Abstract
Lipopolysaccharide (LPS) is known to be a potent activator of mature B cells by signaling through Toll-like receptor 4 (TLR4). Its impact on early B-cell development, however, is not well defined. When comparing to C3H/HeN mice, TLR4-mutant C3H/HeJ mice showed an increase in the number of pro-B and pre-B cells in the bone marrow. When cultured in the presence of IL-7, the proliferation of pro-B and large pre-B cells was significantly inhibited by LPS, possibly due to reduced IL-7 receptor-α (IL-7Rα) expression. Meanwhile, the generation of IgM+/IgD+ B cells was greatly enhanced in IL-7 cultures of pro-B and pre-B cells. Consistent with these results, treatment with LPS facilitated the progression of adoptively transferred B220+IgM−IgD− precursors into IgD+ cells. Overall, these data suggest that LPS has a profound influence on early B-cell development, which may contribute to the deregulated B-cell development under physiological and pathological conditions such as bacterial infections.
Keywords: B-cell differentiation, B lymphopoiesis, IL-7, lipopolysaccharide, Toll-like receptor 4
Introduction
B-cell development is a complex process. Through multiple, immediate stages, hematopoietic progenitor cells eventually give rise to a B-cell pool with a diverse yet self-tolerant repertoire of antigen receptors.1 At the pro-B stage, cells rearrange their immunoglobulin heavy chain (IgH) loci. The productive rearrangement and expression of Igh leads to the assembly of a pre-B-cell receptor (pre-BCR), which delivers a signal that is essential for the transition from the pro-B- to pre-B-cell stages. The large pre-B cells undergo several rounds of proliferation before exiting the cell cycle to begin rearrangement of the Ig light chain (IgL) loci at the small, pre-B-cell stage.2,3 Upon successful rearrangement of Igl, cells begin to express a functional BCR that is capable of antigen recognition, which marks the progression to the immature stage. Immature B cells are then exported to the periphery, where they undergo further maturation4 and acquire surface markers such as IgD, CD21 and CD23.5,6
The intrinsic developmental program of B cells is regulated by extrinsic signals. Among these, IL-7 secreted by bone marrow stromal cells plays a particularly important role.7 The disruption of IL-7 signaling in mice with targeted deletion of the gene encoding IL-7 or components of the IL-7 receptor (IL-7R) results in impaired V–DJ recombination and a developmental arrest at the pro-B-cell stage.8,9 IL-7 primarily acts to drive the proliferation of pro-B and pre-B cells. Moreover, it may regulate the differentiation of B-cell precursors because its withdrawal has been reported to facilitate the generation of sIgM+ immature B cells.
Cells of the B lineage, including developing B cells, also express multiple pathogen recognition receptors, such as Toll-like receptors (TLRs).10 Previous studies mainly focused on their functions in mature B cells. Engagement of TLR4 by lipopolysaccharide (LPS), for example, has been shown to affect many aspects of B-cell activity, including cell proliferation, Ig-class switch, plasma-cell differentiation and antibody production as well as their antigen-presenting capacity.11,12 In contrast, limited information is currently available regarding the potential role of TLR4-mediated signaling in early B-cell development. In one report, LPS was shown to exert an inhibitory effect on B lymphopoiesis by promoting myeloid differentiation of hematopoietic progenitors through a Myd88-dependent mechanism.13 A more recent study, however, indicates that LPS may facilitate B-cell maturation by acting as an accessory stimulus to complement the BAFF physiological pathway of B-cell development.14 Therefore, the precise role of TLR4 signaling in the early stages of B-cell development requires more in-depth analysis. In the present study, we used in vitro (cell culture) and in vivo (adoptive transfer) approaches to systemically analyze the impact of TLR4 signaling on the proliferation, survival and differentiation of B-cell precursors.
Materials and methods
Mice
C57BL/6, C3H/HeN and C3H/HeJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the animal breeding facility at Peking University Health Science Center under specific, pathogen-free conditions. The experimental procedures on the use and care of animals were approved by the ethics committee of Peking University Health Science Center. All animals were used at the age of 6–8 weeks.
Flow cytometry and cell sorting
Bone marrow was removed and cell suspensions were prepared in balanced salt solution (phosphate-buffered saline (PBS) containing 2% fetal calf serum). Following depletion of erythrocytes with ACK lysis buffer, cells were stained for 20 min at 4 °C with FITC-, PE-, PerCP-Cy5.5-, APC- or biotin-conjugated monoclonal antibodies that was specific for mouse B220, Mac-1, CD43, IgM, IgD, CD23 and CD21/35 (BD Biosciences, San Jose, CA), to define B-cell subsets. Flow cytometry was performed using a FACSCalibur (Becton Dickinson, Mountain View, CA, USA), and the data were analyzed using the FlowJo (TreeStar, San Carlos, CA) software.
