Abstract
The paramyxovirus family includes many well-known human and animal pathogens as well as emerging viruses such as Hendra virus and Nipah virus. The V protein of simian virus 5 (SV5), a prototype of the paramyxoviruses, contains a cysteine-rich C-terminal domain which is conserved among all paramyxovirus V proteins. The V protein can block both interferon (IFN) signaling by causing degradation of STAT1 and IFN production by blocking IRF-3 nuclear import. Previously, it was reported that recombinant SV5 lacking the C terminus of the V protein (rSV5VΔC) induces a severe cytopathic effect (CPE) in tissue culture whereas wild-type (wt) SV5 infection does not induce CPE. In this study, the nature of the CPE and the mechanism of the induction of CPE were investigated. Through the use of DNA fragmentation, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling, and propidium iodide staining assays, it was shown that rSV5VΔC induced apoptosis. Expression of wt V protein prevented apoptosis induced by rSV5VΔC, suggesting that the V protein has an antiapoptotic function. Interestingly, rSV5VΔC induced apoptosis in U3A cells (a STAT1-deficient cell line) and in the presence of neutralizing antibody against IFN, suggesting that the induction of apoptosis by rSV5VΔC was independent of IFN and IFN-signaling pathways. Apoptosis induced by rSV5VΔC was blocked by a general caspase inhibitor, Z-VAD-FMK, but not by specific inhibitors against caspases 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 13, suggesting that rSV5VΔC-induced apoptosis can occur in a caspase 12-dependent manner. Endoplasmic reticulum stress can lead to activation of caspase 12; compared to the results seen with mock and wt SV5 infection, rSV5VΔC infection induced ER stress, as demonstrated by increased expression levels of known ER stress indicators GRP 78, GRP 94, and GADD153. These data suggest that rSV5VΔC can trigger cell death by inducing ER stress.
Apoptosis, or programmed cell death, is the physiological process by which unwanted cells undergo morphological changes, protease activation, chromosomal DNA fragmentation, and (eventually) cell death. This process is important for normal development, tissue homeostasis, and immune modulation as well as for host defense against viral infection (12). Apoptosis can be initiated and executed through many different pathways, which can be categorized into two main groups: extrinsic and intrinsic pathways (2). Extrinsic pathways sense death signals from outside the cells and consist of caspases, death receptors (DR), and adapter proteins. The caspases (cysteine aspartate-specific proteases) are critical players in regulation of different apoptotic pathways (8, 54). There are 14 known caspases that can be roughly divided into initiator and effector caspases. Initiator caspases are involved in upstream regulatory events resulting in activation of effector caspases that are directly responsible for proteolytic cleavages leading to cell death. Known initiator caspases include caspase 8 and 9; known effector caspases include caspase 3, 6, and 7. Some caspases (such as caspase 2) can be both initiator and effector caspases (54). Effector caspases, the executioners of apoptosis, can be activated by death stimuli through activation of DR, normally a member of the tumor necrosis factor (TNF) receptor superfamily. Upon activation, DR associate with adapter proteins such as the TNF receptor-associated death domain through their death domains. This complex can activate initiator caspases (which in turn can activate effector caspases to trigger cell death) (49). Intrinsic pathways sense death signals such as stress from inside the cells and act mainly through mitochondria. Most intrinsic apoptotic pathways involve the Bcl-2 protein family, mitochondrion-released proteins, and caspases. In intrinsic pathways, death stimuli are sensed by the Bcl-2 protein family and cause damage to mitochondria resulting in release of cytochrome c to the cytosol. The released cytochrome c activates Apaf1 (apoptotic protease-activating factor 1), which activates caspase 9 to trigger cell death. However, knockout of the gene for cytochrome c, Apaf1, or caspase 9 gene does not prevent the cell from undergoing stress-induced apoptosis in some cells, suggesting there might be additional intrinsic apoptotic pathways (reviewed in reference 7). It is known that damage to mitochondria can also cause release of apoptosis-inducing factor, which triggers apoptosis in a caspase-independent manner (5).
SV5 is a member of the Rubulavirus genus of the family Paramyxoviridae. The Paramyxoviridae family includes many well-known human and animal pathogens, such as mumps virus, Newcastle disease virus (NDV), measles virus, and respiratory syncytial (RS) virus, as well as important emerging viruses such as Hendra virus and Nipah virus (26). The negative-stranded RNA genome of SV5 is 15,246 nucleotides long and encodes eight known viral proteins (26). The nucleocapsid protein (NP), phosphoprotein (P), and large RNA polymerase (L) protein are important for transcription and replication of the viral RNA genome. The fusion (F) protein, a glycoprotein, mediates virus entry into cells by virus-cell fusion and causes syncytial formation. The hemagglutinin-neuraminidase protein (HN), a viral glycoprotein, mediates virus-cell attachment and also cleaves sialic acid from complex carbohydrate chain of glycoprotein (necessary for virus release). The matrix (M) protein plays an important role in virus assembly (45, 46). The small hydrophobic (SH) protein is a 44-residue hydrophobic integral membrane protein and is oriented in membranes with its N terminus in the cytoplasm (18). Recombinant SV5 (rSV5) lacking the SH gene (rSV5ΔSH) induces apoptosis in L929 cells through a TNF alpha (TNF-α)-mediated extrinsic apoptotic pathway (14, 15, 30).
The V/P gene of SV5 is transcribed into both the V mRNA and the P mRNA through a process (commonly called “RNA editing”) of pseudo-templated addition of nucleotides (53). The V mRNA is transcribed when the viral RNA polymerase faithfully transcribes the V/P gene. On some occasion during transcription, however, the viral RNA polymerase complex recognizes a specific RNA sequence in the V/P gene and inserts two non-template G residues at the editing site to generate the P mRNA. As a result, the V/P gene is transcribed into two mRNAs that accumulate at about the same abundance level and the two mRNAs are translated into two proteins, V and P, which share identical N termini but have different C termini. The RNA editing at a specific site occurs for almost all members of the subfamily Paramyxoviridae (26). The V mRNA is faithfully transcribed from the genome RNA only for rubulaviruses, however, whereas for the respiroviruses and the morbilliviruses the P mRNA is faithfully transcribed from the genome RNA and the V mRNA is the result of the presence of the additional pseudo-templated G nucleotide(s) (21). The sequences of the C-terminal domain of the V proteins are highly conserved among the paramyxoviruses (56).
The V protein of the paramyxovirus simian virus 5 (SV5) is a multifunctional protein containing an N-terminal 164-residue domain that is shared with the P protein and a distinct C-terminal domain that is cysteine rich. The V protein C-terminal domain contains seven cysteine residues (resembling a zinc finger domain) and binds atomic zinc (31, 40, 48, 53). The V protein of SV5 interacts with soluble nucleocapsid protein (41), and the N-terminal domain of V binds RNA through a basic region (29). The SV5 V protein interacts with a cellular protein, the 127-kDa subunit of the damage-specific DNA-binding protein (DDB1) that is known to be involved in damaged DNA repair. The interaction of the V protein with DDB1 requires the presence of the C-terminal domain of V protein (28). Expression of the SV5 V protein slows down the cell cycle; this effect on the cell cycle is mediated via the V protein C-terminal domain (28). Coexpression of DDB1 can partially restore the changes in cell cycle caused by V (28). The V protein of SV5 also causes degradation of STAT1 protein, an essential regulator of interferon (IFN) signaling, through a proteasome-mediated pathway in human cells but not in mouse cells (10). STAT2 also plays a role in V's ability to cause degradation of STAT1 (37). It seems likely that the degradation of STAT1 in SV5-infected cells and the alterations to the cell cycle are interrelated, as the V, DDB1, Cul4A, STAT1, and STAT2 proteins form a complex which is essential for V-mediated STAT1 degradation (1, 58). The analysis of properties of an rSV5 that lacks the V protein C-terminal-specific domain (rSV5VΔC) shows that the C terminus of the V protein plays an essential role in causing degradation of STAT1 and preventing IFN-β production. Whereas rSV5 grows in many cell types with minimal cytopathic effect (CPE), rSV5VΔC causes extensive CPE in the same cell types. In this study, the nature of the CPE and the mechanism of rSV5VΔC-induced CPE were investigated.
