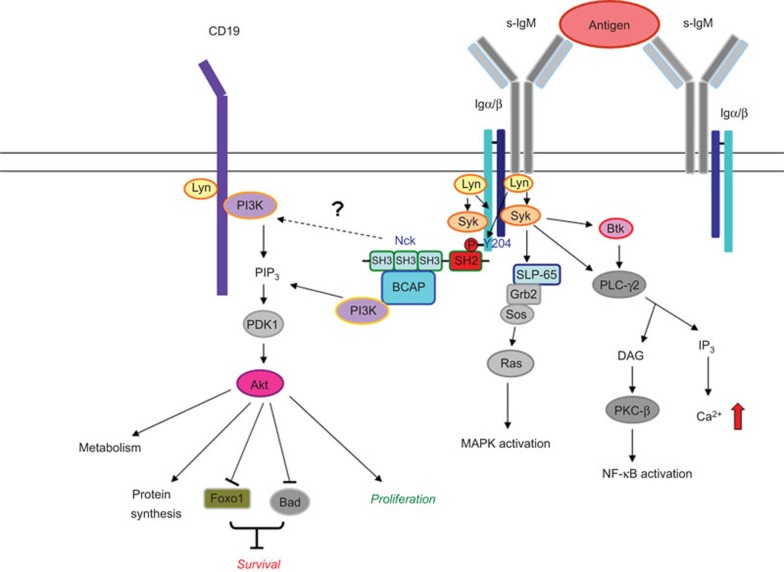
The signaling pathways activated by the B-cell antigen receptor (BCR) play a crucial role in the development, activation, and differentiation of B cells. Among them, the activation of phosphatidylinositol 3-kinase (PI3K)/Akt is particularly important.1,2,3,4,5 The recruitment of PI3K to the plasma membrane is necessary for the generation of phosphatidyl-inositol-3,4,5-triphosphate, which activates PDK1 (Akt/PKB kinase); in turn, PDK1 activates Akt (Figure 1). The BCR coreceptor CD19 and a cytoplasmic adaptor, BCAP (B-cell adaptor for PI3K), are major molecules responsible for the recruitment of PI3K to the BCR complex through direct binding with PI3K.6,7,8 For more than a decade, how BCAP approaches the BCR complex and brings PI3K to the plasma membrane has remained unknown. Recently, Castello et al.9 identified the Nck proteins as key adaptors linking BCAP/PI3K to the BCR complex.9
Figure 1.
Nck proteins as the newly identified adaptors between the BCR complex and BCAP/PI3K/Akt pathway. After BCR crosslinking, Nck proteins bind to phosphorylated Tyr204 at the tail of Igα via their SH2 domains to recruit BCAP through their SH3 domains. Nck may control BCR signaling by activating the BCAP/PI3K/Akt pathway and cross-talking with other pathways. BCAP, B-cell adaptor for PI3K; BCR, B-cell antigen receptor; Igα, immunoglobulin-α PI3K, phosphatidylinositol 3-kinase; SH3, Src-homology 3.
Antigen-triggered BCR crosslinking induces the tyrosine phosphorylation of immunoglobulin-α (Igα, CD79A) and immunoglobulin-β (Igβ, CD79B) by Src family kinases such as Lyn. BCR engagement also stimulates the tyrosine phosphorylation of CD19. The phosphorylation of Igα and Igβ stimulates the recruitment of the Syk tyrosine kinase (Figure 1) to the BCR complex and induces Syk phosphorylation and activation, which subsequently induces the phosphorylation of an adaptor protein, SLP-65 (also known as BLNK).10,11 Phosphorylated SLP-65 provides docking sites for phospholipase C-γ2, Bruton's tyrosine kinase,12,13 and another adaptor protein, Grb2,10 which initiates the activation of the Ras/Raf/MEK/ERK kinase cascade (Figure 1).
The Nck family is another group of adaptors that contains three Src-homology 3 (SH3) domains at its N-terminus and 1 SH2 domain at its C-terminus. Two members in the Nck family exist in mice and humans (Nck1 and Nck2). Nck proteins control the cytoskeletal rearrangement and mobility of kidney podocytes and neurons.14,15 In the immune system, Nck is known to associate with the T-cell receptor and regulate T-cell receptor signaling and T-cell development.16 However, the role of Nck in B cells is largely unknown, although SLP-65 is known to bind Nck.10
Based on these previous observations, Castello and colleagues proposed that Nck might play a role in BCR signaling, and they tested this hypothesis using biochemical and genetic methods. First, they purified Nck-interacting proteins after BCR activation by affinity purification and identified the co-precipitated proteins by mass spectrometry. They found that the μ-heavy chain of the BCR associated with Nck. Furthermore, they deleted the NCK gene in the chicken DT40 B-cell line and observed significant reductions in protein tyrosine phosphorylation and calcium influx in the Nck-deficient cell line (Ref. 9, Figure 1c–e), suggesting an important role for Nck in BCR signaling.
To study whether Nck is recruited to BCR signalosomes, which are platforms for efficient B-cell activation, the authors used total internal reflection fluorescence microscopy to observe the dynamic interactions of the surface molecules on B cells. Because the resolution of total internal reflection fluorescence microscopy can reach the single-molecule level, it can be used to quantitate the association of Nck with other molecules in BCR signalosomes. Meanwhile, to mimic the interaction of B cells with antigen-presenting cells, Castello and colleagues used a planar lipid bilayer containing a fluorochrome-labeled anti-IgM antibody to induce BCR signaling. The analyses showed the colocalization of Nck with SLP-65, Syk and antigens in a Lyn-dependent manner in B cells. In summary, Nck is recruited to BCR signalosomes and is required for efficient activation of signal transduction.
