Abstract
Aim:
Downregulation of androgen biosynthesis genes StAR (steroidogenic acute regulatory) and 3β-HSD (3β-hydroxysteroid dehydrogenase) contributes to low testosterone levels in hypoxic mice and is possibly related to increased expression of pro-inflammatory cytokines in the testis. The aim of this study is to investigate the effects of CPU86017-RS that block Ca2+ influx on hypoxia-induced testis insult in mice.
Methods:
Male ICR mice were divided into 5 groups: control group, hypoxia group, hypoxia group treated with nifedipine (10 mg/kg), hypoxia groups treated with CPU86017-RS (60 or 80 mg/kg). Hypoxia was induced by placing the mice in a chamber under 10%±0.5% O2 for 28 d (8 h per day). The mice were orally administered with drug in the last 14 d. At the end of experiment the testes of the mice were harvested. The mRNA and protein levels of StAR, 3β-HSD, connexin 43 (Cx43), matrix metalloprotease 9 (MMP9), endothelin receptor A (ETAR) and leptin receptor (OBRb) were analyzed using RT-PCR and Western blotting, respectively. The malondialdehyde (MDA), lactate dehydrogenase (LDH), succinate dehydrogenase (SDH) and acid phosphatase (ACP) levels were measured using biochemical kits. Serum testosterone concentration was measured with radioimmunoassay.
Results:
Hypoxia significantly increased the MDA level, and decreased the LDH, ACP and SDH activities in testes. Meanwhile, hypoxia induced significant downregulation of StAR and 3β-HSD in testes responsible for reduced testosterone biosynthesis. It decreased the expression of Cx43, and increased the expression of MMP9, ETAR and OBRb, leading to abnormal testis function and structure. These changes were effectively diminished by CPU86017-RS (80 mg/kg) or nifedipine (10 mg/kg).
Conclusion:
Low plasma testosterone level caused by hypoxia was due to downregulation of StAR and 3β-HSD genes, in association with an increased expression of pro-inflammatory cytokines. These changes can be alleviated by CPU86017-RS or nifedipine.
Keywords: intermittent hypoxia, testicular injury, CPU86017-RS, nifedipine, StAR, 3β-hydroxysteroid dehydrogenase, matrix metalloprotease 9, endothelin receptor A, connexin 43, OBRb
Introduction
Oxygen is essential and critical for life in humans and other mammals1, 2. When subjected to a low supply of oxygen, a series of pathological changes are initiated in cells and tissues, leading to ischemia, stroke, hemorrhage and many other cardiopulmonary diseases3, 4. Certain genes whose transcription is activated by exposure of the cell to hypoxia accelerate the appearance of pro-inflammatory cytokines such as ROS (reactive oxygen species), and induce the activation of the endothelin system5, 6. Hypoxia, which is a ubiquitous pathological process, has serious adverse effects on the respiratory and cardiovascular systems7, and possibly damages the reproductive system as well. A decline in the ability of conceive has frequently been noticed in population lived in highland area.
Male hypogonadism causes degenerative changes in the testes and is characterized by low-concentrations of serum testosterone associated with testicular lesions8. An over-activated ET-ROS (endothelin–reactive oxygen species) system enhances activities of some cellular inflammatory factors responsible for the genesis of male hypogonadism. Emerging data suggest that the ET system mediates pathological changes leading to male hypogonadism 9 and testopathy in individuals with diabetic mellitus10, 11, 12. Matrix metalloprotease (MMPs) are the most important proteases participating in extracellular matrix degradation and remodeling and MMP9 takes an important part in the maintenance of the normal structure and function of the seminiferous tubules. Intercellular gap junction communication is operated by a family of proteins known as connexins, among them connexin 43 (Cx43) is the most abundant and extensively distributed of the connexins and it is known to be affected in disease13, 14. An alteration of Cx43 is concerned in abnormal function and structure of the testis15. Testicular disorders resulting from intermittent hypoxia exposure may be caused by a deficiency in Cx43-mediated gap junctional signaling in the testis.
CPU86017 is synthesized by China Pharmaceutical University through modification of the berberine moiety. In pulmonary hypertension induced by hypoxia, or by the inflammatory reagent monocrotaline, CPU86017 suppresses the activation of the endothelin system and the formation of oxygen free radicals7, 16. CPU86017 also blocks L-type calcium channels17. This calcium- antagonism activity is thought to underlie its effect of relieving cardiovascular disorder18. CPU86017 and its isomer CPU86017-RS were found to act as a protection against tissue damage from various pathological factors. They confer this protection by blocking the ET-ROS pathway and subsequently attenuating the pro-inflammatory cytokines involved in the pathologies caused by hypoxia-induced pulmonary hypertension and cardiovascular abnormalities7, 16, 17, 18, 19, 20, 21. Hypoxia-induced pulmonary hypertension causes ROS production22, and nifedipine has been recommended as an effective therapy for managing hypoxic pulmonary hypertension in clinical settings23, 24, 25.
We hypothesized that the male hypogonadism caused by intermittent hypoxia is a result of a chronic inflammatory response in which over-activated cytokines such as ET, leptin, and MMPs, in addition to the downregulation of Cx43, contribute to the pathology in hypoxic testes. We further hypothesized that these changes could be initiated by an increase in calcium influx. In this study, we aimed to determine whether the hypoxia-induced upregulation of inflammatory cytokines and downregulation of testosterone biosynthesis genes in the testis, which are attributed to an enhanced influx of calcium, could respond to nifedipine or CPU 86017-RS.
Materials and methods
Animals and treatment
Male ICR mice, weighing 22–25 g, were bought from the Zhejiang Experimental Animal Center [License Number: SCXK(Z)20080033]. All procedures performed on the animals were conducted in accordance with the Animal Regulations of Jiangsu Province, China.