MATERIALS AND METHODS
Viruses and cells.
Generation of rSV5VΔC was described previously. rSV5VΔC were grown in Vero cells and harvested 5 to 7 days postinfection (dpi) as described previously (16, 17, 39). Wild-type (wt) SV5 was grown in MDBK cells. Virus titers were determined by plaque assays using BHK 21F and Vero cells (39). To infect cells, monolayers were washed with phosphate-buffered saline (PBS) and then inoculated with viruses in Dulbecco's modified Eagle's medium (DMEM)-1% bovine serum albumin (BSA) at a multiplicity of infection (MOI) of 5 for 1 to 2 h at 37°C. The monolayers were washed and incubated with DMEM containing 2% fetal calf serum (FCS) at 37°C with 5% CO2. HeLa cells, U3A cells, and Vero cells were maintained in DMEM-10% FCS. BHK 21F cells were maintained in DMEM-10% tryptose phosphate broth-10% FCS. Virus-infected cells were grown in DMEM-2% FCS.
Transfections were carried using Lipofectamine-PLUS (Invitrogen, Carlsbad, Calif.) following the manufacturer's instructions. HeLa cells in 6-cm-diameter plates at about 70% confluency were washed once with Opti-MEM medium and incubated in 2 ml of Opti-MEM. A total of 2 μg of plasmid DNA (vector or pBH361 [which encodes V]) was mixed with 0.25 ml of Opti-MEM and 8 μl of PLUS for 15 min at room temperature. Opti-MEM (0.25 ml) with 12 μl of Lipofectamine was added to the DNA-PLUS mixture and incubated for 15 min at room temperature. The mixture of DNA, PLUS, and Lipofectamine was added to the cells and incubated for 16 h. The transfected cells were infected with rSV5VΔC. The transfected-infected cells were analyzed for V expression and apoptosis by flow cytometry (described below).
DNA fragmentation assay, propidium iodide staining, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, and trypan blue staining.
Fragmented DNAs were purified as described in reference 64. Confluent HeLa cells in 6-cm-diameter plates were mock infected or infected with SV5 or rSV5VΔC at an MOI of 5. At 24 and 48 h postinfection (hpi), similar numbers of HeLa cells in 60-mm-diameter dishes were washed twice with PBS (−) and then incubated in 0.5 ml of TTE buffer (0.2% Triton X-100, 10 mM Tris, 15 mM EDTA, pH 8.0) at room temperature for 15 min. Cell lysates were harvested into microtubes and subjected to centrifugation at 20,000 × g for 20 min. Supernatants were digested with 100 μg of RNase A/ml at 37°C for 1 h. Samples were purified with phenol-chloroform extraction, precipitated, and then washed with 70% ethanol. Pellets were air dried and redissolved in 10 μl of Tris-EDTA buffer each. Electrophoresis was performed on 2% agarose gels with DNA size markers.
For propidium iodide (PI) staining, confluent HeLa cells in 6-cm-diameter plates were mock infected or infected with SV5 or rSV5VΔC at an MOI of 5. Cells in monolayers were trypsinized and combined with the floating cells in the medium at different time points. The harvested cells were then centrifuged at 250 × g for 8 min at 4°C and washed with PBS between each subsequent pair of steps. The cells were fixed with 0.25% formaldehyde for 2 h at 4°C. The fixed cells were resuspended in 0.5 ml of 50% DMEM-50% FCS and permeabilized by adding 1.5 ml of 70% ethanol at 4°C for at least 2 h and for up to 3 days. To monitor expression of viral proteins, the permeabilized cells were incubated with 0.5 ml of anti-V monoclonal antibody 11C6 (40) at 1:500 in PBS-1% BSA at 4°C for 1 h and then with 0.5 ml of fluorescein isothiocyanate-labeled anti-mouse secondary antibody (Organon-Teknika Corp., Charlotte, N.C.) at 1:1,000 in PBS-1% BSA for 1 h at 4°C. For PI staining, the cells were incubated with 500 μl of 50 μg of propidium iodide (Sigma-Aldrich)/ml for 1 h at 4°C. The cells were then analyzed using a flow cytometer (EPICS XL; Beckman-Coulter). Single cells were selected on FL2-W (cell width) versus FL2-A (DNA content) plots. Infected cells were selected on FL2-A (DNA content) versus FL1-H (V expression) plots.
For TUNEL assays, the cells were fixed and permeabilized as described above. The cells were then incubated with 25 μl of TUNEL reaction mixture (in situ fluorescein cell death detection kit; Boehringer-Mannheim) for 2 to 3 h in the dark at 37°C. The cells were analyzed by flow cytometry.
To examine cell viability, the adherent cells were washed with PBS− and trypsinized and were combined with the cells in the medium to obtain the entire cell population. The cells were centrifuged 960 × g with a tabletop centrifuge and resuspended in 0.08% trypan blue solution in DMEM. The cells were placed on a hemacytometer and examined under a microscope.
UV inactivation of virus.
Confluent HeLa cells in 60-mm-diameter plates were mock infected or infected with SV5 or rSV5VΔC at an MOI of 5. The infected cells were incubated in 5 ml of 2% FCS-DMEM for 2 days. Medium from infected cells was placed inside a Fisher Hamilton class II biological safety cabinet and UV treated for 30 min. The media were then filtered through a 0.22-μm-pore-size filter to remove cell debris. The effectiveness of the UV treatment with respect to inactivation of SV5 was confirmed by plaque assays.
Antibody treatment of infected cells.
Confluent HeLa cells in 6-well plates were mock infected or infected with SV5 or rSV5VΔC at an MOI of 5 as described before and incubated in 1 ml of DMEM-2% FCS with neutralizing antibody against IFN-β (CalBiochem, San Diego, Calif.) at 105 kU/ml. At 2 dpi, the cells were collected, stained with trypan blue, and counted.
Confluent L929 cells in 6-well plates were mock infected or infected with SV5, rSV5ΔSH, or rSV5VΔC at an MOI of 5 as described before and incubated in 1 ml of DMEM-2% FCS with neutralizing antibody against TNF-α (BD Pharmingen, San Diego, Calif.) at 20 μg/ml. At 2 dpi, the cells were photographed using a light microscope equipped with a digital camera.
Caspase assays.
For caspase 3 and 7 assays, triplicates of infected cells (∼2 × 106 to 10 × 106 cells/ml) were lysed with cell lysis buffer containing 10 mM Tris-HCl, 10 mM NaH2PO4/NaHPO4 (pH 7.5), 130 mM NaCl, 1% Triton X-100, and 10 mM NaPPi (sodium pyrophosphate). Protein concentrations of lysates were determined using a BCA protein assay kit (Pierce, Rockford, Ill.). For each reaction in a 96-well microtiter plate, 30 μg of cell lysate was added to 200 μl of reaction buffer (20 mM HEPES [pH 7.5], 20% glycerol, 4 mM dithiothreitol) and 20 μM caspase 3 substrate Ac-DEVD-AMC (BD Biosciences Pharmingen). For caspase 2 assays, assay buffer containing 100 mM HEPES (pH 7.5), 10 mM dithiothreitol, and 100 μM caspase 2 substrate Ac-LDESD-AMC (CalBiochem) were used. Samples in the plates were mixed for 30 s and incubated for 1 h at 37°C. The plates were then read on a microtiter plate reader with an excitation wavelength of 380 nm and an emission wavelength of 430 to 460 nm. Similarly, subsequent caspase assays were carried out using 20 μM Ac-YVAD-AMC substrate (BD Bioscience) for caspase 1, 50 μM Ac-LEVD-AMC substrate (A.G. Scientific Inc., San Diego, Calif.) for caspase 4, 50 μM Ac-LEHD-AMC substrate (A.G. Scientific Inc.) for caspases 5, 9, 10, and 11, 50 μM Ac-VEID-AMC substrate (A. G. Scientific Inc.) for caspase 6 assay, 20 μM Ac-LETD-AMC substrate (BD Bioscience) for caspase 8, and 100 μM Ac-LEED-AFC substrate (Enzymesys, Aurora, Ohio) for caspase 13.