For cell sorting, bone marrow cells were stained with antibodies specific for B220, CD43, IgM, and IgD, and pro-B and pre-B cells were defined as B220+CD43+IgM−IgD− and B220+CD43−IgM−IgD− cells, respectively. Large and small pre-B cells were distinguished according to forward scattering, and cell sorting was performed using a FACSAria cytometer (BD Bioscience) with a purity >95%.
B-cell culture
Sorted pro-B or pre-B cells were cultured in 96-well, flat-bottom plates at 2×105 cells/well in Opti-MEM (Invitrogen, San Diego, CA) supplemented with 10% fetal calf serum (FCS) and gentamycin (200 U/ml) in a humidified atmosphere of 5% CO2 at 37 °C. LPS (10 µg/ml; Sigma-Aldrich, St. Louis, MO) and IL-7 (10 ng/ml; R&D Systems, Minneapolis, MN) were added to the culture at final concentrations of 10 µg/ml and 10 ng/ml, respectively.
Proliferation assay
For the proliferation assay, 5×106 B cells were incubated at room temperature for 5 min in 1 ml of PBS containing 5 µM carboxy fluorescein diacetate succinimide ester (CFSE; Sigma). Cells were then washed twice to remove free dye before being put into culture. After culturing for 24–72 h, the cells were monitored for CFSE dilution using flow cytometry.
Apoptosis assay
Apoptosis of the cultured cells was determined by staining with FITC-coupled Annexin V (Beijing Biosea Biotechnology Co. Ltd, Beijing, China) followed by analysis on a FACSCalibur.
Adoptive transfer of B cells
B220+IgM−IgD− cells were isolated from the bone marrow of adult C3H/HeN mice by cell sorting to a purity >95%. Sorted B cells (6×106) were labeled with CFSE (0.5 µM) and then intravenously transferred into C3H/HeJ-recipient mice. Immediately afterwards, the recipient received intraperitoneal injection of LPS (2.5 µg/g weight) or an equal volume of PBS. Bone marrow cells were then harvested 18 h after transfer, and IgM and IgD expression by CFSE+ donor cells were analyzed by flow cytometry.
Statistical analysis
The data were collected from at least three independent experiments. The unpaired Student's t-test was performed using the GraphPad Prism software (GraphPad, La Jolla, CA, USA), and differences were considered significant when the P value was <0.05.
Results
Increased pro-B and pre-B cells in C3H/HeJ mice
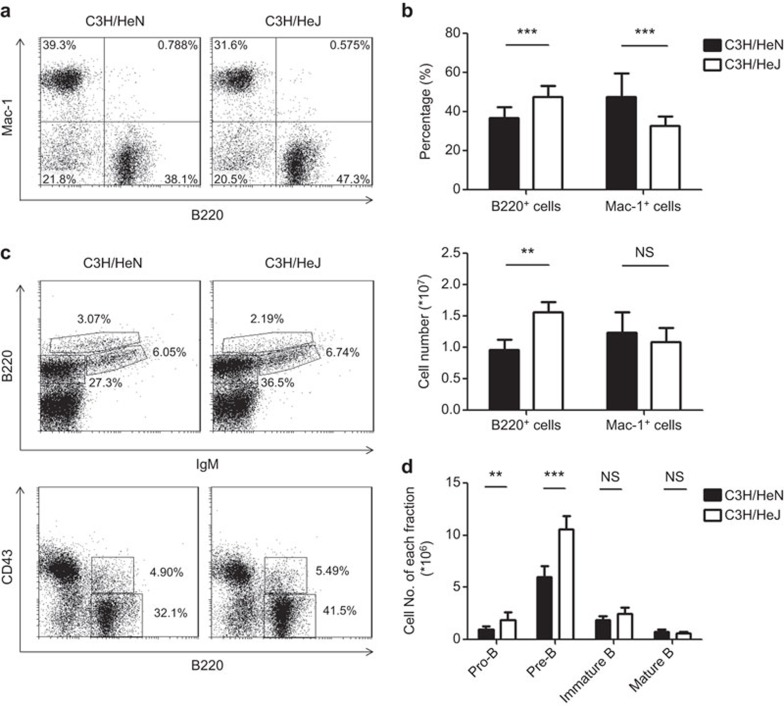
To reveal the potential influence of TLR4 signaling on early B-cell development, we first compared the bone marrow cell populations in C3H/HeN mice and C3H/HeJ mice that harbored a mutation in Tlr4. The mutant mice showed an increase in the percentage and absolute number of B220+ B-lineage cells. In contrast, the number of Mac-1+ myeloid cells remained within the normal range, despite a relative decrease in frequency (Figure 1a and b). The B-cell compartment in the bone marrow was further dissected based on surface expression of B220, CD43 and IgM. The Tlr4 mutation caused an expansion of the pro-B (B220+CD43+IgM−) and pre-B (B220+CD43−IgM−) populations, whereas the numbers of immature B (B220+CD43−IgM+) and mature B (B220hiCD43−IgM+) cells were comparable to that of C3H/HeN mice (Figure 1c and d). These results suggest that TLR4-mediated signals may have a modulatory effect on the development of early B-cell precursors.