Mice were randomly divided into 5 groups (n=6). The groups were as follows: a control group, a hypoxia group, a hypoxia group also treated with nifedipine (10 mg/kg po, Changzhou Kangpu Pharmaceutical Co, Ltd, China, batch number: 0910004), and two groups treated with CPU86017-RS (RS60, 60 mg/kg; RS80, 80 mg/kg po, supplied by the Department of Medicinal Chemistry of China Pharmaceutical University, batch number: 20090301). Chronic exposure to intermittent hypoxia was performed as previously described6, 7. Briefly, for 8 h per day for 28 d, the mice were placed in a normobaric hypoxic chamber in which the oxygen content was maintained at 10%±0.5%. The interventions by nifedipine or CPU86017-RS were applied during the last 2 weeks of hypoxia exposure.
Biochemical assays
The testis was harvested from mice after cervical dislocation and 100 mg of testicular tissue was homogenized in 10 volume of physiological saline. MDA, LDH, SDH, and ACP levels were measured by using biochemical kits (Nanjing Jiancheng Institute of Bio-engineering Company, Nanjing, China). Serum testosterone concentrations were measured by radioimmunoassay (RIA) with kits purchased from the institute of Jiuding Medical Bio-engineering company (Tianjin, China). These measurements were made in accordance with previous reports9, 10, 11.
RT-PCR
The testes were harvested and total RNA was extracted using the Trizol solution. cDNA was synthesized as described previously (Invitrogen, Carlsbad, CA, USA)14. The primer (Invitrogen Trading Co, Ltd, Shanghai, China) sequences were used as follows: sense: 5′-ATCGGGATCCCCTTGATTAC-3′ and antisense: 5′-ACAGCAACAGAGGCAGGACT-3′ for ETAR, sense: 5′-CGACATAGACGGCATCCAG-3′ and antisense: 5′-CTGTCGGCTGTGGTTCAGT-3′ for MMP9, sense: 5′-TACCACGCCACCGGCCCA-3′ and antisense: 5′-GCATTTTGGCTGTCGTCAGG-3′ for Cx43, sense: 5′-AGAATTGTTCCTGGGCACAAG-3′ and antisense: 5′-ACACTCATCCTCACAGGTTCC-3′ for OBRb, sense: 5′-AACTTTGGCATTGTGGAAGG-3′ and antisense: 5′-GGAGACAACCTGGTCCTCAG-3′ for GAPDH.
Western blotting
After the mice were sacrificed, proteins of testes were extracted (Biouniquer Biotechnology Co, Ltd, Hangzhou, China) and Western blotting was conducted as described previously6. The sources of antibodies were listed as below: polyclonal rabbit anti-ETAR-IgG, polyclonal rabbit anti-StAR-IgG and polyclonal rabbit anti-MMP9-IgG were purchased from Santa Cruz Biotechnology Inc, USA; polyclonal rabbit anti-Cx43-IgG, polyclonal rabbit anti-β-actin-IgG, polyclonal rabbit anti-OBRb-IgG and horseradish peroxidase (HRP)-conjugated goat secondary antibody IgG were purchased from Wuhan Boster Biological Technology, Wuhan, China.
Assessment of testis pathology
The testis tissues, fixed with neutral 10% formalin, were embedded in paraffin and sliced into 4-μm-thick sections and stained with hematoxylin-eosin (HE). All pictures were viewed under an inverted microscope (Nikon TE 2000-U, Japan) by a pathologist blinded to the experiment.
Immunohistochemical fluorescence assay
The immunohistochemical fluorescence assay were conducted as previously described26. Paraffin-embedded testicular tissue was sliced into 4-μm-thick sections, dewaxed with xylene 2 times and dehydrated with decreasing concentrations of ethanol (100%, 95%, 90%, 80%, and 70%). The sections were then placed in a 3:4 ratio solution of sodium-citrate:citrate, microwaved on full power for 8 min and soaked in a 3% H2O2-methanol solution for 15 min to inactivate enzymes. The slices were then incubated in primary antibody (1:100, polyclonal rabbit anti-3β-hydroxysteroid dehydrogenase (3β-HSD)-IgG, Novus Biological LLC, USA) for 2 h, washed 3 times with PBS, incubated with FITC-labeled secondary antibodi+es (1:400, KeyGEN BioTECH, Nanjing, China) for 1 h at 37 °C, washed 3 times and stained with DAPI (KeyGEN BioTECH, Nanjing, China) for 10 min at room temperature in the dark. All slides were viewed with a fluorescence microscope (OLYMPUS IX51, Japan).
Statistical analysis
All data were analyzed using SPSS11.5 (USA) software and are presented as the mean±SD. Statistics were evaluated using one-way analysis of variance followed by a Dunnett's test. The Student Newman-Keuls test was performed when the variances were equal, and the Games-Howell test was used when the variances were not equal. A probability value of P<0.05 was considered statistically significant.