Treatment of cells with caspase inhibitors.
HeLa cells in 24-well plates were mock infected or infected with SV5 or rSV5VΔC and incubated with DMEM-2% FCS containing dimethyl sulfoxide (DMSO) or 40 μM inhibitors dissolved in DMSO. The cells were photographed at 2 dpi and counted after trypan blue staining. General caspase inhibitor Z-VAD-FMK, caspase 1 and 4 inhibitor Z YVAD-FMK, caspase 2 inhibitor Z-VAVAD-FMK, caspase 5, 9, 10, and 11 inhibitor Z-LEHD-FMK, caspase 8 inhibitor Z-LETD-FMK, and caspase 13 inhibitor Z-LEED-FMK were from Enzymesys. Caspase 3, 6, and 7 inhibitor Z-DEVD-FMK was from CalBiochem.
Immunoblotting.
HeLa cells in 6-cm-diameter plates were mock infected or infected with rSV5VΔC or rSV5. At 1 or 2 dpi, cells were lysed in 0.5 ml of protein lysis buffer (2% sodium dodecyl sulfate, 62.5 mM Tris-HCl [pH 6.8], 2% dithiothreitol) and sonicated briefly to shear DNA. Up to 100 μl of the lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 15% gel. Polypeptides were transferred (using a wet-gel transfer apparatus) to a polyvinylidene difluoride membrane. The membrane was first blocked with 5% dry fat-free milk and then incubated with primary antibodies against GRP 78 at 1: 200 dilution (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), GRP 94 at 1 μg/ml (CalBiochem), GADD153 at 1:200 dilution (Santa Cruz Biotechnology), or actin (Santa Cruz Biotechnology). Two caspase 12 antibodies were used. One (which recognizes residues 95 to 318 of mouse caspase 12) (Sigma) was used at 1:500; the other (which recognizes residues 100 to 116 of mouse caspase 12) (Oncogene) was used at 1:500. A mixture of anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase was used to detect primary antibodies. The proteins on the membrane were detected using an ECL+ kit (Amersham Pharmacia, Piscataway, N.J.), and chemiluminescence was detected using a Storm System PhosphorImager (Molecular Dynamics Inc, Sunnyvale, Calif.).
RESULTS
Induction of apoptosis by rSV5VΔC infection.
Previously, we observed that rSV5VΔC infection of several cell types (e.g., HeLa cells, CV1 cells, Vero cells, and L929 cells) caused a severe CPE by 30 hpi. Whereas rSV5 infection of HeLa cells produced small syncytial foci from 24 hpi onwards, rSV5VΔC-infected cells at times >30 hpi were detaching from the monolayer. Although CPE induced by rSV5VΔC in Vero and HeLa cells was evident by 30 hpi, protein synthesis did not appear to be affected at this time and neither was virus particle production (16) (excluding expression of viral proteins or production of virions as possible causes for increased CPE in rSV5VΔC-infected cells).
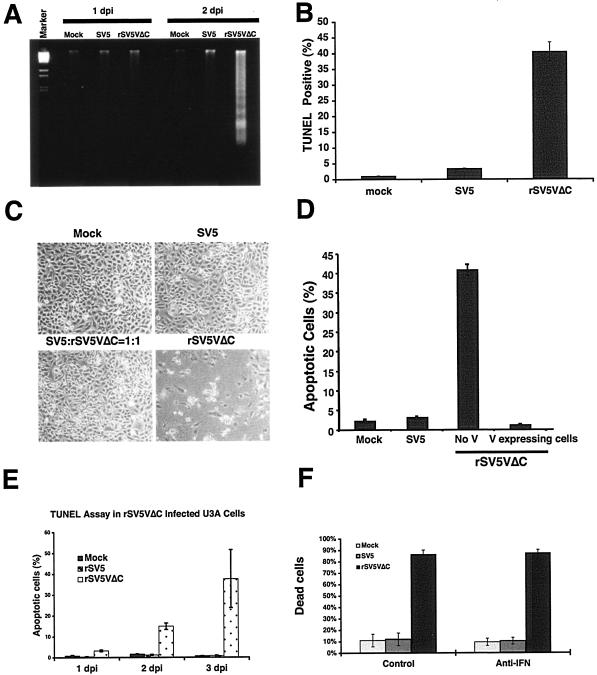
To examine whether the CPE was due to induction of apoptosis, the presence of fragmented chromosomal DNA in rSV5VΔC-infected cells was examined. HeLa cell were mock infected or infected with SV5 or rSV5VΔC viruses. DNAs were purified and resolved in agarose gel (Fig. 1A). Increasing amounts of fragmented DNA was detected in rSV5VΔC-infected cells in comparison with that found in mock- or SV5-infected cells, suggesting that rSV5VΔC induced apoptosis in cells (Fig. 1A). To confirm that the CPE was due to induction of apoptosis in the rSV5VΔC-infected cells and to quantify the number of apoptotic cells, the TUNEL assay (which directly measures the presence of nicked chromosomal DNA, a hallmark of apoptotic cells) was employed. The percentages of TUNEL-positive cells, i.e., apoptotic cells, were quantified using a flow cytometer as described previously (15). As shown in Fig. 1B, at 2 dpi about 45% of cells infected by rSV5VΔC were TUNEL positive, indicating that the cells were apoptotic.
FIG. 1.
Induction of apoptosis by rSV5VΔC. HeLa cells were infected with SV5 or rSV5VΔC or mock infected and were subjected to apoptosis assays. (A) DNA fragmentation. The infected cells were collected at 1 and 2 dpi, and DNAs were purified and resolved in agarose gel as described in Materials and Methods. The DNA marker was λ DNA digested with BstEII enzyme. (B) TUNEL assay. The infected cells were collected at 2 dpi and subjected to TUNEL assays as described in Materials and Methods. (C) Inhibition of rSV5VΔC induced CPE by SV5 coinfection. HeLa cells were infected with SV5, rSV5VΔC, or SV5 plus rSV5VΔC at an MOI of 5 or were mock infected. The cells were photographed at 2 dpi. (D) Inhibition of rSV5VΔC induced apoptosis by V. HeLa cells were transfected with plasmid encoding the V protein and then infected with rSV5VΔC at an MOI of 5. The cells were collected at 2 dpi, labeled with anti-V-specific antibody and stained with PI. rSV5VΔC-infected cells expressing V were gated and analyzed using a flow cytometer. (E) Increased levels of apoptotic cells in rSV5VΔC-infected U3A cell populations. Mock-, SV5-, or rSV5VΔC-infected U3A cells were collected at 1, 2, and 3 dpi and examined using TUNEL assays as described before (15). (F) Treatment of infected cells with anti-IFN-β. The cells were infected and incubated in the presence of anti-IFN-β (105 kU/ml) for 2 days. The cells were then collected and counted after being stained with trypan blue as described in Materials and Methods.