Figure 1.
The TLR4 mutation is accompanied with an increase in pro-B and pre-B cells in the bone marrow. Bone marrow cells from C3H/HeN and TLR4 mutant C3H/HeJ mice were analyzed by flow cytometry following staining with antibodies against B220, Mac-1, CD43 and IgM. (a) Representative dot plots for B220 and Mac-1 staining. (b) The percentage of B220+ and Mac-1+ cells and their absolute numbers harvested from one femur and one tibia. The data are presented as the means±s.d. (n=7–13). (c) Representative dot plots for CD43, B220 and sIgM staining. The number indicates the percentage of the gated population. (d) The absolute numbers of pro-B (B220+CD43+IgM−), pre-B (B220+CD43−IgM−), immature B (B220+CD43−IgM+) and mature B (B220hiCD43−IgM+) cells harvested from one femur and one tibia. The data are presented as the means±s.d. (n=7–13). *P<0.05, **P<0.01, ***P<0.0001, NS: not significant. IgM, immunoglobulin M; TLR4, Toll-like receptor 4.
Inhibition of IL-7-driven proliferation of pro-B and large pre-B cells by LPS
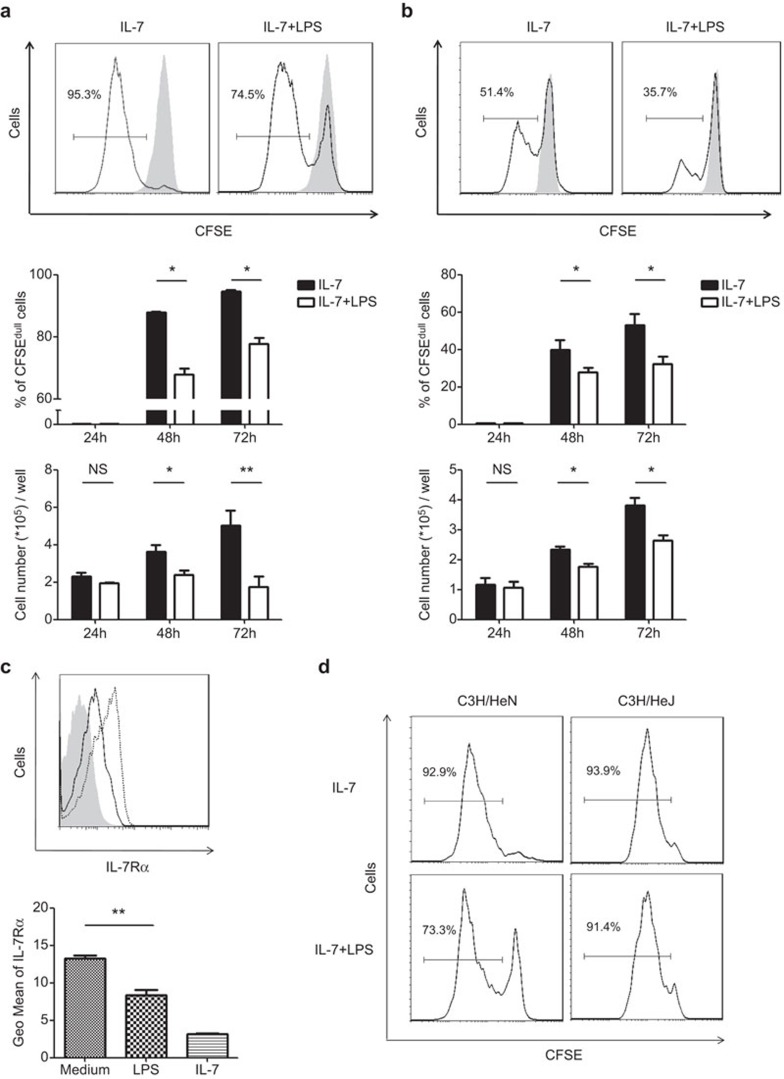
To inquire into the cellular mechanisms behind the expanded pro-B and pre-B-cell subsets in C3H/HeJ mice, we examined the potential impact of TLR4 signaling on the proliferative response of B-cell precursors to IL-7, a key cytokine for B lymphopoiesis.15 To this end, purified pro-B cells were cultured in medium containing IL-7 in the presence or absence of LPS, and cell proliferation was monitored by CFSE dilution. As expected, pro-B cells underwent robust proliferation upon IL-7 stimulation. In cultures with LPS, however, a significant fraction of cells were prevented from entry into the cell cycle, resulting in a marked decrease in the number of cells that were harvested at 48 and 72 h (Figure 2a). A similar inhibitory effect was also observed in the cultures of large pre-B cells (Figure 2b), which was another IL-7-responsive population of B-cell precursors.16 The reduced proliferative response prompted us to examine the expression of IL-7 receptors in LPS-treated cells. Indeed, culture with LPS alone caused a substantial reduction in the cell surface expression of IL-7Rα in pro-B cells (Figure 2c) and large pre-B cells (data not shown). Notably, an even more dramatic reduction was observed upon IL-7 stimulation that was most likely due to receptor internalization. To verify the contribution of TLR4, we monitored proliferation of pro-B cells in TLR4-mutant C3H/HeJ mice. While LPS significantly attenuated IL-7-driven proliferation of pro-B cells from C3H/HeN mice, the inhibitory effect was abolished in TLR4-mutant pro-B cells (Figure 2d).