Results
Testicular MDA, ACP, SDH, and LDH
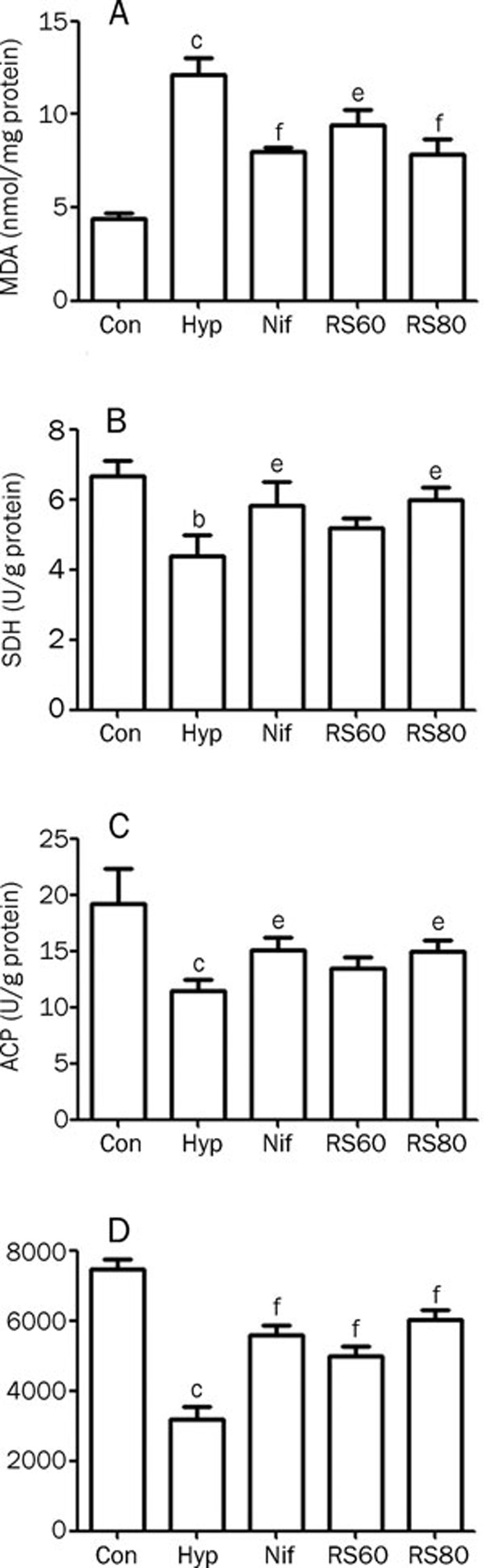
Oxidative stress occurs in testicular tissues following exposure to intermittent hypoxia. In the testis, the production of MDA, an indication of oxidized lipid substances from an excess of reactive oxygen species (ROS), was increased by 177.1% (P<0.01) compared to controls. By contrast, the activities of ACP, SDH, and LDH, which are the main energy suppliers for both testosterone biosynthesis and cell maturation during spermatogenesis, were decreased in hypoxic testes by 42.1% (P<0.01), 34.3% (P<0.01) and 57.1% (P<0.01), respectively, compared to controls. A reduction in these enzymatic activities was, at least in part, related to the testicular lesions induced by the 28-d exposure to intermittent hypoxia, which led to low testosterone production in these mice. These abnormalities were decreased by interventions with either CPU86017-RS or nifedipine (Figure 1).
Figure 1.
Testicular MDA increased significantly in hypoxic mice, accompanied with a reduction of SDH, ACP, and LDH dramatically, indicating that both oxidative stress and short of energy supply in the hypoxic testis were significant. These changes were obviously alleviated by the medication of CPU86017-RS and nifedipine. n=6. Mean±SD. bP<0.05, cP<0.01 vs control. eP<0.05, fP<0.01 vs hypoxia.
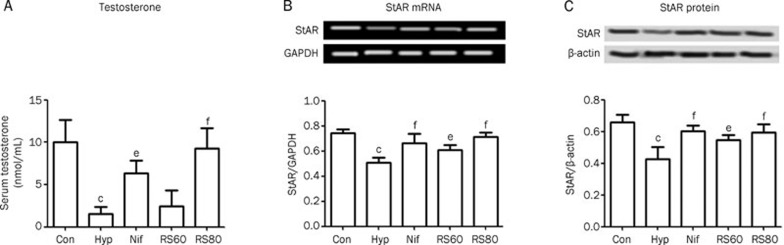
Serum testosterone and StAR
Low serum testosterone has been recognized as the main criterion for clinical diagnosis of male hypogonadism. Therefore, we measured serum testosterone levels. In this study, serum testosterone levels, which are very sensitive to hypoxia, decreased by 85.0% (P<0.01) in the hypoxic testis compared to the control group (Figure 2A). The biosynthesis of testosterone depends on a series of gene activities that convert cholesterol to testosterone. The first step in this series of reactions involves the StAR-controlled uptake of cholesterol into the mitochondria. The expression of the testosterone biosynthesis gene StAR was downregulated by 32% (P<0.01) at the mRNA level and 36.4% (P<0.01) at the protein level relative to controls (Figures 2B, 2C).
Figure 2.
Serum testosterone content and expressions of StAR were decreased distinctly in mice suffering from hypoxia, which were neutralized by medication of CPU86017-RS. n=6. Mean±SD. cP<0.01 vs control. eP<0.05, fP<0.01 vs hypoxia.
