T lymphocytes are major players in immunity, providing protection against pathogenic microorganisms and tumors. They achieve this goal because they express clonally distributed receptors (T-cell receptor, TCR) on their surface capable of recognizing antigens displayed on the surface of specialized antigen-presenting cells, in association with antigen presenting molecules.1
The situation for γδ T cells, an unconventional subset of so called innate memory T cells, has long remained obscure.2 In the blood of most healthy individuals, the predominant γδ T cell population consists of T cells expressing the Vδ2 gene paired with the Vγ9 chain.
Vγ9Vδ2 T cells recognize host- and microbe-derived phosphorylated prenyl metabolites (phosphoantigens, PAgs),3 but the molecular basis for PAg recognition has long remained a major unanswered question in the biology of Vγ9Vδ2 T cells.
In a study published in the September issue of Nature Immunology, De Libero and colleagues4 shed light on the mechanism of Vγ9Vδ2 T-cell recognition of PAgs and identify butyrophilin (BTN) 3A1 as the PAg-presenting molecule.
Many previous studies have been consistent with the existence of a PAg-presenting molecule:
The small size of the stimulatory PAgs and the requirement for cell-to-cell contact have suggested that PAgs bind a specialized antigen presenting molecules. Such a putative molecule should be species specific, ubiquitously expressed and functionally nonpolymorphic.5
None of the known human presenting molecules (major histocompatibility complex (MHC) class I and class II, MR1 or CD1) is involved in PAg presentation5,6 and antigen processing is not required, likely explaining why PAgs can be presented by non professional antigen-presenting cell, including Vγ9Vδ2 T cells themselves.5,6
The findings that PAgs fail to bind directly to the soluble Vγ9Vδ2 TCR and that the putative antigen-binding groove of the Vγ9Vδ2 TCR is much larger than that occupied by the PAg alone, further suggest interactions with a complex formed by the PAg and a PAg-presenting molecule.7,8
Therefore, all these observations strongly support the requirement for a surface protein(s) distinct from all known antigen-presenting molecules for PAg presentation.
Identification of a PAg-presenting molecule, however, has been complicated by the fact the human Vγ9Vδ2 T cells are able to ‘autopresent' PAgs to themselves, thus preventing results' interpretation.5,9 De Libero and colleagues4 have developed a new strategy to avoid the autopresentation phenomenon: they have generated mice with transgenic expression of the Vγ9Vδ2 TCR and have tested mouse–human hybrid lines for their ability to present PAgs to Vγ9Vδ2 T cells from the transgenic mice.
Using this approach, De Libero and colleagues4 have found that the gene coding for a candidate PAg-presenting molecule is located in the 3–27.4 telomeric megabases region (p25.2–p22.1) of human chromosome 6. Exploiting the ubiquitous expression and the presence of two immunolgobulin-like extracellular domains of the putative PAg-binding molecule to narrow the search, De Libero and colleagues4 identify several members of the BTN (B7-related molecules of the immunoglobulin superfamily) family as potential candidate. Finally, using transfection and small interfering RNA, they finally demonstrate that BTN3A1 is the key PAg-presenting molecule.
Extensive biophysical and structural experiments provide evidence for a direct role for BTN3A1 in PAg presentation. By mass spectroscopy and surface plasmon resonance, De Libero and colleagues4 demonstrate binding of PAgs to the immunoglobulin V domain of BTN3A1 and crystal structures of the immunoglobulin V domain of BTN3A1 with PAgs identify a shallow groove on the protein surface that accommodates a single PAg.
Finally, by measuring surface-enhanced Raman scattering, the authors show that the Vγ9Vδ2 TCR interacts directly, but weakly, with BTN3A1, and that PAg enhances this interaction.
Despite the study by De Libero et al. makes key advances in antigen recognition by Vγ9Vδ2 T cells, several questions remain unanswered. The major one concerns the role of the intracellular B30.2 domain of BTN3A1, which is required for Vγ9Vδ2 T-cell activation.
Earlier study suggested that PAgs modify the BTN3A1 molecule via interaction with the B30.2 cytoplasmic region, which would potentially involve dimerization and clustering.10,11
Another study by Morita and colleagues12 found that a predicted PAg-binding pocket only exists on the intracellular B30.2 domain of the BTN3A1. Nonetheless, BTN3A1 did not bind a photo affinity-labeled PAg, thus indicating that neither extracellular nor the cytoplasmic B30.2 domains actually was capable to bind PAgs.12
De Libero and colleagues4 now find that only the extracellular V domain of BTN3A1 contains a PAg-binding pocket and suggest that the B30.2 domain might be involved in the trafficking of BTN3A1 to the proper intracellular compartments where it is loaded with PAgs. In support to this, De Libero et al.4 show that BNT3A2, which shares a similar V domain with BTN3A1, but lacks B30.2, fails to stimulate Vγ9Vδ2 T cells.