Deletion of the C-terminal domain of the V protein caused increased CPE and apoptosis in virus-infected HeLa cells, indicating that full-length V is required to prevent apoptosis in virus-infected cells. It is most likely that the phenotype of increased apoptosis was a result of loss of function of the C-terminal domain. However, it is possible that the N-terminal domain had acquired a novel function even though the half-life of the N-terminal domain is relative short (less than 45 min). To test whether intact V protein can inhibit apoptosis induced by rSV5VΔC, SV5 V protein was expressed in rSV5VΔC-infected cells by coinfecting cells with wt SV5 or by transfecting plasmid DNA expressing the V protein. Coinfection of rSV5VΔC with wt SV5 did not result in CPE (as shown in Fig. 1C), suggesting that V from a wt SV5 infection was able to block CPE induced by rSV5VΔC infection and that the truncated form of the V protein was unlikely to be the reason for the increased apoptosis observed in rSV5VΔC-infected cells. The ability of rSV5VΔC to induce apoptosis in HeLa cells was further confirmed by PI staining, which detects cellular DNA content. DNA fragments due to apoptosis are lost during PI staining (in which lipid membranes of cells are extracted). As a result, the DNA content profile of apoptotic cells is the same as that of sub G0-G1 cells. Increased populations of sub-G0 and -G1 cells, i.e., apoptotic cells, were detected only in rSV5VΔC-infected cells at 2 dpi (Fig. 1D).
Furthermore, HeLa cells were transfected with a plasmid encoding V protein and then infected with rSV5VΔC. V-expressing cells were gated using a flow cytometer. The DNA content of the V-expressing cells was compared (using PI staining) with that of the rSV5VΔC-infected cells that did not express V in trans. In V-expressing cells, rSV5VΔC infection did not induce apoptosis (Fig. 1D). Thus, V expressed alone can inhibit the apoptosis induced by rSV5VΔC.
Activation of intrinsic apoptotic pathway by rSV5VΔC infection.
The major apoptotic pathways can be categorized into intrinsic and extrinsic pathways. IFN is thought to be an essential mediator of apoptosis in virus-infected cells, and increased expression of IFN-β was detected in the media of rSV5VΔC-infected cells (16). Thus, apoptosis induced by rSV5VΔC infection could be caused by IFN via an extrinsic apoptotic pathway. To test this notion, U3A cells whose IFN signaling pathways are impaired due to STAT1 protein deficiency (47) were mock infected or infected with SV5 or rSV5VΔC and apoptotic cell populations were examined using TUNEL assays. rSV5VΔC induced apoptosis in U3A cells whereas wt SV5 did not induce apoptosis (Fig. 1E), suggesting that the induction of apoptosis by rSV5VΔC does not require IFN signaling. Furthermore, neutralizing antibody against IFN-β did not block rSV5VΔC-induced apoptosis (Fig. 1F), suggesting that the apoptosis induced by rSV5VΔC did not require IFN signaling. This is consistent with the observation that rSV5VΔC induced cell death in Vero cells, a cell line defective in IFN production.
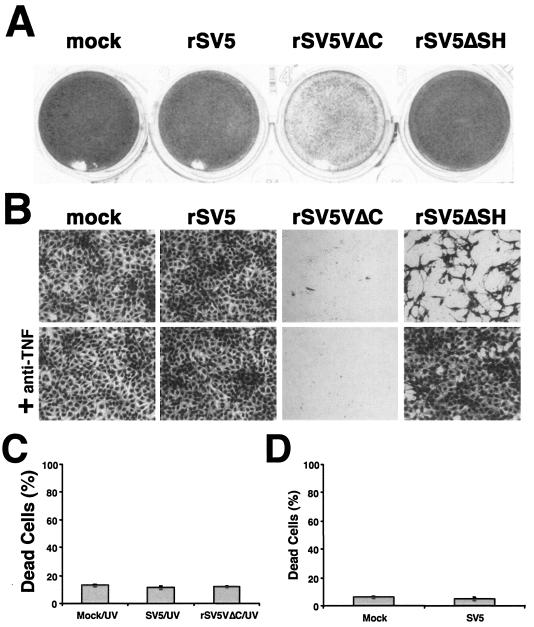
The rSV5 lacking the SH gene grows as well as wt SV5, but this virus causes increased apoptosis in MDBK cells (a bovine cell line) and L929 cells (a mouse cell line) but not in human cell lines (such as HeLa and A549 cells) while growing as well as wt virus (15) (Fig. 2A). One possible explanation is that other SV5-encoded proteins also inhibit apoptotic pathways activated by SV5 infection in different cell types. Because rSV5VΔC caused CPE and apoptosis in HeLa cells, the V protein may block apoptosis in HeLa cells. SH and V could inhibit the same apoptotic pathway or different apoptotic pathways in different cell types. To investigate whether the V protein and the SH protein block the same apoptotic pathway, we compared apoptotic pathways activated by rSV5VΔC and rSV5ΔSH in L929 cells in which both viruses induced apoptosis (data not shown). rSV5ΔSH infection induces expression of TNF-α, and TNF-α is responsible for the induction of apoptosis (30). Treating infected L929 cells with neutralizing antibody against TNF-α blocked apoptosis induced by rSV5ΔSH but not that induced by rSV5VΔC (Fig. 2B), suggesting that rSV5VΔC induced apoptosis through a pathway different from that of rSV5ΔSH.
FIG. 2.
Induction of apoptosis by rSV5VΔC and rSV5ΔSH occurs by different mechanisms. (A) rSV5VΔC but not rSV5ΔSH induces cell death in HeLa cells. HeLa cells were mock infected or infected with SV5, rSV5VΔC, or rSV5ΔSH at an MOI of 5 and were stained with Hema 3 at 2 dpi. (B) rSV5VΔC induced apoptosis in a TNF-independent manner. L929 cells were mock infected or infected with SV5, rSV5VΔC, or rSV5ΔSH at an MOI of 5 and were treated with neutralizing antibody against TNF-α (20 μg/ml). The cells were stained at 2 dpi and photographed. (C) Incubation of HeLa cells with conditioned medium from rSV5VΔC-infected cells. Medium from mock-, SV5-, or rSV5VΔC-infected cells was collected at 2 dpi and then UV irradiated to kill virus. (D) Mock- or SV5-infected cells were inoculated with the conditioned medium from rSV5VΔC-infected cells. The percentages of dead cells were counted using trypan blue staining as described in Materials and Methods. Errors were standard deviations of means.
To examine whether soluble death factors of extrinsic apoptotic pathways in the media of rSV5VΔC-infected cells played a role in rSV5VΔC-induced apoptosis, media from mock-, SV5-, or rSV5VΔC-infected cells were collected at 2 dpi and UV irradiated. The conditioned media were applied to fresh HeLa cells. There was no increase of CPE in cells incubated with the conditioned media from rSV5VΔC-infected cells compared to the results seen with conditioned media from mock- or SV5-infected cells, suggesting that soluble death factors in the conditioned media alone were not sufficient to induce cell death (Fig. 2C). Furthermore, the conditioned media from the rSV5VΔC-infected cells were used to treat SV5-infected cells and there was no increase of CPE compared to the results seen with normal SV5 infection, suggesting that the conditioned media did not sensitize the infected cells for apoptosis induction (Fig. 2D).
Activation of caspases in rSV5VΔC-infected cells.