Pathological changes
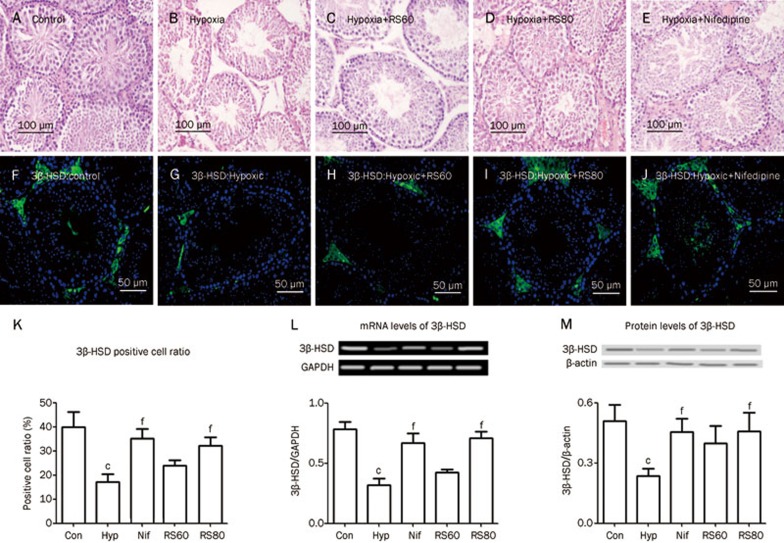
The morphological arrangement of the seminiferous tubules was precisely layered and contained the spermatogenetic and Sertoli's cells associated with an intact basement. As in normal physiological conditions, these tubules were filled with spermatozoa that were concentrated at the center of the lumen and were surrounded by a clearly structured reproductive epithelium. The inter-tubule space contained an abundance of Leydig's cells, which are responsible for the active production of androgen. In contrast, the seminiferous tubules in the hypoxic group were badly damaged; their multi-layer reproductive endothelium was destroyed; they had greatly decreased amounts of spermatozoa in the lumen, and they had distorted reproductive epithelial and Sertoli's cells. The hypoxic mice also had a decreased number of Leydig's cells in the intra-tubular space. CPU86017-RS and nifedipine treatment preserved the normal appearance of the seminiferous tubules and the number of Leydig's cells in the inter-tubular space (Figure 3A–3E).
Figure 3.
Histological changes of the testes and abnormal expression of 3β-HSD (green fluorescence) were observed. A–E: Histological changes in the testis were found in H+E stained slices. (A) Control; (B) Hypoxia; (C) Hypoxia+RS60; (D) Hypoxia+RS80; (E) Hypoxia+nifedipine. The cellular distribution and expression of 3β-HSD were detected by immunofluorescence assay in green color (F–J), and RT-PCR and western blotting were also employed. (F) 3β-HSD in normal control; (G) in hypoxic mice; (H) Hypoxia+RS60; (I) Hypoxia+RS80; (J) Hypoxia+nifedipine; (K) positive cell ratio of 3β-HSD; L & M, mRNA and protein levels of 3β-HSD.
Immunofluorescence imaging and Western blotting of 3β-HSD
To collect further evidence of the existence of hypoxic lesions in the testis, 3β-HSD, a specific biomarker of Leydig's cell in the testis, was targeted. 3β-HSD is involved in many of the steps in the synthesis of testosterone from cholesterol that occur in the mitochondria of Leydig's cells and is therefore critical for androgen biogenesis. The location and abundance of 3β-HSD conjugated to Leydig's cells in the space within the tubules were clearly observed with the green signal from the immunofluorescence assay, and functioning Leydig's cells were clearly observed in the control mice (Figure 3F). The green fluorescence intensity of Leydig's cells was greatly reduced following exposure to intermittent hypoxia, in agreement with the changes in histology and biomarkers and the reduced serum testosterone concentration. The recovery of the Leydig's cells labeled with immunofluorescence was significant in mice treated with either nifedipine or CPU86017-RS (Figure 3G–3K). In parallel experiments, Western blots revealed a significant decrease in the protein expression of 3β-HSD (P<0.01) compared to controls. CPU86017-RS and nifedipine treatment increased the mRNA and protein expression of 3β-HSD (Figure 3L, 3M).
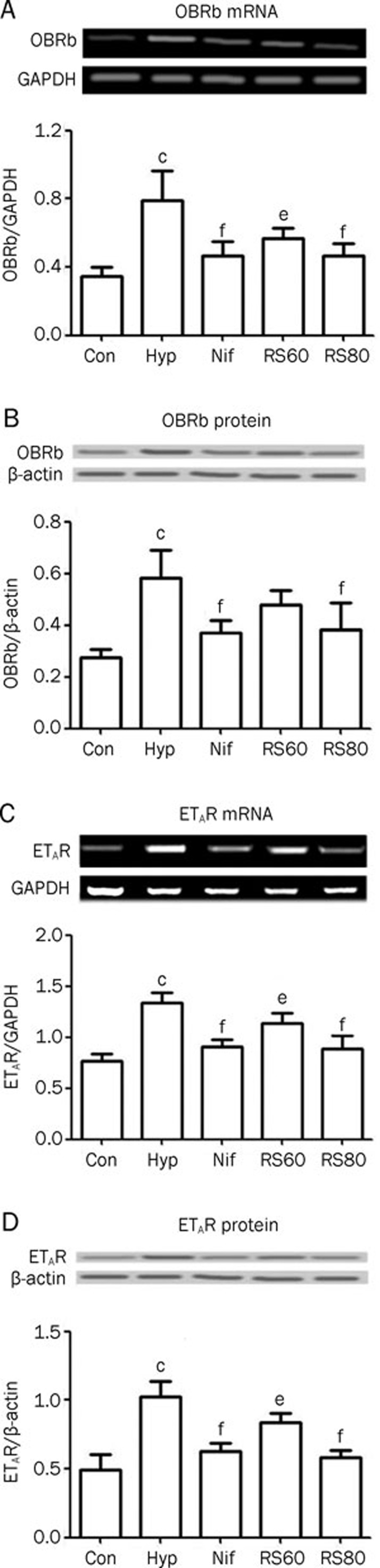
ETAR and OBRb expression
Leptin and its receptors greatly affect the male reproductive epithelium during developmental and postnatal periods. In the present study, we found elevated levels of OBRb mRNA and protein expression in the testis (Figure 4A, 4B), as well as an upregulation of mRNA and protein levels of ETAR (Figure 4C, 4D). Activated ETAR is always accompanied by an increase in oxidants. Therefore, the upregulation of ETAR is indicative of a state of oxidative stress in the hypoxic testes. CPU86017-RS and nifedipine neutralized the abnormal expression of OBRb (P<0.01) and ETAR (P<0.01) compared with hypoxic mice that did not receive treatment (Figure 4).
