polystyrene round bottom tubes (Falcon; BD Biosciences). Each tube contained 106 PBMC, anti-CD107a antibody (BD Biosciences), Monensin (‘Golgistop’, BD Biosciences), one of the stimulants (SEB, one of 16 different PepMix solutions, or tuberculin) plus complete media to a final volume of 250 μl. Stimulations were incubated at 37 °C in 5% CO2, humidified atmosphere, for 2 h prior to the addition of 250 μl Brefeldin A (BFA, final concentration 10 μg/ml; Sigma-Aldrich). Incubation was stopped after an additional 14 h.
4.4. Flow cytometry
After 14 h of incubation, 100 μl 20 mM EDTA was added to each tube and incubation continued for 10 min at 37 °C. Cells were then washed with FACS buffer and stained with v500-conjugated anti-CD3, APC-H7-conjugated anti-CD8, PE-conjugated anti-CD27 (BD Biosciences), peridinin–chlorophyll–protein complex (PerCp)-conjugated anti-CD4 (BioLegend), ECD-conjugated anti-CD45RA (Beckman Coulter, High Wycombe, UK), and live/dead yellow stain (Invitrogen). After 30 min incubation at 4 °C, cells were treated with BD FACS lysing solution and BD FACS permeabilizing Solution II according to manufacturer's instructions (BD Biosciences). For intracellular cytokine staining, the following antibodies were used: Pacific Blue (PB)-conjugated anti-CD154, PE-Cy7-conjugated anti-IFN-γ (BioLegend), Alexa 700-conjugated anti-TNF-α, and FITC-conjugated anti-IL-2 (BD Biosciences). After 30 min incubation at 4 °C, cells were washed, fixed with 0.5% PFA for 5 min, RT, then washed again and kept at 4 °C in the dark until acquisition. Cells were analyzed on an LSRII flow cytometer using FACSDiva-v6.1 software (BD Biosciences) and analyzed using FlowJo-v9 software (TreeStar Inc., Ashland, USA). In order to identify functional combinations of markers, Boolean gates were used on separate gates set for each activation marker on CD4 and CD8 T-cells (IL-2, degranulation, CD154, TNF-α, and IFN-γ). In this study ‘activated’ effector T-cells are represented by the percentage of cells displaying at least one of the markers in the sum of all Boolean gates. For each individual gate, values for unstimulated samples (negative control) were subtracted from those for stimulated samples.
4.5. Statistical analysis
Statistical tests were performed with SPSS-20. A two-sided p < 0.05 was considered statistically significant, unless multiple end-point correction (Bonferoni) was performed as indicated.
For the calculation of Pearson correlation coefficients (SPSS bivariate correlation procedure) log-transformed T-cell subset percentages were used. Log transformation of T-cell subset percentages improved the normality of distributions.
Conflict of interest
The authors have no conflicts of interests.
Footnotes
This work was supported by The Dunhill Medical Trust [grant number R107/0209].
References
- Sylwester A.W., Mitchell B.L., Edgar J.B., Taormina C., Pelte C., Ruchti F., Sleath P.R., Grabstein K.H., Hosken N.A., Kern F., Nelson J.A., Picker L.J. Broadly targeted human cytomegalovirus-specific CD4 + and CD8 + T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidrawar S., Khan N., Wei W., McLarnon A., Smith N., Nayak L., Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanzar G., Schwaiger S., Jenewein B., Keller M., Herndler-Brandstetter D., Wurzner R., Schonitzer D., Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8 + T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney R.J., Falsey A., Campbell D., Torres A., Kolassa J., Brower C., McCann R., Menegus M., McCormick K., Frampton M., Hall W., Abraham G.N. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin. Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E., Maier A.B., Hahnel K., Beck R., de Craen A.J., Slagboom E.P., Westendorp R.G., Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4 + and CD8 + T-cells in humans. J. Gen. Virol. 2011;92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- Fagnoni F.F., Vescovini R., Passeri G., Bologna G., Pedrazzoni M., Lavagetto G., Casti A., Franceschi C., Passeri M., Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- Moro-Garcia M.A., Alonso-Arias R., Lopez-Vazquez A., Suarez-Garcia F.M., Solano-Jaurrieta J.J., Baltar J., Lopez-Larrea C. Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age (Dordr) 2012;34:479–495. doi: 10.1007/s11357-011-9240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurwein-Teissl M., Lung T.L., Marx F., Gschosser C., Asch E., Blasko I., Parson W., Bock G., Schonitzer D., Trannoy E., Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Appay V., Dunbar P.R., Callan M., Klenerman P., Gillespie G.M., Papagno L., Ogg G.S., King A., Lechner F., Spina C.A., Little S., Havlir D.V., Richman D.D., Gruener N., Pape G., Waters A., Easterbrook P., Salio M., Cerundolo V., McMichael A.J., Rowland-Jones S.L. Memory CD8 + T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E., Theeten H., Hahnel K., Van Damme P., Cools N., Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31:685–690. doi: 10.1016/j.vaccine.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Lachmann R., Bajwa M., Vita S., Smith H., Cheek E., Akbar A., Kern F. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J. Virol. 