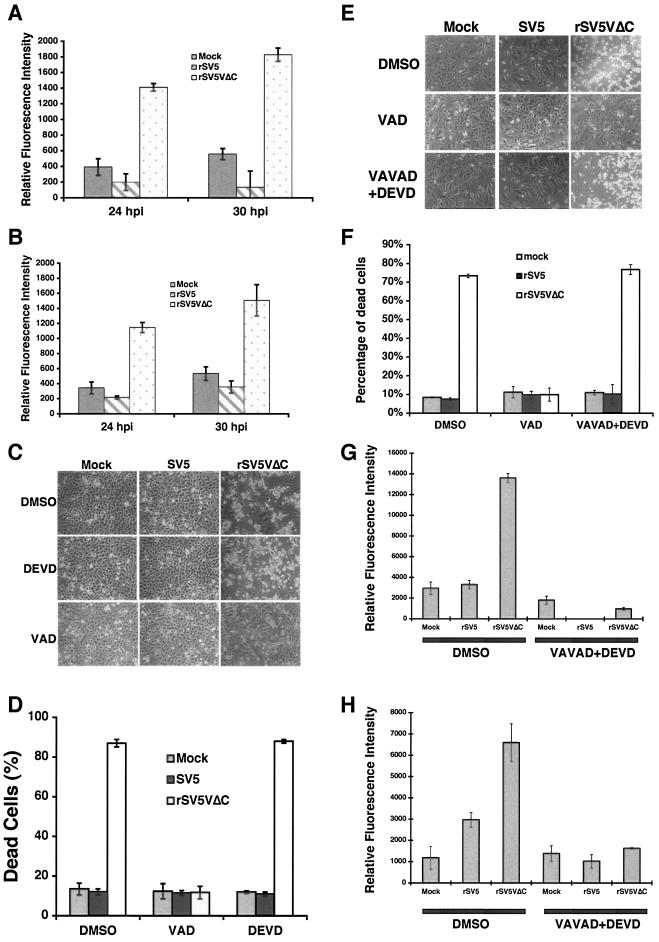
Caspases are key players in the regulation of apoptotic pathways. Different apoptotic pathways activate different caspases. An understanding of which caspase is activated provides information about which apoptotic pathway is activated in apoptotic cells. The activity of caspase 3, a major effector caspase, in mock-, SV5-, or rSV5VΔC-infected cells was examined. Increased caspase 3 activity was detected in rSV5VΔC-infected HeLa and U3A cells but not in mock- or SV5-infected HeLa or U3A cells, suggesting that caspase 3 might play a role in apoptosis induced by rSV5VΔC (Fig. 3A and B). Furthermore, activation of caspase 3 was confirmed by immunoblotting using anti-caspase 3 antibody (data not shown). DEVD, a specific inhibitor against caspase 3, did not block apoptosis induced by rSV5VΔC (Fig. 3C), however, suggesting that caspase 3 is not an essential regulator for rSV5VΔC-induced apoptosis. Interestingly, a general caspase inhibitor was able to block apoptosis induced by rSV5VΔC, suggesting that a caspase or caspases other than caspase 3 are responsible for rSV5VΔC-induced apoptosis.
FIG. 3.
Caspase activities in rSV5VΔC-infected cells. HeLa or U3A cells were mock infected or infected with SV5 or rSV5VΔC at an MOI of 5. The cells were collected at the times indicated, and caspase assays were performed as described in reference 30. (A) HeLa cells; (B) U3A cells. (C and D) Inhibition of CPE by caspase inhibitors. Mock-, SV5-, or rSV5VΔC-infected HeLa cells were treated with DMSO (the solvent for caspase inhibitor), DEVD (a caspase 3 inhibitor) (100 μM), or VAD (a general caspase inhibitor) (50 μM) and photographed at 2 dpi. (E) Combinations of caspase 2 and caspase 3 inhibitors did not block rSV5VΔC-induced cell death. Mock-, SV5-, or rSV5ΔC-infected HeLa cells were treated with DMSO, VAD (at 50 μM), or VAVAD (caspase 2 inhibitor at 80 μM) plus DEVD (caspase 3 inhibitor at 80 μM). Pictures were taken at 1 dpi. (F) The cells shown in panel E were stained with trypan blue and counted. (G) The effectiveness of caspase 2 inhibitor. The caspase 2 activities of the cells shown in panel E were measured using activity assays as described in Materials and Methods. (H) Caspase 3 activities of the cells shown in panel E.
To identify the caspase that is activated in rSV5VΔC-infected HeLa cells, caspase activity assays were carried out; the results are shown in Table 1. There are 14 known caspases, and activity assays were carried out on 12 caspases. Caspase 14, a keratinocyte-specific caspase, is not expressed in differentiated adult cells (19), and there is no available activity assay for caspase 12. While increased caspase 2 and caspase 3 activities were detected in rSV5VΔC-infected cells compared to the results seen with mock- or SV5-infected cells, neither individual inhibitor against the caspases nor the combination of caspase 2 and 3 inhibitors inhibited rSV5VΔC-induced apoptosis (Fig. 3E and F) (Table 2), suggesting that caspases 2 and 3 were not responsible for rSV5VΔC-induced apoptosis. Activities of caspase 1, 4, 5, 6, 7, 8, 9, 10, 11, and 13 were not detected in the infected cells, although it is possible the assays were not sufficiently sensitive. To examine the involvement of these caspases in rSV5VΔC-induced apoptosis, specific inhibitors against these caspases were applied to the infected cells; the results are in shown in Table 2. None of the specific caspase inhibitors blocked CPE caused by rSV5VΔC infection. The levels of activity of caspase 2 and 3 inhibitors were measured, and the inhibitors were found to be effective (Fig. 3G and H).
TABLE 1.
Caspase activities of mock-, SV5-, and rSV5VΔC-infected cellsa
| Caspase | Target sequence | Tested substrate | Result with indicated infection
|
||
|---|---|---|---|---|---|
| Mock | rSV5 | rSV5VΔC | |||
| 1 | YVAD | Ac-YVAD-AMC | − | − | − |
| 2 | DESD | Ac-LDESD-AMC | − | − | + |
| 3 | DEVD | Ac-DEVD-AMC | − | − | + |
| 4 | LEVD | Ac-LEVD-AMC | − | − | − |
| 5 | LEHD | Ac-LEHD-AMC | − | − | − |
| 6 | VEID | Ac-VEID-AMC | − | − | − |
| 7 | DEVD | Ac-DEVD-AMC | − | − | − |
| 8 | LETD | Ac-LETD-AMC | − | − | − |
| 9 | LEHD | Ac-LEHD-AMC | − | − | − |
| 10 | LEHD | Ac-LEHD-AMC | − | − | − |
| 11 | LEHD | Ac-LEHD-AMC | − | − | − |
| 12 | NAb | NA | NA | NA | NA |
| 13 | LEED | Ac-LEED-AFC | − | − | − |
The caspase enzymatic activity assay was carried out as described in Materials and Methods.
NA, not available.
TABLE 2.
Effects of caspase inhibitors on the virus-infected cells
| Inhibitor(s)a | Targeted caspase(s) | Result with indicated infectionb
|
||
|---|---|---|---|---|
| Mock | rSV5 | rSV5VΔC | ||
| DMSO | None | − | − | + |
| Z-VAD-FMK | All caspases | − | − | − |
| Z-DEVD-FMK | Caspases 3, 6, 7 | − | − | + |
| Z-VAVAD-FMK | Caspase 2 | − | − | + |
| Z-LEHD-FMK | Caspases 5, 9, 10, 11 | − | − | + |
| Z-YVAD-FMK | Caspase 1, 4 | − | − | + |
| Z-LETD-FMK | Caspase 8 | − | − | + |
| Z-VDVAD-FMK | Caspase 2 | − | − | + |
| Z-LEED-FMK | Caspase 13 | − | − | + |
| Z-VAVAD-FMK + Z-DEVD-FMK | Caspases 2, 3, 6, 7 | − | − | + |
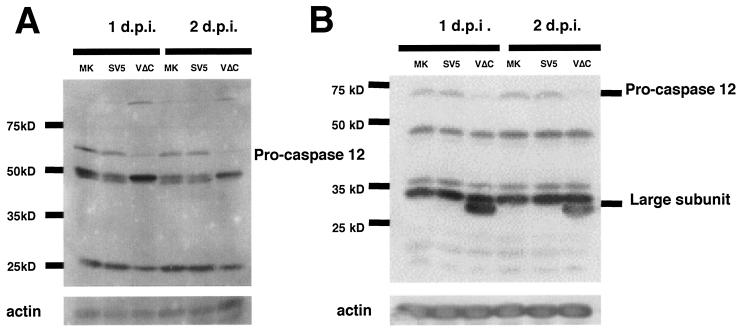
Due to the lack of an enzymatic activity assay for caspase 12, the activation of caspase 12 was examined by immunoblotting using antibodies specific for caspase 12. The inactive form of caspase 12 (pro-caspase 12) is ∼60 kDa and is activated by cleavage of its N-terminal prodomain. The active form of caspase 12 is ∼50 kDa, and it can be further cleaved into a small subunit and a ∼35-kDa subunit (42). When two different antibodies against pro-caspase 12 were used, the inactive form of caspase 12 was not detected in rSV5VΔC-infected cells whereas proteins of the expected mass of pro-caspase 12 were detected in mock or SV5-infected cells, suggesting that caspase 12 was activated in rSV5VΔC-infected cells (Fig. 4A). A polypeptide species of ∼35 kDa, corresponding to the mass of the large subunit of active form of caspase 12, was observed in rSV5VΔC-infected cells (Fig. 4B).