Figure 4.
An increase in expression of ETAR and OBRb was found in hypoxic mice, which was blunted by CPU86017-RS compound and nifedipine respectively. n=6. Mean±SD. cP<0.01 vs dontrol. eP<0.05, fP<0.01 vs hypoxia.
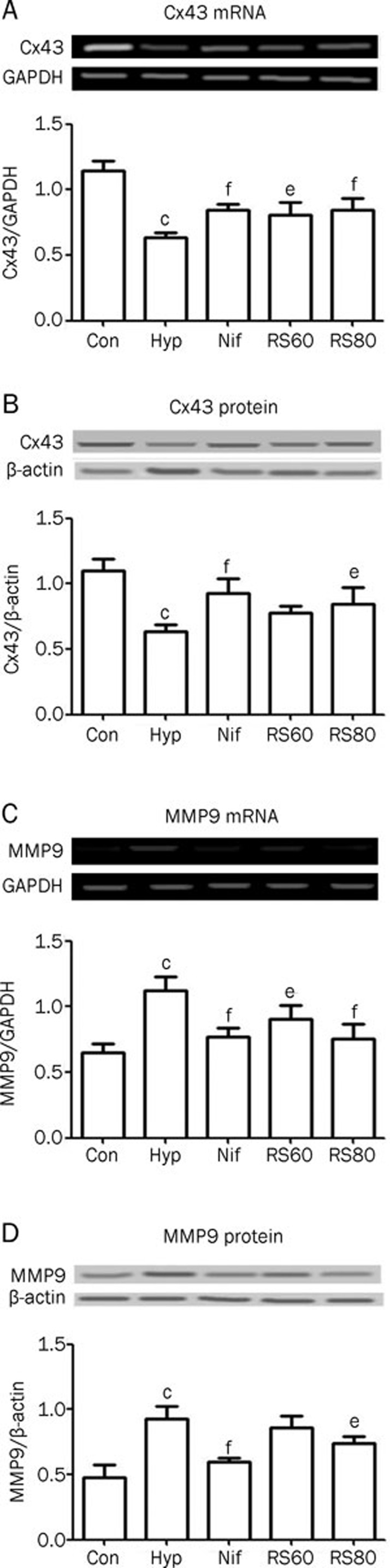
Cx43 and MMP9
Hypoxia-induced male hypogonadism may be the consequence of impairment of the extracellular matrix. This is relevant because the extracellular matrix is important in gap junctional communication through the intercellular space of cells in the testes. Cx43 mRNA and protein expression decreased by 43.6% and 42.7% (P<0.01), respectively, in the hypoxia group compared to controls (Figure 5A, 5B), indicating that the intercellular gap junctional communications in the testis were insufficient. This finding coincided with the depressed testicular function observed after episodes of hypoxia. This Cx43 impairment contributed to the decreased testosterone production in the testes. In contrast, MMP9 mRNA and protein expression was increased by 70.8% and 93.8% (P<0.01), respectively (Figure 5C, 5D), providing evidence for the idea that intermittent hypoxia induces remodeling of the extracellular matrix. Extracellular matrix remodeling was significant and resulted in an alteration in the intercellular space that affected signal transduction through intercellular gap junctions. CPU86017-RS and nifedipine relieved the hypoxia-induced insults to the reproductive system, partly by normalizing the extracellular matrix and gap junctional communication in the testes.
Figure 5.
Abnormal expressions of MMP9 and Cx43 were found in the testis after hypoxia exposure and were reversed by CPU86017-RS and nifedipine dramatically. n=6. Mean±SD. cP<0.01 vs control. eP<0.05, fP<0.01 vs hypoxia.
Discussion
Normobaric hypoxia is a common pathological process that damages multiple metabolic and cardiovascular systems27. Hypoxia inhibits spermatogenesis by damaging the seminiferous epithelium and promoting the apoptosis of spermatogenic cells in the reproductive system.
Serum testosterone concentrations less than 300 ng/dL serve as a criterion for the diagnosis of male hypogonadism28. Testosterone serum concentrations decreased significantly after exposure to intermittent hypoxia, and this decrease was due to decreased expression of the androgen biosynthesis genes, StAR and 3β-HSD. Decreased testosterone production induced by intermittent hypoxia may result from inhibition of cholesterol desmolase activity (cleavage of cholesterol side-chains by cytochrome P450 and P450scc) and the cAMP pathway; therefore, decreased testosterone production may be related to abnormal intracellular calcium levels29, 30. Testosterone replacement therapy, which is widely used to treat male hypogonadism in clinical settings, elevates serum testosterone levels directly and improves erectile dysfunction. However, although this therapeutic approach suppresses the hypothalamus-pituitary-testis feed back loop, it does not alleviate testicular lesions10, 11. Increasing the gene expression of both StAR and 3β-HSD represents a basic approach to normalize the hypoxia-induced decrease in testicular function. In the present study, the hypoxia-induced downregulation of these two genes was significantly attenuated by the calcium antagonists nifedipine and CPU86017-RS.
In the testes, the activity of ACP, LDH, and SDH is a crucial source of energy for both testosterone biosynthesis and spermatogenic epithelium maturation. Therefore, the activities of ACP, LDH, and SDH can be used as markers for predicting testis function. ACP is essential for protein synthesis and is actively involved in germ development and Sertoli's cell function. LDH and SDH are present during spermatogenesis and are found in Sertoli's cells, where they take an active part in supplying energy by metabolizing glucose28. They also play an important role in the transformation of testosterone from cholesterol in Leydig's cells.