2012;86:1001–1009. doi: 10.1128/JVI.00873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelic A., Verschoor C.P., Ventresca M., Parsons R., Evelegh C., Bowdish D., Betts M.R., Loeb M.B., Bramson J.L. The polyfunctionality of human memory CD8 + T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog. 2012;8:e1003076. doi: 10.1371/journal.ppat.1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q., Wagner W.M., Zheng W., Wikby A., Remarque E.J., Pawelec G. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp. Gerontol. 2004;39:607–613. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Vescovini R., Biasini C., Fagnoni F.F., Telera A.R., Zanlari L., Pedrazzoni M., Bucci L., Monti D., Medici M.C., Chezzi C., Franceschi C., Sansoni P. Massive load of functional effector CD4 + and CD8 + T cells against cytomegalovirus in very old subjects. J. Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- Kaufmann S.H. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Khan N., Hislop A., Gudgeon N., Cobbold M., Khanna R., Nayak L., Rickinson A.B., Moss P.A. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. Journal of immunology. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- Horsburgh C.R., Jr., O'Donnell M., Chamblee S., Moreland J.L., Johnson J., Marsh B.J., Narita M., Johnson L.S., von Reyn C.F. Revisiting rates of reactivation tuberculosis: a population-based approach. Am. J. Respir. Crit. Care Med. 2010;182:420–425. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Turner J., Szomolay B. A model on the influence of age on immunity to infection with Mycobacterium tuberculosis. Exp. Gerontol. 2008;43:275–285. doi: 10.1016/j.exger.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Hervert L.P., Garcia-Garcia L., Ferreyra-Reyes L., Bobadilla-del-Valle M., Cano-Arellano B., Canizales-Quintero S., Ferreira-Guerrero E., Baez-Saldana R., Tellez-Vazquez N., Nava-Mercado A., Juarez-Sandino L., Delgado-Sanchez G., Fuentes-Leyra C.A., Montero-Campos R., Martinez-Gamboa R.A., Small P.M., Sifuentes-Osornio J., Ponce-de-Leon A. Tuberculosis in ageing: high rates, complex diagnosis and poor clinical outcomes. Age Ageing. 2012;41:488–495. doi: 10.1093/ageing/afs028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J.M., Vukmanovic-Stejic M., Dunne P.J., Birch K.E., Cook J.E., Jackson S.E., Salmon M., Rustin M.H., Akbar A.N. Cytomegalovirus-specific CD4 + T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- Day C.L., Abrahams D.A., Lerumo L., Janse van Rensburg E., Stone L., O'Rie T., Pienaar B., de Kock M., Kaplan G., Mahomed H., Dheda K., Hanekom W.A. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 2011;187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley W.W., Shafiani S., Wittmer S.T., Tucker-Heard G., Moon J.J., Jenkins M.K., Urdahl K.B., Winslow G.M., Woodland D.L. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A.N., Reed J.R., Lacy K.E., Jackson S.E., Vukmanovic-Stejic M., Rustin M.H. Investigation of the cutaneous response to recall antigen in humans in vivo. Clin. Exp. Immunol. 2013;173:163–172. doi: 10.1111/cei.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V., van Lier R.A., Sallusto F., Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- Roederer M., Nozzi J.L., Nason M.C. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitz M., Tesfa L., Yildirim V., Yahyazadeh A., Ulrichs T., Lenkei R., Quassem A., Liebetrau G., Nomura L., Maecker H., Volk H.D., Kern F. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS ONE. 2007;2:e735. doi: 10.1371/journal.pone.0000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfa L., Koch F.W., Pankow W., Volk H.D., Kern F. Confirmation of Mycobacterium tuberculosis infection by flow cytometry after ex vivo incubation of peripheral blood T cells with an ESAT-6-derived peptide pool. Cytometry. 2004;60B:47–53. doi: 10.1002/cyto.b.20007. [DOI] [PubMed] [Google Scholar]
- Huebner R.E., Schein M.F., Bass J.B., Jr. The tuberculin skin test. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- Wills M., Akbar A., Beswick M., Bosch J.A., Caruso C., Colonna-Romano G., Dutta A., Franceschi C., Fulop T., Gkrania-Klotsas E., Goronzy J., Griffiths S.J., Henson S., Herndler-Brandstetter D., Hill A., Kern F., Klenerman P., Macallan D., Macualay R., Maier A.B., Mason G., Melzer D., Morgan M., Moss P., Nikolich-Zugich J., Pachnio A., Riddell N., Roberts R., Sansoni P., Sauce D., Sinclair J., Solana R., Strindhall J., Trzonkowski P., van Lier R., Vescovini R., Wang G., Westendorp R., Pawelec G. Report from the second cytomegalovirus and immunosenescence workshop. Immun. Ageing. 2011;8:10. doi: 10.1186/1742-4933-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E., Maier A.B., Beck R., Jahn G., Hahnel K., Slagboom P.E., de Craen A.J., Westendorp R.G., Pawelec G. Hallmark features of immunosenescence are absent in familial longevity. J. Immunol. 2009;185:4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- den Elzen W.P., Vossen A.C., Cools H.J., Westendorp R.G., Kroes A.C., Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29:4869–4874. doi: 10.1016/j.vaccine.2011.03.086. [DOI] [PubMed] [Google Scholar]