FIG. 4.
Activation of caspase 12 in rSV5VΔC-infected cells. HeLa cells were mock (MK) infected or infected with SV5 or rSV5VΔC. The infected cells were collected at 1 and 2 dpi and subjected to immunoblotting as described in Materials and Methods. (A) Antibody specific for pro-caspase 12 from Sigma (clone 14F7; catalog no. C 7611). (B) Antibody specific for pro-caspase 12 from Oncogene (catalog no. PC557T). Nonspecific proteins were also detected in mock-, SV5-, or rSV5VΔC-infected cells by the antibodies.
ER stress in rSV5VΔC-infected cells.
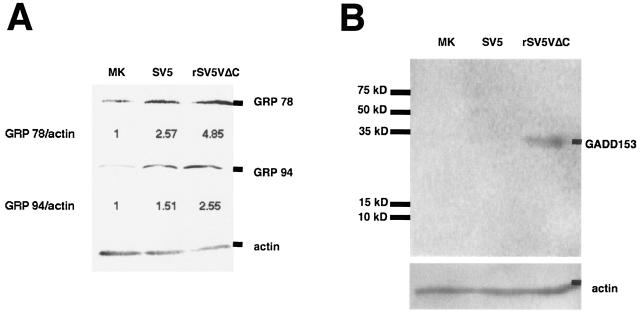
Activation of caspase 12 often associates with increased endoplasmic reticulum (ER) stress (35, 42). To examine ER stress of virus-infected cells, expression levels of major ER stress indicators such as GRP 78, GRP 94, and GADD153 (CHOP) were measured using immunoblotting (20, 32, 59). Whereas expression levels of GRP 78 and GRP 94 increased moderately in SV5-infected cells compared with the results seen with mock-infected cells, expression levels of these proteins were increased dramatically in rSV5VΔC-infected cells, suggesting that rSV5VΔC induced ER stress (Fig. 5A). Furthermore, expression of GADD153, which is often associated with cell death after ER stress, was examined. Expression of GADD153 was increased only in rSV5VΔC-infected cells (Fig. 5B).
FIG. 5.
Increased expression levels of ER stress-related proteins. HeLa cells were mock (MK) infected or infected with SV5 or rSV5VΔC. The infected cells were collected at 1 dpi and subjected to Western blotting as described in Materials and Methods. Numbers below the bands are protein expression levels relative to the value for mock-infected cells (defined as 1). (A) Anti-GRP 78 and anti-GRP 94; (B) anti-GADD153.
DISCUSSION
SV5 V is known to interrupt host IFN signaling and production. However, increased CPE caused by rSV5VΔC in Vero cells, an IFN production-defective cell line, suggests that V has other functions in addition to circumventing the host IFN response. Recently, He et al. used four- to six-week-old BALB/c mutant mice homozygous for a targeted disruption of STAT1 to establish a small animal model system for studying SV5 pathogenesis (16). In STAT1−/− mice, rSV5 grew to a titer of 0.44 × 107 PFU/g of lung tissue whereas rSV5VΔC grew only to a titer of 0.14 × 106 PFU/g. The higher titer of rSV5 compared to that seen with rSV5VΔC in mice that lack a STAT1 signaling pathway suggests that the C terminus of the V protein has another function in the virus life cycle in addition to mediating the anti-IFN activities. We hypothesize that the additional role of the V protein is that of preventing apoptosis in infected cells. rSV5VΔC was less pathogenic in vivo than wt rSV5 (even though it caused vastly greater CPE in mouse and human cells), a finding consistent with the notion of virus clearance by apoptosis of infected cells in an infected animal (15). In this study, we found that HeLa cells infected with rSV5VΔC underwent apoptosis and that the apoptosis was inhibited by V, suggesting that V had an anti-apoptosis function. Sendai virus, another paramyxovirus that lacks the V gene (rSeVΔV), is viable and grows as well as wt SeV. However, rSeVΔV induces increased CPE in culture-grown cells and is attenuated in animal hosts (24). The phenotype of rSeVΔV is very similar to that of rSV5VΔC (i.e., increased CPE in culture and attenuated in vivo), suggesting that SeV V protein might have a function similar to that of SV5 V protein in preventing cell death. Recently, a recombinant NDV lacking its V gene (rNDVΔV) has been recovered that induces increased apoptosis in CEF cells, suggesting that NDV V has an anti-apoptosis function (38). The mechanism by which V protein blocks virus-induced apoptosis is not clear.
Virus infection can activate a variety of cellular signaling pathways that lead to apoptosis. IFN produced in response to virus infection plays an essential role in inducing apoptosis in virus-infected cells (3, 9, 52). The infected host organisms are thought to inhibit and eliminate viral infection by sacrificing virus-infected cells through apoptosis. However, many viruses have developed means to delay and inhibit apoptosis to avoid being eliminated along with their host cells (4, 44). The exact mechanisms of IFN-promoted apoptosis in virus-infected cells are not clear. It is thought that IFN can activate caspases and up-regulate expression of genes with proapoptotic functions such as death factors (3, 9, 52). In rSV5VΔC-infected HeLa cells, increased IFN production was detected and the IFN signaling pathway was intact. However, the induction of apoptosis by rSV5VΔC also occurred in U3A cells and in Vero cells and in the presence of neutralizing antibody against IFN, suggesting that rSV5VΔC induced apoptosis in an IFN-independent manner. As the apoptotic pathway activated by rSV5VΔC was blocked by V expression (Fig. 1), we hypothesize that V protein has an antiapoptotic function and that the antiapoptotic function is distinct from its anti-IFN function. Thus, SV5 (an RNA virus) can activate IFN-independent apoptotic pathways in virus-infected cells.
Examination of possible involvement of death factors showed that medium components from rSV5VΔC-infected cells were not sufficient to induce cell death, suggesting that rSV5VΔC induced apoptosis through an intrinsic apoptotic pathway. To identify the intrinsic apoptotic pathway activated by rSV5VΔC, the involvement of caspases was examined. Whereas a caspase is undoubtedly involved in rSV5VΔC-induced apoptosis (as demonstrated by inhibition of the apoptosis by the pan-caspase inhibitor Z-VAD-FMK) (Fig. 3 and Table 2), it is very intriguing that caspase 3 and caspase 9, two major caspases in the intrinsic pathway, were not responsible for rSV5VΔC-induced apoptosis. Examination (using immunoblotting) of caspase 12 indicated that caspase 12 is activated. Caspase 12 was first identified in the mouse and was found to be important for ER stress-induced apoptosis (35). Although many reports indicated that caspase 12 has a similar function in human cells (6, 25, 57), there is one report indicating that (due to a mutation in the active site of caspase 12) there is no functional human caspase 12 (13). At present, unfortunately, there is no specific inhibitor available against caspase 12 to aid in resolving this uncertainty.