Figure 2.
IL-7-driven proliferation of pro-B and large pre-B cells is suppressed by LPS. (a) Purified pro-B (B220+CD43+IgM−IgD−) cells from C57BL/6 mice were plated at a density of 2×105/well and cultured in the presence of IL-7 (10 ng/ml) with (10 µg/ml) or without LPS, and cell proliferation was monitored using the CFSE dilution assay. Representative histograms are shown for CSFE intensity before (shaded) and 72 h after (blank) culture in the top panel. The percentage of CFSEdull cells and the number of live cells that were recovered from different time points are shown in the middle and bottom panels, respectively. The data are presented as the means±s.d. from three independent experiments. (b) Similar analyses were performed using large pre-B cells (B220+CD43−IgM−IgD−FSChi). (c) Pro-B cells were cultured in medium alone (dotted line) or in the presence of IL-7 (shaded area) or LPS (solid line) for 72 h, and subsequently analyzed for IL-7Rα expression. IL-7Rα levels are expressed as the mean fluorescence intensity. Data are from three independent experiments. (d) Pro-B cells from C3H/HeN and C3H/HeJ mice were cultured for 72 h as described above, harvested and analyzed for CFSE fluorescence. The histogram plots show the results of a representative experiment of the 3 independent experiments. *P<0.05, **P<0.01, NS: not significant. CFSE, carboxy fluorescein diacetate succinimide ester; FSC, forward scattering; IgD, immunoglobulin D; IgM, immunoglobulin M; IL-7R, interleukin-7 receptor; LPS, lipopolysaccharide.
Synergism of LPS and IL-7 signals in the promotion of pre-B-cell differentiation
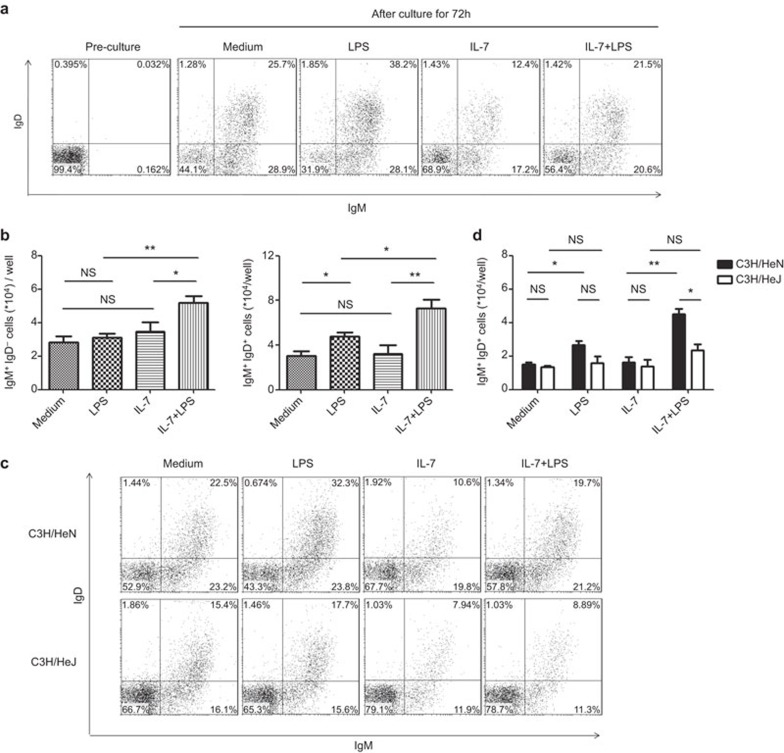
In contrast to its well-characterized mitogenic effect, the role of IL-7 in the differentiation of pro-B and pre-B cells is highly debated. On the one hand, IL-7 was reported to inhibit their differentiation into IgM+ immature B cells because the latter was markedly increased upon withdrawal of IL-7 from cultures. On the other hand, there is evidence that IgM+ B cells could be generated in the presence of IL-7 and that the increased representation of these cells upon IL-7 withdrawal was simply the result of reduced precursor cells in the culture.17 We revisited this issue in the context of potential interactions of LPS and IL-7 signals. Purified pre-B (B220+CD43−IgM−IgD−) cells were cultured for 72 h in medium alone or in the presence of IL-7 (10 ng/ml), LPS (10 µg/ml) or both and subsequently stained for surface expression of IgM and IgD. As reported by Hayashi et al.,14 the generation of IgM+ cells, especially IgM+IgD+ cells, was enhanced by the addition of LPS. When cultured with IL-7, 70% of the cells remained IgM−IgD− at the end of the culture, with a concomitant reduction in the percentage of IgM+IgD− and IgM+IgD+ cells (Figure 3A). However, the absolute number of IgM+IgD− or IgM+IgD+ cells was comparable to that in medium alone if the approximately twofold increase in total cellularity in the IL-7 culture were taken into consideration (Figure 3b). This result supports that idea IL-7 does not necessarily inhibit pre-B-cell differentiation, but induces a preferential expansion of IL-7-responsive, large pre-B cells. Most importantly, addition of LPS into IL-7 cultures resulted in a significant increase in the absolute number of IgM+IgD− or IgM+IgD+ cells (Figure 3b). A similar trend was observed when additional maturation markers, such as CD23 and CD21, were analyzed (data not shown). Therefore, LPS and IL-7 seem to have a synergistic effect on the further differentiation of pre-B cells, which stands in contrast to the inhibitory effect of LPS on IL-7-driven proliferation of B-cell precursors.