Calcium channels in the cell membrane actively control the influx of Ca2+, which influences the intracellular levels of free Ca2+. Consequently, the intracellular levels of free Ca2+ markedly affect the genesis and maturation of sperm cells31, 32. Sperm and Leydig's cells containing L-type voltage-dependent calcium channels are crucial for sperm-egg fusion and testosterone biosynthesis. In this study, nifedipine served as the positive control that limited the calcium influx and reversed the increase in calcium influx observed following exposure to low levels of oxygen. Under physiological conditions, calcium concentrations in the extracellular space are about a million times higher than those in the cytosol. Voltage-dependent L-type channels are important in living cells, and upon activation under physiological conditions, a small amount of Ca2+ moves through these channels and promotes a series of Ca2+-activated events necessary for normal testicular function. An overload of Ca2+ influx caused by hypoxia contributes significantly to hypoxia-induced testicular damage. Blocking this calcium influx with nifedipine reverses the hypoxia-induced increase in overloaded free Ca2+ levels in the testicular cells, thereby decreasing the pathological changes secondary to hypoxia. In addition, CPU86017 has been found to act as a calcium antagonist in the cardiovascular system17, 18, and this calcium antagonism may underlie the mechanism by which this compound acts to relieve hypoxia-induced testicular abnormalities. The disturbance of calcium homeostasis in reproductive cells is the main cause of the low testosterone concentrations and abnormal pro-inflammatory biomarkers observed in hypoxic testes. These changes were relieved by the CPU86017-RS isomer, and the relief was comparable to that observed with nifedipine. We also emphasize that under physiological conditions calcium influx is necessary for the maintenance of normal testicular size and function. Large doses of nifedipine caused a decrease in testosterone biosynthesis and a reduction in testicular size in prepubertal mice33.
An over-activated endothelin system has been found to be involved in several pathologies in the cardiovascular system and to participate in myocardium fibrosis through the activation of MMP2/9 in heart fibroblasts14. Our previous studies have suggested that an upregulation of ETAR is closely linked to an increase in ROS production, which forms a so-called ET-ROS pathway in diabetic cardiomyopathy, vascular disorder and nephropathy34, 35. ROS are over-produced in hypoxia and facilitates the activation of ETAR. ETAR then provides positive feedback to stimulate the NADPH oxidase and to initiate oxidative stress in the testis. The rhythmic contraction of seminiferous tubules propels sperm cells to the epididymis and depends on the stimulation of ET-1, which is secreted mainly by Sertoli's cells and Leydig's cells. Under intermittent hypoxia conditions, over-activation of ETAR leads to an impairment in the movement of the tubules, impeding the final maturation of sperm cells in the epididymis.
Abnormal expression of MMP9 and Cx43 in isoproterenol-induced cardiovascular abnormality is related to ETAR activation14. Spermatogenesis is a highly programmed process that requires well-regulated degradation and remodeling of the extracellular matrix. The regulation of CD147 by MMP2 and MMP9 is essential for germ cell migration during spermatogenesis36. MMP9 is a key effector molecule in the increased neural stem cell proliferation and migration that occur during low O2 conditions. Decreased MMP9 expression adversely influences cell proliferation, cell migration and cell cycle kinetics in the testes. Gap junctional communication is achieved through a family of proteins named connexins, through which small molecules, such as ions and second messengers, and various other signaling molecules can be exchanged between cells37. Cx43 is found in a variety of tissues, including the testes, and is responsible for conducting cell-to-cell communication via electrical and chemical coupling. Gap junctional communication via Cx43 plays an important role in normal testicular function because it normalizes the distribution of electrical heterogeneity38. The effectiveness of nifedipine and CPU86017-RS in relieving the hypoxia-induced abnormality of testicular Cx43 is likely the same as diltiazem39. Cx43 function is dependent on the connection of the two halves of the channels across the extracellular space; therefore, changes in the extracellular matrix may greatly impair this connection, resulting in a blockade of gap junctional communication and a dysfunctional testis.
Leptin, and its receptor OBRb, significantly modulates the function of reproductive cells in the testis. Leptin also affects the proliferation and differentiation of germ cells and modulates testicular steroidogenesis through autocrine/paracrine factors40. Over-activation of testicular OBRb caused by intermittent hypoxia reflects a status of hyperleptinemia, which indicates the presence of oxidative stress related to over-activity of ETAR and the sympathetic nerve system. As a result, over-expressed OBRb may damage testosterone biosynthesis by increasing Ca2+ influx in the testis. Therefore, treatment with nifedipine and CPU86017-RS is appropriate for the treatment of abnormal OBRb and ETAR, thereby ameliorating the dysfunction observed in hypoxic testes.
In conclusion, hypoxic testopathy is characterized by a downregulation of StAR, 3β-HSD, and Cx43 expression and an upregulation of ETAR, MMP9 and OBRb expression. These changes are sensitive to both nifedipine and CPU86017-RS, which implies that the primary cause of the hypoxia-induced damage to the testicular cells is likely mediated by an increase in Ca2+ influx. CPU86017-RS and nifedipine attenuated the hypoxia-induced testicular dysfunction by normalizing the testicular expression of genes and pro-inflammatory biomarkers. Further investigation is needed to obtain direct evidence of the calcium-influx-blocking effect of CPU86017-RS and nifedipine in Leydig's cells.
Author contribution
Guo-lin ZHANG conducted the project, processed the data and prepared the manuscript. Feng YU assisted with the data processing. Yu-si CHENG and Can ZHANG provided the compounds. De-zai DAI and Yin DAI designed the project, discussed the underlying mechanisms and revised the manuscript.