In our studies, we used immunoblotting analysis with two different antibodies to detect activation of caspase 12 in rSV5VΔC-infected cells; inhibitors against all other known caspases did not inhibit rSV5VΔC-induced apoptosis. We speculate that caspase 12 is responsible for rSV5VΔC-induced apoptosis. However, we cannot rule out the possibility that an as-yet-unidentified mammalian caspase might be responsible for rSV5VΔC-induced apoptosis. As activation of caspase 12 is often associated with ER stress, expression levels of ER stress-related proteins were examined and found to be up-regulated in rSV5VΔC-infected cells, supporting the hypothesis that caspase 12 is activated in rSV5VΔC-infected cells and that it might play an essential role in the induction of apoptosis. It is not surprising that viral infection can cause ER stress, as large amounts of viral glycoproteins are made in virus-infected cells (as reported previously for SV5 and for cells infected with other viruses) (22, 36, 62, 63). It is interesting that wt SV5 caused a mild increase of ER stress, as was evident in small increases in expression levels of GRP 78 and 94 (Fig. 5A) (two major host proteins involved in unfolded protein response [UPR]), and that wt SV5 did not induce cell death.
It has been reported that increased expression of GRP 78 can prevent apoptosis by downregulation of Bax protein, a proapoptotic protein (43). It is also known that UPR can activate proapoptotic gene expression (such as that of GADD153), which in turn can induce infected cells to undergo apoptosis (23, 33, 61). Expression levels of GADD153 were increased only in rSV5VΔC-infected cells (consistent with the induction of apoptosis seen in rSV5VΔC-infected cells). It is not clear how cells regulate expression of GRP 78 and GRP 94 to have an antiapoptotic effect at moderate expression levels and to have a proapoptotic effect at higher expression levels. We speculate that expression levels of GRP 78 and GRP 94 might determine how cells respond to ER stress. Regardless of how ER stress and cell death is regulated, it is conceivable that it is beneficial for viruses to prevent ER stress signaling to prevent host cells from undergoing apoptosis. The V protein of SV5 may play a role in eliminating ER stress signaling and enabling SV5 replication in nonapoptotic cells. Interestingly, it has been reported that human RS virus, which does not encode a V protein, induces expression of GRP 78 (a major indicator of ER stress in A549 cells) and causes cell death (6). However, there are other reports indicating that infection by RS virus does not induce apoptosis (11, 50).
It has been reported that mutant SV5 containing mutations in the shared N termini of V and P protein (known as CPI− virus) accelerates viral gene expression and causes increased cell death, with characteristics of apoptosis (60). Even though the mutant SV5 (CPI− virus) is different from rSV5VΔC, they may induce cell death through similar pathways. CPI− virus encodes two mutant proteins, whereas rSV5VΔC encodes a truncated V protein with a very short half-life. Thus, the phenotype of rSV5VΔC is likely a result of loss of V function whereas phenotypes of CPI− virus may be due to gains as well as losses of functions of both V and P mutant proteins. It is not unexpected that mutations in the shared N termini of P and V proteins of CPI− virus affect viral gene expression, as SV5 P is essential for viral RNA replication and transcription. It is possible that overexpression and early expression of viral proteins (including glycoproteins F and HN) in CPI− virus-infected cells can cause ER stress and trigger cell death in a fashion similar to that seen with the induction of apoptosis by rSV5VΔC infection, in which viral gene expressions are not affected in rSV5VΔC-infected cells (since it still encodes a wt P protein). Alternatively, increased UPR response in rSV5VΔC-infected cells may be a result of increased ER stress induced by overexpression of viral glycoproteins even though the increase was not obvious in rSV5VΔC-infected cells.
Mitochondria play an essential role in intrinsic apoptotic pathways through caspase-dependent and caspase-independent pathways. In rSV5VΔC-infected cells, apoptosis was caspase dependent but caspase 9 activity was not detected and an inhibitor against caspase 9 did not affect rSV5VΔC-induced apoptosis, suggesting that a previously unidentified caspase was activated through mitochondria or that mitochondria did not play a determining role in rSV5VΔC-induced apoptosis. Caspase 12 localizes to ER, and there is no evidence of its association with mitochondria (34, 35). At present, it is not clear how ER stress induced by rSV5VΔC infection affects mitochondrial function. Studies of the relationship between ER stress and mitochondrion damage in virus-infected cells are in progress.
Acknowledgments
We thank George Stark for providing U3A cells. We are grateful to Michael Teng for critically reading the manuscript. We appreciate other members of Biao He's lab for discussion and technical help. The services provided by the General Clinical Research Center of the Pennsylvania State University are appreciated.
The General Clinical Research Center of the Pennsylvania State University was partially supported by National Institutes of Health grant M01 RR 10732. R.A.L. is an Investigator of the Howard Hughes Medical Institute. The work was supported by a seed grant from the College of Agricultural Sciences of the Pennsylvania State University to B.H. and grants from the National Institute of Allergy and Infectious Diseases to R.A.L. (R01 AI 23173) and to B.H. (R01 AI 051372).
REFERENCES
- 1.Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 3.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed] [Google Scholar]
- 4.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. [DOI] [PubMed] [Google Scholar]
- 5.Bernardi, P., V. Petronilli, F. Di Lisa, and M. Forte. 2001. A mitochondrial perspective on cell death. Trends Biochem. Sci. 26:112-117. [DOI] [PubMed] [Google Scholar]
- 6.Bitko, V., and S. Barik. 2001. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 80:441-454. [DOI] [PubMed] [Google Scholar]
- 7.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 8.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. (Erratum, 13:371, 1999.) [DOI] [PubMed] [Google Scholar]
- 9.Dai, C., and S. B. Krantz. 1999. Interferon gamma induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood 93:3309-3316. [PubMed] [Google Scholar]
- 10.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domachowske, J. B., C. A. Bonville, A. J. Mortelliti, C. B. Colella, U. Kim, and H. F. Rosenberg. 2000. Respiratory syncytial virus infection induces expression of the anti-apoptosis gene IEX-1L in human respiratory epithelial cells. J. Infect. Dis. 181:824-830. [DOI] [PubMed] [Google Scholar]
- 12.Evan, G., and T. Littlewood. 1998. A matter of life and cell death. Science 281:1317-1322. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, H., U. Koenig, L. Eckhart, and E. Tschachler. 2002. Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 293:722-726. [DOI] [PubMed] [Google Scholar]
- 14.He, B., G. P. Leser, R. G. Paterson, and R. A. Lamb. 1998. The paramyxovirus SV5 small hydrophobic (SH) protein is not essential for virus growth in tissue culture cells. Virology 250:30-40. [DOI] [PubMed] [Google Scholar]
- 15.He, B., G. Y. Lin, J. E. Durbin, R. K. Durbin, and R. A. Lamb. 2001. The SH integral membrane protein of the paramyxovirus simian virus 5 is required to block apoptosis in MDBK cells. J. Virol. 75:4068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 17.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237:249-260. [DOI] [PubMed] [Google Scholar]
- 18.Hiebert, S. W., C. D. Richardson, and R. A. Lamb. 1988. Cell surface expression and orientation in membranes of the 44 amino acid SH protein of simian virus 5. J. Virol. 62:2347-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, S., S. J. Snipas, C. Vincenz, G. Salvesen, and V. M. Dixit. 1998. Caspase-14 is a novel developmentally regulated protease. J. Biol. Chem. 273:29648-29653. [DOI] [PubMed] [Google Scholar]
- 20.Hurtley, S. M., D. G. Bole, H. Hoover-Litty, A. Helenius, and C. S. Copeland. 1989. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J. Cell Biol. 108:2117-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacques, J. P., and D. Kolakofsky. 1991. Pseudo-templated transcription in prokaryotic and eukaryotic organisms. Genes Dev. 5:707-713. [DOI] [PubMed] [Google Scholar]
- 22.Jordan, R., O. V. Nikolaeva, L. Wang, B. Conyers, A. Mehta, R. A. Dwek, and T. M. Block. 2002. Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions. Virology 295:10-19. [DOI] [PubMed] [Google Scholar]
- 23.Jordan, R., L. Wang, T. M. Graczyk, T. M. Block, and P. R. Romano. 2002. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 76:9588-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato, A., K. Kiyotani, Y. Sakai, T. Yoshida, and Y. Nagai. 1997. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 16:578-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura, Y., A. Miyamura, K. Takata, M. Inden, D. Tsuchiya, K. Nakamura, and T. Taniguchi. 2003. Possible involvement of both endoplasmic reticulum- and mitochondria-dependent pathways in thapsigargin-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Pharm. Sci. 92:228-236. [DOI] [PubMed] [Google Scholar]
- 26.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, Pa.