Unfortunately however, the authors do not provide evidence of the capability of BTN3A2 to bind PAg, which leaves unsolved the question of whether the intracytoplasmic B30.2 domain influences PAg binding or PAg/BTN3A1 complexes are necessary, but not sufficient to activate Vγ9Vδ2 T cells.
Solving this issue may help in understanding how PAgs are trafficked to and loaded onto BTN3A1, and whether or not this requires specialized enzymes. Many different chaperons promote loading of peptides into MHC class I and II, and of glycolipids into CD1 molecules: specialized enzymes are also capable of covalent addition of isoprenoids to proteins (a process known as protein penylation), but whether or not similar mechanisms also promote PAgs loading into BTN3A1 is worth of additional studies.
Finally, De Libero et al.4 show that the Vγ9Vδ2 TCR has a very weak affinity for BTN3A1 and PAg only modestly enhances it: thus, additional structural studies are needed to understand how PAg–BTN3A1 complexes are recognized by the Vγ9Vδ2 TCRs.
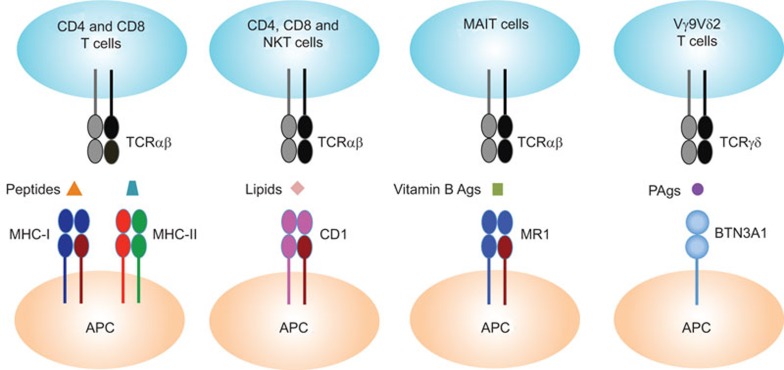
The thymus ‘educates' T lymphocytes to recognize foreign antigens in a genetically restricted fashion. It is already known that there are three families of molecules involved in this:1 MHC class I and II molecules present peptides to CD8 and CD4 T cells, respectively; another MHC-like family of proteins called CD1 present glycolipids to CD4, CD8 and natural killer T cells; a third MHC-related protein called MR1 (MHC-related 1) presents vitamin B metabolites to a class of immune T cells called MAIT (mucosa-associated invariant T) cells:13 the paper by De Libero et al.4 now provides evidence that BTN3A1 presents PAg to Vγ9Vδ2 T cells (Figure 1), and it therefore defines a fourth model of antigen presentation to immune cells.
Figure 1.
TCR recognition of peptides, lipids, vitamin B Ags and PAgs. MHC class I and II molecules present peptides to CD8 and CD4 T cells, respectively; MHC class I-like molecules CD1 present lipids to CD4, CD8 and NKT cells; the MHC class I-related protein MR1 presents vitamin B metabolites to MAIT cells and BTN3A1 presents PAgs to Vγ9Vδ2 T cells. Ag, antigen; BTN, butyrophilin; MHC, major histocompatibility complex; NKT, natural killer T; PAg, phosphoantigen; TCR, T-cell receptor.
Acknowledgments
This work was supported by grants from the Ministry of University and Research (MIUR-PRIN 2008) and the Ministry of Health ‘Ricerca Finalizzata 2007' to FD.
References
- Eckle SB, Turner SJ, Rossjohn J, McCluskey J. Predisposed αβ T cell antigen receptor recognition of MHC and MHC-I like molecules. Curr Opin Immunol. 2013;25:1–7. doi: 10.1016/j.coi.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol. 2013;14:908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- Sireci G, Champagne E, Fournié JJ, Dieli F, Salerno A. Patterns of phosphoantigen stimulation of human Vγ9Vδ2 T cell clones include Th0 cytokines. Hum Immunol. 1997;58:70–82. doi: 10.1016/s0198-8859(97)00211-5. [DOI] [PubMed] [Google Scholar]
- Sireci G, Espinosa E, Di Sano C, Dieli F, Fournié JJ, Salerno A. Differential activation of human γδ cells by nonpeptide phosphoantigens. Eur J Immunol. 2001;31:1628–1635. doi: 10.1002/1521-4141(200105)31:5<1628::AID-IMMU1628>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human γδ T-cell antigen receptor. Nature. 2001;411:820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- Wang H, Fang Z, Morita CT. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol. 2010;184:6209–6222. doi: 10.4049/jimmunol.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Harly C, Guillaume Y, Nedellec S, Peigné CM, Mönkkönen H, Mönkkönen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palakodeti A, Sandstrom A, Sundaresan L, Harly C, Nedellec S, Olive D, et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J Biol Chem. 2012;287:32780–32790. doi: 10.1074/jbc.M112.384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Henry O, Distefano MD, Wang YC, Räikkönen J, Mönkkönen J, et al. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J Immunol. 2013;191:1029–1042. doi: 10.4049/jimmunol.1300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]