Figure 3.
LPS promotes differentiation of pre-B cells in IL-7 culture. (a) Purified pre-B (B220+CD43−IgM−IgD−) cells were cultured at a density of 2×105/well for 72 h in medium alone or in the presence of IL-7 (10 ng/ml), LPS (10 µg/ml) or both. Cells were then stained for surface expression of IgM and IgD. Dot plots show the staining profile of a representative experiment of four independent experiments. The numbers indicates the percentage of cells in each quadrant. (b) The number of IgM+IgD− and IgM+IgD+ cells generated in the cultures. (c) Similar experiments were performed with pre-B cells from C3H/HeN and C3H/HeJ mice. Dot plots show the results of a representative experiment of three independent experiments. (d) The number of IgM+IgD+ cells in cultures. *P<0.05, **P<0.01, NS: not significant. IgD, immunoglobulin D; IgM, immunoglobulin M; LPS, lipopolysaccharide.
We also tested the effect of LPS on pre-B cells derived from C3H/HeJ mice. LPS-induced acceleration of pre-B-cell maturation was no longer observed in the absence of a functional TLR4 (Figure 3c and d), further validating the implication of signaling through TLR4.
No preferential expansion or survival of IgM+ cells in the pre-B-cell culture with LPS
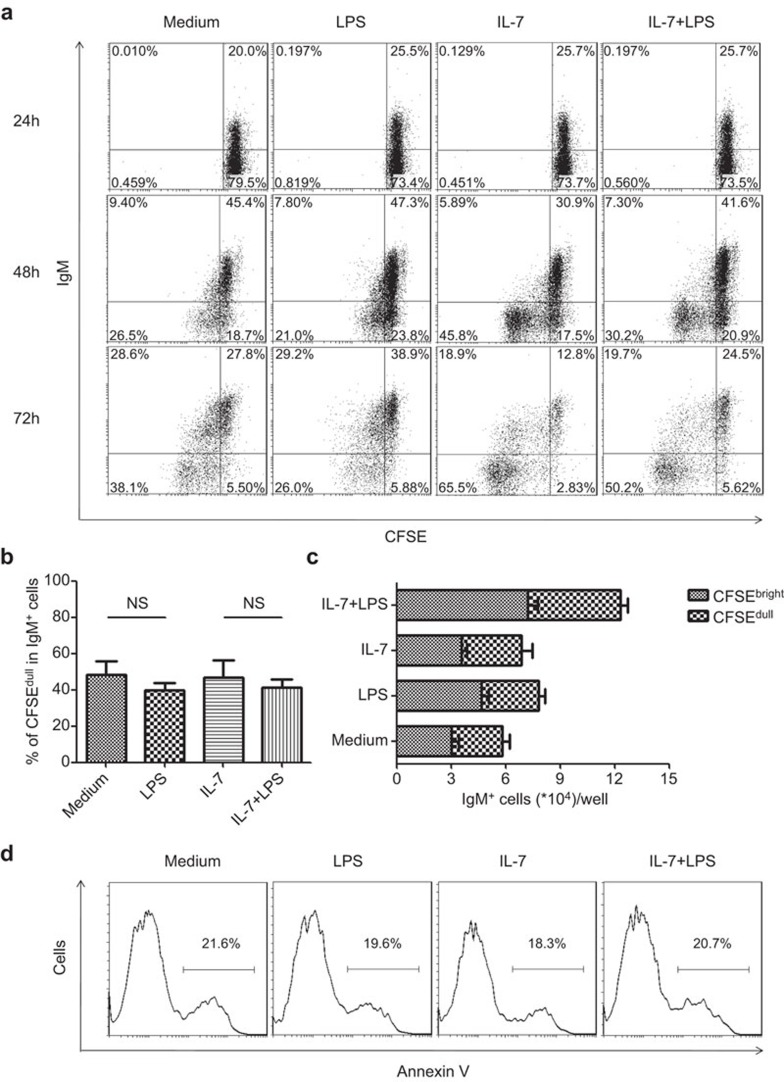
Theoretically, the increase of IgM+ cells in the pre-B-cell culture could be due to either accelerated developmental progression or preferential survival/proliferation of newly formed IgM+ B cells. To distinguish between these two possibilities, we used CFSE dilution to follow the proliferation of IgM+ cells and Annexin V staining to monitor cell apoptosis under various conditions. Virtually all IgM+ cells arising from the pre-B-cell culture appeared as a single, non-dividing population until 48 h, whereas IgM− B cells, especially those in the IL-7 and IL-7+LPS cultures, underwent active division (Figure 4a). At 72 h after culture, a distinct population of IgM+CFSEdull cells emerged in the IL-7 and IL-7+LPS cultures. Such kinetics suggests that these cells were more likely to be derived from the maturation of divided IgM− precursors rather than by division of preformed IgM+ cells. Notably, although the number of IgM+ cells was markedly increased in the IL-7+LPS culture, the percentage of CFSEdull cells in the IgM+ cells was comparable to that in IL-7 culture without LPS (Figure 4b), further arguing against the possibility of preferential expansion of IgM+ cells after LPS stimulation. Concerning cell survival, similar percentages of Annexin V+ cells were observed in all culture conditions (Figure 4d). Taken together, these data support the idea that accumulation of IgM+ cells is the consequence of the accelerated maturation of precursor cells instead of the enhanced proliferation or survival of their progeny.