Acknowledgments
This work was supported by the National Key New Drug Innovation Program, the Ministry of Science and Technology of China (Grant No: 2009ZXJ09004-070) and the National Natural Science Foundation of China (Grant No: 81070145).
References
- Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci. 2009;66:3539–54. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahling M. Cellular oxygen sensing, signalling and how to survive translational arrest in hypoxia. Acta Physiol. 2009;195:205–30. doi: 10.1111/j.1748-1716.2008.01894.x. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Osredkar D, Lee CL, Jiang X, Mu D, Ferriero DM. HIF-1 alpha-deficient mice have increased brain injury after neonatal hypoxia-ischemia. Dev Neurosci. 2009;31:452–8. doi: 10.1159/000232563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa M, Moustafa RR, Baron JC. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke. 2008;39:1629–37. doi: 10.1161/STROKEAHA.107.485938. [DOI] [PubMed] [Google Scholar]
- Guo S, Miyake M, Liu KJ, Shi H. Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J Neurochem. 2009;108:1309–21. doi: 10.1111/j.1471-4159.2009.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Dai DZ, Dai Y. CPU86017 and its isomers improve hypoxic pulmonary hypertension by attenuating increased ETA receptor expression and extracellular matrix accumulation. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:541–52. doi: 10.1007/s00210-008-0309-4. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Cui B, Dai DZ, Tang XY. Pharmacological efficacy of CPU 86017 on hypoxic pulmonary hypertension in rats: mediated by direct inhibition of calcium channels and antioxidant action, but indirect effects on the ET-1 pathway. J Cardiovasc Pharmacol. 2005;46:727–34. doi: 10.1097/01.fjc.0000184470.58047.79. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Kawa G, Kinoshita H, Matsuda T, Asai A. Male hypogonadotropic hypogonadism (MHH) outpatient with testosterone deficiency syndrome: a case report. Hinyokika kiyo. 2009;55:107–9. [PubMed] [Google Scholar]
- Feng Y, Zhang Q, Dai DZ, Ying HJ, Dai Y. Strontium fructose 1,6-diphosphate rescues adenine-induced male hypogonadism and upregulates the testicular endothelin-1 system. Clin Exp Pharmacol Physiol. 2007;34:1131–7. doi: 10.1111/j.1440-1681.2007.04693.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Dai DZ, Zhang Q, Cheng YS, Dai Y. Upregulated NADPH oxidase contributes to diabetic testicular complication and is relieved by strontium fructose 1,6-diphosphate. Exp Clin Endocrinol Diabetes. 2010;118:459–65. doi: 10.1055/s-0030-1248325. [DOI] [PubMed] [Google Scholar]
- Tang XY, Zhang Q, Dai DZ, Ying HJ, Wang QJ, Dai Y. Effects of strontium fructose 1,6-diphosphate on expression of apoptosis-related genes and oxidative stress in testes of diabetic rats. Int J Urol. 2008;15:251–6. doi: 10.1111/j.1442-2042.2007.01980.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu HR, Ying HJ, Dai DZ, Tang XY, Dai Y. Strontium fructose 1,6-diphosphate alleviates early diabetic testopathy by suppressing abnormal testicular matrix metalloproteinase system in streptozocin-treated rats. J Pharm Pharmacol. 2009;61:229–36. doi: 10.1211/jpp/61.02.0013. [DOI] [PubMed] [Google Scholar]
- Hu C, Cong XD, Dai DZ, Zhang Y, Zhang GL, Dai Y. Argirein alleviates diabetic nephropathy through attenuating NADPH oxidase, Cx43, and PERK in renal tissue. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:309–19. doi: 10.1007/s00210-010-0593-7. [DOI] [PubMed] [Google Scholar]
- Peng HJ, Dai DZ, Ji H, Dai Y. The separate roles of endothelin receptors participate in remodeling of matrix metalloproteinase and connexin 43 of cardiac fibroblasts in maladaptive response to isoproterenol. Eur J Pharmacol. 2010;634:101–6. doi: 10.1016/j.ejphar.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Steiner M, Weipoltshammer K, Viehberger G, Meixner EM, Lunglmayr G, Schöfer C. Immunohistochemical expression analysis of Cx43, Cx26, c-KIT and PlAP in contralateral testis biopsies of patients with non-seminomatous testicular germ cell tumor. Histochem Cell Biol. 2011;135:73–81. doi: 10.1007/s00418-010-0769-8. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Cui B, Dai DZ, Su W. CPU 86017, p-chlorobenzyltetrahydroberberine chloride, attenuates monocrotaline-induced pulmonary hypertension by suppressing endothelin pathway. Acta Pharmacol Sin. 2005;26:1309–16. doi: 10.1111/j.1745-7254.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- Dai DZ, Hu HJ, Zhao J, Hao XM, Yang DM, Zhou PA, et al. Blockade of L-type calcium channel in myocardium and calcium-induced contractions of vascular smooth muscle by CPU 86017. Acta Pharmacol Sin. 2004;25:416–23. [PubMed] [Google Scholar]
- Dai DZ. CPU86017: a novel class III antiarrhythmic agent with multiple actions at ion channels. Cardiovasc Drug Rev. 2006;24:101–15. doi: 10.1111/j.1527-3466.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Du RH, Yi HW, Dai DZ, Tang WH, Dai Y. Inflammatory factors that contribute to upregulation of ERG and cardiac arrhythmias are suppressed by CPU86017, a class III antiarrhythmic agent. J Pharm Pharmacol. 2008;60:1089–95. doi: 10.1211/jpp.60.8.0015. [DOI] [PubMed] [Google Scholar]