- 27.Lee, P., J. Lee, S. Kim, M. S. Lee, H. Yagita, S. Y. Kim, H. Kim, and K. Suk. 2001. NO as an autocrine mediator in the apoptosis of activated microglial cells: correlation between activation and apoptosis of microglial cells. Brain Res. 892:380-385. [DOI] [PubMed] [Google Scholar]
- 28.Lin, G. Y., and R. A. Lamb. 2000. The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 74:9152-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, G. Y., R. G. Paterson, and R. A. Lamb. 1997. The RNA binding region of the paramyxovirus SV5 V and P proteins. Virology 238:460-469. [DOI] [PubMed] [Google Scholar]
- 30.Lin, Y., A. C. Bright, T. A. Rothermel, and B. He. 2003. Induction of apoptosis by paramyxovirus simian virus 5 lacking a small hydrophobic gene. J. Virol. 77:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liston, P., and D. J. Briedis. 1994. Measles virus V protein binds zinc. Virology 198:399-404. [DOI] [PubMed] [Google Scholar]
- 32.Little, E., M. Ramakrishnan, B. Roy, G. Gazit, and A. S. Lee. 1994. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 4:1-18. [DOI] [PubMed] [Google Scholar]
- 33.McCullough, K. D., J. L. Martindale, L. O. Klotz, T. Y. Aw, and N. J. Holbrook. 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21:1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa, T., and J. Yuan. 2000. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150:887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa, T., H. Zhu, N. Morishima, E. Li, J. Xu, B. A. Yankner, and J. Yuan. 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98-103. [DOI] [PubMed] [Google Scholar]
- 36.Ng, D. T., R. E. Randall, and R. A. Lamb. 1989. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J. Cell Biol. 109:3273-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parisien, J. P., J. F. Lau, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2002. Selective STAT protein degradation induced by paramyxoviruses requires both STAT1 and STAT2 but is independent of alpha/beta interferon signal transduction. J. Virol. 76:4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, M. S., A. García-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paterson, R. G., T. J. R. Harris, and R. A. Lamb. 1984. Analysis and gene assignment of mRNAs of a paramyxovirus, simian virus 5. Virology 138:310-323. [DOI] [PubMed] [Google Scholar]
- 40.Paterson, R. G., G. P. Leser, M. A. Shaughnessy, and R. A. Lamb. 1995. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology 208:121-131. [DOI] [PubMed] [Google Scholar]
- 41.Randall, R. E., and A. Bermingham. 1996. NP:P and NP:V interactions of the paramyxovirus simian virus 5 examined using a novel protein:protein capture assay. Virology 224:121-129. [DOI] [PubMed] [Google Scholar]
- 42.Rao, R. V., E. Hermel, S. Castro-Obregon, G. del Rio, L. M. Ellerby, H. M. Ellerby, and D. E. Bredesen. 2001. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol. Chem. 276:33869-33874. [DOI] [PubMed] [Google Scholar]
- 43.Rao, R. V., A. Peel, A. Logvinova, G. del Rio, E. Hermel, T. Yokota, P. C. Goldsmith, L. M. Ellerby, H. M. Ellerby, and D. E. Bredesen. 2002. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 514:122-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 45.Schmitt, A. P., B. He, and R. A. Lamb. 1999. Involvement of the cytoplasmic domain of the hemagglutinin-neuraminidase protein in assembly of the paramyxovirus simian virus 5. J. Virol. 73:8703-8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt, A. P., G. P. Leser, D. L. Waning, and R. A. Lamb. 2002. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J. Virol. 76:3952-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stark, G. R. 1997. Genetic analysis of interferon and other mammalian signaling pathways. Harvey Lect. 93:1-16. [PubMed] [Google Scholar]
- 48.Steward, M., A. C. R. Samson, W. Errington, and P. T. Emmerson. 1995. The Newcastle disease virus V protein binds zinc. Arch. Virol. 140:1321-1328. [DOI] [PubMed] [Google Scholar]
- 49.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi, R., H. Tsutsumi, M. Osaki, K. Haseyama, N. Mizue, and S. Chiba. 1998. Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1β-converting enzyme gene expression but does not cause apoptosis. J. Virol. 72:4498-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talanian, R. V., C. Quinlan, S. Trautz, M. C. Hackett, J. A. Mankovich, D. Banach, T. Ghayur, K. D. Brady, and W. W. Wong. 1997. Substrate specificities of caspase family proteases. J. Biol. Chem. 272:9677-9682. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka, N., M. Sato, M. S. Lamphier, H. Nozawa, E. Oda, S. Noguchi, R. D. Schreiber, Y. Tsujimoto, and T. Taniguchi. 1998. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells 3:29-37. [DOI] [PubMed] [Google Scholar]
- 53.Thomas, S. M., R. A. Lamb, and R. G. Paterson. 1988. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell 54:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 55.Thornberry, N. A., T. A. Rano, E. P. Peterson, D. M. Rasper, T. Timkey, M. Garcia-Calvo, V. M. Houtzager, P. A. Nordstrom, S. Roy, J. P. Vaillancourt, K. T. Chapman, and D. W. Nicholson. 1997. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272:17907-17911. [DOI] [PubMed] [Google Scholar]
- 56.Tidona, C. A., H. W. Kurz, H. R. Gelderblom, and G. Darai. 1999. Isolation and molecular characterization of a novel cytopathogenic paramyxovirus from tree shrews. Virology 258:425-434. [DOI] [PubMed] [Google Scholar]
- 57.Tong, W. G., X. Z. Ding, and T. E. Adrian. 2002. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 296:942-948. [DOI] [PubMed] [Google Scholar]
- 58.Ulane, C. M., and C. M. Horvath. 2002. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 304:160-166. [DOI] [PubMed] [Google Scholar]
- 59.Wang, X. Z., B. Lawson, J. W. Brewer, H. Zinszner, A. Sanjay, L. J. Mi, R. Boorstein, G. Kreibich, L. M. Hendershot, and D. Ron. 1996. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 16:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 76:10109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe, Y., O. Suzuki, T. Haruyama, and T. Akaike. 2003. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell. Biochem. 89:244-253. [DOI] [PubMed] [Google Scholar]
- 62.Watowich, S. S., R. I. Morimoto, and R. A. Lamb. 1991. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J. Virol. 65:3590-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wooden, S. K., L. J. Li, D. Navarro, I. Qadri, L. Pereira, and A. S. Lee. 1991. Transactivation of the grp78 promoter by malfolded proteins, glycosylation block, and calcium ionophore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-I. Mol. Cell. Biol. 11:5612-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou, G., and B. Roizman. 2002. Cation-independent mannose 6-phosphate receptor blocks apoptosis induced by herpes simplex virus 1 mutants lacking glycoprotein D and is likely the target of antiapoptotic activity of the glycoprotein. J. Virol. 76:6197-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]