Figure 4.
The increase of IgM+ cells in LPS-treated pre-B-cell cultures is not due to their preferential expansion or survival. (a) CFSE-labeled pre-B cells from C57BL/6 mice were cultured in medium alone or in the presence of IL-7 (10 ng/ml), LPS (10 µg/ml) or both. Cells were then harvested at different time points and monitored for surface IgM expression and CFSE dilution. Dot plots show the results of a representative experiment of four independent experiments. (b) The percentage of the CFSEdull subset among IgM+ cells in cultures at 72 h. (c) The absolute number of CFSEbrightIgM+ and CFSEdullIgM+ cells in cultures at 72 h. (d) Pre-B cells were cultured as described above. Annexin V staining was performed at 72 h. Similar results were obtained in four independent experiments. Representative histograms are shown for one experiment. The number denotes the percentage of Annexin V+ cells. CFSE, carboxy fluorescein diacetate succinimide ester; IgM, immunoglobulin M; LPS, lipopolysaccharide.
Enhanced maturation of adoptively transferred B-cell precursors in the bone marrow of LPS-treated mice
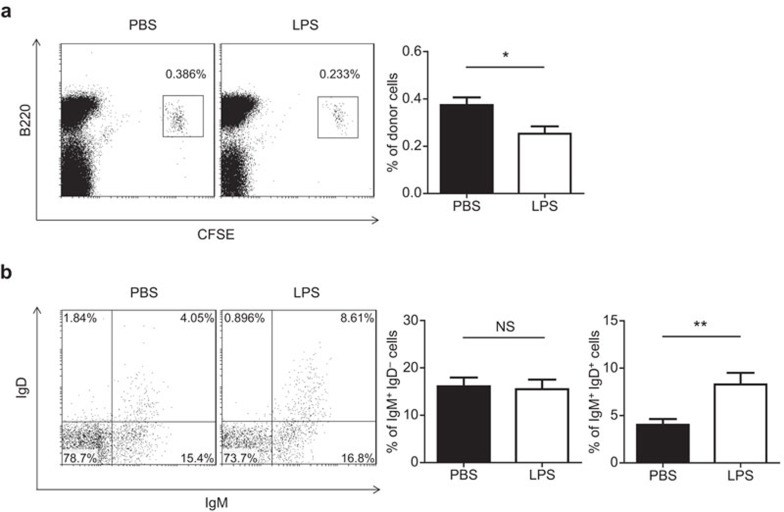
We then examined whether LPS had similar effects on the differentiation of B-cell precursors in vivo using an adoptive transfer assay. B220+IgM−IgD− B cells were sorted from C3H/HeN mice, labeled with CFSE and intravenously injected into TLR4-mutant, C3H/HeJ mice. The recipient mice received intraperitoneal injection of LPS immediately afterwards, and the donor cells were retrieved 18 h post-injection. This method allowed us to test whether any impact on B-cell development was due to direct action on developing B cells. As shown in Figure 5a, the percentage of CFSE-labeled donor cells in the bone marrow of LPS-treated recipients was only about half of that in the PBS-treated controls 18 h after LPS administration. This decrease could result from impaired homing, increased cell death or reduced proliferation of donor cells. Given the data that were obtained in in vitro studies, we favor the explanation of suppressed cell proliferation. Despite a decrease in absolute number, the donor cells that were recovered from LPS-treated mice contained a much higher percentage of IgM+IgD+ cells than those from the control mice (Figure 5b), rendering further support for a potential role of LPS in the maturation of pre-B cells.
Figure 5.