- Li N, Yang L, Dai DZ, Wang QJ, Dai Y. Chiral separation of racemate CPU86017, an anti-arrhythmic agent, produces stereoisomers possessing favourable ion channel blockade and less alpha-adrenoceptor antagonism. Clin Exp Pharmacol Physiol. 2008;35:643–50. doi: 10.1111/j.1440-1681.2007.04854.x. [DOI] [PubMed] [Google Scholar]
- Na T, Huang ZJ, Dai DZ, Zhang Y, Dai Y. Abrupt changes in FKBP12.6 and SERCA2a expression contribute to sudden occurrence of ventricular fibrillation on reperfusion and are prevented by CPU86017. Acta Pharmacol Sin. 2007;28:773–82. doi: 10.1111/j.1745-7254.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Dehnert C, Luks AM, Menold E, Castell C, Schendler G, et al. High-altitude pulmonary hypertension is associated with a free radical-mediated reduction in pulmonary nitric oxide bioavailability. J Physiol. 2010;588:4837–47. doi: 10.1113/jphysiol.2010.194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen AK, Geiran O, Madsen S, Hognestad A, Simonsen S. Pulmonary arterial hypertension treated with prostacyclin or calcium blockers. Tidsskr Nor Laegeforen. 2003;123:3393–6. [PubMed] [Google Scholar]
- Rosati E, Butera G, Bossone E, De Felice C, Latini G. Inhaled nitric oxide and oral nifedipine in a preterm infant with bronchopulmonary dysplasia and pulmonary hypertension. Eur J Pediatr. 2007;166:737–8. doi: 10.1007/s00431-006-0292-z. [DOI] [PubMed] [Google Scholar]
- Maggiorini M. High altitude-induced pulmonary oedema. Cardiovasc Res. 2006;72:41–50. doi: 10.1016/j.cardiores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Richardson A, Watt P, Maxwell N. Hydration and the physiological responses to acute normobaric hypoxia. Wild Environ Med. 2009;20:212–20. doi: 10.1580/09-WEME-OR-272R1.1. [DOI] [PubMed] [Google Scholar]
- Hodkinson PD, Hunt BJ, Parmar K, Ernsting J. Is mild normobaric hypoxia a risk factor for venous thromboembolism. J Thromb Haemost. 2003;1:2131–3. doi: 10.1046/j.1538-7836.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Dai DZ, Dai Y. Testis dysfunction by isoproterenol is mediated by upregulating endothelin receptor A, leptin and protein kinase Cvarepsilon and is attenuated by an endothelin receptor antagonist CPU0213. Reprod Toxicol. 2010;29:421–6. doi: 10.1016/j.reprotox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl. 2005;26:25–31. [PubMed] [Google Scholar]
- Rangel PL, Rodriguez A, Rojas S, Sharp PJ, Gutierrez CG. Testosterone stimulates progesterone production and STAR, P450 cholesterol side-chain cleavage and LH receptor mRNAs expression in hen (Gallus domesticus) granulosa cells. Reproduction. 2009;138:961–9. doi: 10.1530/REP-09-0071. [DOI] [PubMed] [Google Scholar]
- Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–66. doi: 10.1095/biolreprod.108.070367. [DOI] [PubMed] [Google Scholar]
- Boni R, Gualtieri R, Talevi R, Tosti E. Calcium and other ion dynamics during gamete maturation and fertilization. Theriogenology. 2007;68:S156–64. doi: 10.1016/j.theriogenology.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ahn HJ, Lee SJ, Gye MC, Min CK. Effects of L- and T-type Ca2+ channel blockers on spermatogenesis and steroidogenesis in the prepubertal mouse testis. J Assist Reprod Genet. 2011;28:23–30. doi: 10.1007/s10815-010-9480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XY, Liu Q, Dai DZ, Dai Y. CPU0213, a novel endothelin receptor antagonist, suppresses the upregulation of matrix metalloproteinases and connexin 43 in hyperthyroid myocardium. Pharmacol Rep. 2008;60:524–31. [PubMed] [Google Scholar]
- Su W, Dai DZ, Liu HR, Na T, Dai Y. Upregulated endothelin system in diabetic vascular dysfunction and early retinopathy is reversed by CPU0213 and total triterpene acids from Fructus Corni. Clin Exp Pharmacol Physiol. 2007;34:1228–33. doi: 10.1111/j.1440-1681.2007.04698.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Fok KL, Yu S, Jiang J, Chen Z, Gui Y, et al. CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis. Mol Hum Reprod. 2011;17:405–14. doi: 10.1093/molehr/gar013. [DOI] [PubMed] [Google Scholar]
- Izzo G, Ferrara D, Delrio G, d'Istria M, Minucci S. Connexin43 expression in the testis of frog Rana esculenta: sex hormonal regulation. Ann NY Acad Sci. 2009;1163:425–7. doi: 10.1111/j.1749-6632.2008.03651.x. [DOI] [PubMed] [Google Scholar]
- Guan K, Wagner S, Unsold B, Maier LS, Kaiser D, Hemmerlein B, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–25. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Kurihara H, Watanabe M, Okada T, Sakai T, Amano A. Inhibition of connexin43 dephosphorylation is involved in protective effects of diltiazem on cardiac function during hypoxic injury. Histol Histopathol. 2011;26:315–22. doi: 10.14670/HH-26.315. [DOI] [PubMed] [Google Scholar]
- Herrid M, O'Shea T, McFarlane JR. Ontogeny of leptin and its receptor expression in mouse testis during the postnatal period. Mol Reprod Dev. 2008;75:874–80. doi: 10.1002/mrd.20796. [DOI] [PubMed] [Google Scholar]