The next key question was how Nck was recruited to the BCR complex. Analytical mass spectrometry demonstrated the association of Igα and Igβ with Nck. To confirm this result, the authors used His-tagged Nck proteins as probes to perform a binding assay with immobilized peptides corresponding to the human Igα cytoplasmic domain. They found that Nck proteins bound Igα peptides containing phosphorylated Tyr204, which is a non-ITAM tyrosine residue. Castello et al. further used an assay called ‘biolayer interferometry' to measure the avidity of Nck proteins and phosphorylated Tyr204 peptides. Biolayer interferometry is a method used to assess the affinity and kinetics of protein–protein interactions in real time. In this method, one protein is coated on the tip of a biosensor, and the other is added to the solution. Any protein binding to the immobilized protein will generate a wavelength shift in the interference pattern of white light that is reflected from the inside and outside surfaces of the biosensor. Using this technique, this group confirmed that Nck proteins could bind to phosphorylated Tyr204 on Igα in vitro.
To study the role of Nck in B cells in vivo, the authors generated conventional Nck1-knockout (KO) and conditional Nck2-KO mice lacking Nck2 in the B lineage. B-cell development appeared to be normal in Nck1-KO and Nck1–Nck2-KO mice, although a decrease in B1a cells was observed. However, Nck-deficient B cells were demonstrated to have defects in BCR-induced calcium influx, proliferation, and cell survival. In contrast, IL-4- and BAFF-mediated cell survival and the antigen presentation ability of Nck-deficient B cells were comparable to that of wild-type B cells in vitro. More importantly, T-independent and early T-dependent antibody responses were suppressed in the Nck1- and Nck1–Nck2-KO mice after immunization. In accordance with the reduced humoral response, the number of hapten-specific, IgM-secreting cells was significantly reduced in the KO mice. However, no substantial differences in the frequencies of antigen-specific, IgG-secreting cells or germinal center B cells, the affinity maturation or the memory responses were observed in the Nck-KO mice. Overall, these experiments demonstrated the importance of Nck in B-cell function in vivo, particularly in the T-independent response.
To delineate the mechanism whereby Nck controls B-cell activation, the authors re-examined their mass spectrometry data and observed an association between BCAP and Nck. Using the peptide-binding assay, they found that the Nck SH3 domains, particularly the second domain, interacted with the proline-rich domain of BCAP. The analyses of biolayer interferometry and total internal reflection fluorescence microscopy confirmed the binding of BCAP to Nck after BCR stimulation. Notably, they showed that Nck was necessary for BCAP recruitment to BCR signalosomes and for the tyrosine phosphorylation of BCAP (Ref. 9, Figure 6e–g). This discovery reveals how BCAP is linked to the BCR complex for the first time.
To consolidate their findings, the phosphorylation of Akt and its substrate, Foxo-1, was examined. As expected, the phosphorylation of Akt and Foxo-1 was partially reduced in Nck1-deficient B cells; however, it was nearly completely abolished in Nck1–Nck2-deficient B cells (Ref. 9, Figure 7d and e). These results were somewhat striking because BCAP-deficient B cells showed apparently normal Akt activation after BCR crosslinking.8 It has been demonstrated that CD19 plays a major role in the activation of the PI3K/Akt signaling pathway and that Akt activation is completely blocked in B cells lacking CD19 and BCAP.8 The severe defects in Akt activation in the Nck1–Nck2-KO B cells indicate that Nck may use other means to activate the PI3K/Akt pathway in addition to the recruitment of BCAP to the BCR complex. One possible downstream candidate of Nck is CD19, as the authors note, but they did not examine whether the recruitment or phosphorylation of CD19 were regulated by Nck. Because CD19 contains PI3K-binding sites, Nck deficiency is more likely to hinder CD19 phosphorylation. Another possibility is that the lack of Nck protein affects cytoskeletal reorganization (Ref. 6, Figure 1c), which might influence the clustering of BCR signalosomes and reduce the efficiency of signal transduction.
In addition, it is notable that Nck1 is ubiquitously expressed in many cell types in the immune system, such as T cells, macrophages, dendritic cells and NK cells. In the study conducted by Castello et al., the ablation of Nck1 in other cell types may have disturbed their interactions with B cells, which could have led to the observed reductions in the T-dependent and T-independent humoral responses. Because Nck proteins regulate T-cell signaling and synapse formation,16 we should be careful when interpreting these results from Nck1–Nck2-KO mice. Conditional deletion of the Nck1 and Nck2 genes in B cells will provide further evidence to verify the roles of Nck proteins in the activation and function of B cells in vivo.
References
- Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, et al. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260:986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- Castello A, Gaya M, Tucholski J, Oellerich T, Lu KH, Tafuri A, et al. Nck-mediated recruitment of BCAP to the BCR regulates the PI3K–Akt pathway in B cells. Nat Immunol. 2013;14:966–975. doi: 10.1038/ni.2685. [DOI] [PubMed] [Google Scholar]
- Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen PJ, Reth M. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Maeda A, Ishiai M, Hashimoto A, Inabe K, Takata M. Regulation of the phosphlipase C-γ2 pathway in B cells. Immunol Rev. 2000;176:19–29. doi: 10.1034/j.1600-065x.2000.00605.x. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Kurosaki M. Transphosphorylation of Bruton's tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem. 1997;272:15595–15598. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- Jones N, Basutig IM, Eremina V, Ruston JM, Bladt F, Li H, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Georgiou J, Ruston J, Bladt F, Sherman A, Warner N, et al. Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc Natl Acad Sci USA. 2007;104:20973–20978. doi: 10.1073/pnas.0710316105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]