LPS accelerates the maturation of adoptively transferred B-cell precursors in vivo. Six million CSFE-labeled, B220+IgM−IgD− bone marrow cells from C3H/HeN mice were adoptively administered into C3H/HeJ mice through intravenous injection. Immediately afterwards, LPS (2.5 µg/g weight) or PBS was administered immediately afterwards. Bone marrow cells were harvested 18 h after transfer. (a) The percentage of donor cells in the bone marrow. (b) Donor cells were analyzed for surface IgM and IgD expression. The percentage of IgM+IgD− and IgM+IgD+ cells are shown as the mean±s.d. from five recipients. *P<0.05, **P<0.01, NS: not significant. CFSE, carboxy fluorescein diacetate succinimide ester; IgD, immunoglobulin D; IgM, immunoglobulin M; LPS, lipopolysaccharide; PBS, phosphate-buffered saline.
Discussion
B-cell development in the adult bone marrow is a well-defined process that is regulated by the coordinated action of cytokine signaling, pre-BCR/BCR signaling, and transcriptional activation/suppression.1,20,21 Signal transduction initiated by IL-7 and its downstream effectors is essential for the expansion and differentiation of B-cell precursors.22,23 In this study, we present evidence that LPS-induced signals via TLR4 crosstalk with IL-7 signals have a profound influence on early B-cell development.
LPS is known as a potent activator of murine mature B cells because it induces massive cell proliferation and antibody production by triggering TLR4 signaling.24 Recent studies by Hayashi et al. indicate that TLR4 signaling also plays a role during late stages of B-cell development by facilitating the generation of immature or transitional B cells.14 The present study further demonstrates that early B-cell development is subject to regulation by TLR4 signaling. The pro-B- and pre-B-cell population was found to be enlarged in the bone marrow of TLR4-mutant, C3H/HeJ mice, indicating a physiological role of TLR4 in early B lymphopoiesis. In addition to the best-known ligand LPS, TLR4 can be activated by endogenous ligands, such as the extracellular matrix component heparan sulfate.25 Developing B cells are positioned in specific cellular niches in the bone marrow that supply the microenvironment that is essential for their differentiation.26 Endogenous ligands of TLR4 can be a part of the network modulating B lymphopoiesis. Given the multiple functions of IL-7 during early B-cell development, regulation of IL-7-induced signals is critical. A large number of studies have illustrated that IL-7R signaling is modified at various stages along the B-cell developmental pathway.27,28 Proinflammatory cytokines such as interferon-γ, for example, are shown to inhibit IL-7-driven proliferation of pre-B cells and induce cell death.29,30 In vitro data in this study demonstrated that TLR4 signals limited IL-7-dependent proliferation of pro-B and large pre-B cells, which was consistent with the phenotype that was observed in C3H/HeJ mice. This inhibitory effect is at least partly attributable to the downregulation of IL-7Rα expression by LPS.
Withdrawal of IL-7 has been reported to induce recombination activating gene expression, light chain rearrangement and emergence of IgM+ cells during in vitro culture,18,31,32 which leads to the speculation that IL-7 inhibits the developmental progression of B-cell precursors by keeping them in a proliferative state. The study by Paige and colleagues,17 however, shows that surface IgM+ cells can be effectively generated in IL-7 cultures. Consistent with this observation, we found that pre-B-cell cultures gave rise to virtually equal numbers of IgM+ or more advanced IgD+ cells in the presence or absence of IL-7, although their relative frequencies were lower in the presence of IL-7, possibly due to selective expansion of IL-7-responsive IgM−IgD− cells. Notably, co-stimulation with IL-7 and LPS markedly enhanced the generation of IgM+ and IgD+ cells in pre-B-cell cultures, suggesting a crosstalk between TLR4 and IL-7 signals. This is reminiscent of the synergism between pre-BCR and IL-7 signals that was revealed in previous studies.16,33 Although signal transduction downstream of pre-BCR remains to be fully characterized, similar signaling complexes are formed following the engagement of BCR and pre-BCR.34,35,36 Because TLR4 signaling is shown to synergize with BCR-delivered signals during the activation of mature B cells,37,38 it would not be surprising if the TLR4 signaling pathway also interacts with pre-BCR signaling during pre-B-cell development. Thus, signals mediated by IL-7R, pre-BCR and TLR4 may converge to modulate pre-B-cell maturation.
LPS-induced, systematic inflammation is often associated with rapid contraction of the pro-B- and pre-B-cell populations; however, the mechanism behind this is not clearly defined. The results from the present study suggest that suppressed proliferation of pro-B/pre-B cells and accelerated progression of these cells to more mature cells may contribute to LPS-induced reduction in pro-B and pre-B cells. Thus, while pathogen-mediated stimulation of preferential myeloid differentiation pathways may provide a means for the rapid replenishment of the innate immune system, the inhibition of proliferation and promotion of maturation of B-cell precursors may provide another way for instant supplementation of the adaptive immune response during infection.
Altogether, the current study has demonstrated that TLR4 signals are implicated in regulating the proliferation and differentiation of pro-B and pre-B cells. LPS limits IL-7-dependent proliferation of pro-B and large pre-B cells and synergizes with IL-7 signals to promote pre-B-cell maturation in vitro and in vivo. Further studies are warranted to elucidate the detailed molecular mechanisms underlying the crosstalk between TLR4 and IL-7 signals.
Acknowledgments
We thank Xiaoping Qian and Xuewen Pang for excellent technical assistance. We also thank Yanhui Yin and Qing Ge for valuable discussions and critical comments. This work was supported by grants from the National Basic Research Program of China (2011CB946100), National Natural Sciences Foundation of China (30830091 to YZ and 30960357 to RL) and the 111 Project of China (B07001).
The authors have no financial conflicts of interest.
References
- Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose–response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- Wang YH, Stephan RP, Scheffold A, Kunkel D, Karasuyama H, Radbruch A, et al. Differential surrogate light chain expression governs B-cell differentiation. Blood. 2002;99:2459–2467. doi: 10.1182/blood.v99.7.2459. [DOI] [PubMed] [Google Scholar]
- Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol. 2013;10:103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, et al. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi EA, Granato A, Paiva LS, Bertho AL, Bellio M, Nobrega A. TLR4 promotes B cell maturation: independence and cooperation with B lymphocyte-activating factor. J Immunol. 2010;184:4662–4672. doi: 10.4049/jimmunol.0903253. [DOI] [PubMed] [Google Scholar]
- Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18:20–30. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Ochiai K, Maienschein-Cline M, Mandal M, Triggs JR, Bertolino E, Sciammas R, et al. A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nat Immunol. 2012;13:300–307. doi: 10.1038/ni.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne CD, Fleming HE, Paige CJ. IL-7 does not prevent pro-B/pre-B cell maturation to the immature/sIgM+ stage. Eur J Immunol. 2004;34:2647–2655. doi: 10.1002/eji.200425400. [DOI] [PubMed] [Google Scholar]
- Melamed D, Kench JA, Grabstein K, Rolink A, Nemazee D. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J Immunol. 1997;159:1233–1239. [PubMed] [Google Scholar]
- Kato I, Miyazaki T, Nakamura T, Kudo A. Inducible differentiation and apoptosis of the pre-B cell receptor-positive pre-B cell line. Int Immunol. 2000;12:325–334. doi: 10.1093/intimm/12.3.325. [DOI] [PubMed] [Google Scholar]
- Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- Johnson SE, Shah N, Panoskaltsis-Mortari A, LeBien TW. Murine and human IL-7 activate STAT5 and induce proliferation of normal human pro-B cells. J Immunol. 2005;175:7325–7331. doi: 10.4049/jimmunol.175.11.7325. [DOI] [PubMed] [Google Scholar]
- Malin S, McManus S, Cobaleda C, Novatchkova M, Delogu A, Bouillet P, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A, Gronowicz E, Bullock WW, Moller G. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J Exp Med. 1974;139:74–92. doi: 10.1084/jem.139.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, Chao NJ, et al. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012;120:2899–2908. doi: 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Corfe SA, Rottapel R, Paige CJ. Modulation of IL-7 thresholds by SOCS proteins in developing B lineage cells. J Immunol. 2011;187:3499–3510. doi: 10.4049/jimmunol.1100424. [DOI] [PubMed] [Google Scholar]
- Johnson K, Hashimshony T, Sawai CM, Pongubala JM, Skok JA, Aifantis I, et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28:335–345. doi: 10.1016/j.immuni.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995;3:475–484. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Garvy BA, Riley RL. IFN-gamma abrogates IL-7-dependent proliferation in pre-B cells, coinciding with onset of apoptosis. Immunology. 1994;81:381–388. [PMC free article] [PubMed] [Google Scholar]
- Grawunder U, Haasner D, Melchers F, Rolink A. Rearrangement and expression of kappa light chain genes can occur without mu heavy chain expression during differentiation of pre-B cells. Int Immunol. 1993;5:1609–1618. doi: 10.1093/intimm/5.12.1609. [DOI] [PubMed] [Google Scholar]
- Rolink A, Grawunder U, Haasner D, Strasser A, Melchers F. Immature surface Ig+ B cells can continue to rearrange kappa and lambda L chain gene loci. J Exp Med. 1993;178:1263–1270. doi: 10.1084/jem.178.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson L, Licence S, Gaspal F, Lane P, Corcoran AE, Martensson IL. Both the pre-BCR and the IL-7Ralpha are essential for expansion at the pre-BII cell stage in vivo. . Eur J Immunol. 2005;35:1969–1976. doi: 10.1002/eji.200425821. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Sanjo H, Pages G, Kawano Y, Karasuyama H, Pouyssegur J, et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity. 2008;28:499–508. doi: 10.1016/j.immuni.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Tretter T, Ross AE, Dordai DI, Desiderio S. Mimicry of pre-B cell receptor signaling by activation of the tyrosine kinase Blk. J Exp Med. 2003;198:1863–1873. doi: 10.1084/jem.20030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal 20103a60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, et al. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38:2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]