Abstract
We have shown that Hsp60sp-loaded immature dendritic cells (DC/sp) can protect mice from the induction of experimental allergic encephalomyelitis (EAE) by inducing Qa-1-restricted CD8+ T regulatory (Treg) cells. The binding half-life between Qa-1 and Hsp60sp is particularly short and leads to an unstable Qa-1/peptide complex that significantly decreases the efficacy of this vaccination. To prevent Qa-1/Hsp60sp complex dissociation, we utilized paraformaldehyde (PFA) fixation to stabilize the formation of the Qa-1/Hsp60sp complex and maximize the function of DC/sp as a vaccine to control autoimmune diseases. Compared with the non-fixed DC/sp, the fixed DC/sp (FDC/sp) showed an enhanced ability to activate Qa-1-restricted Hsp60sp-specific CD8+T cells in vitro and prevented EAE in vivo. Importantly, the FDC/sp maintained immune activity following cryopreservation for 1 week or after storage for 72 h at 4 °C. These results indicate that PFA fixation can sustain or increase the efficacy of DC/sp by improving the stability of the Qa-1/Hsp60sp complex on the surface of the DC/sp. In addition, PFA fixation creates a time window for DC/sp storage, transport and application. Our data suggest a potential clinical use of FDC/sp as a vaccine for the prevention and treatment of autoimmune disease.
Keywords: autoimmune disease, dendritic cells, Hsp60sp, PFA fixation, Qa-1-restricted CD8+ T cells
Introduction
Hsp60sp is one of the Qa-1/HLA-E binding peptides.1,2 Our previous studies have shown that Hsp60sp-loaded immature dendritic cells (DC/sp) can induce Qa-1-restricted CD8+ T regulatory (Treg) cells that protect mice from the induction of experimental allergic encephalomyelitis (EAE).3,4 We have also shown that the defect of HLA-E-restricted CD8+ T cells in type 1 diabetes patients could be corrected by activating CD8+ T cells with autologous DCs isolated from type 1 diabetes patients and then loaded with Hsp60sp.5 These studies indicated a potential new approach for the treatment and prevention of autoimmune diseases by DC/sp vaccination. However, the short half-life of the interaction between Qa-1 and its binding peptide6 inevitably led to an unstable Qa-1/peptide complex expressed on the membrane of the DC/sp. Stable expression of the Qa-1/peptide complex is critical for the function of DC/sp vaccinations in controlling autoimmune diseases. Thus, maintaining Qa-1/Hsp60sp complex on the surface of the DCs becomes a crucial step for using DC/sp to activate the Qa-1/HLA-E-restricted CD8+ T cells in vivo. Therefore, we hypothesized that a more effective activation of Qa-1-restricted CD8+ T cells could be achieved by fixing the Hsp60sp-loaded immature DCs.
In this study, we have utilized PFA fixation to form protein–protein crosslinks and stabilize the formation of Qa-1/Hsp60sp complexes on DC/sp. We then evaluated the efficacy of PFA-fixed Hsp60sp-loaded immature dendritic cells (FDC/sp) as a vaccine for preventing EAE induction in mice.
Materials and methods
Mice
C57BL/6 (B6) mice aged 6–8 weeks were purchased from the Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). GFP-Tg mice of the same B6 background were purchased from the Shanghai Research Center for Model Organisms (Shanghai, China). Qa-1 knockout (KO) mice were a generous gift from Dr Harvey Cantor at Harvard Medical School. The mice were maintained under pathogen-free conditions. All animal experiments were approved by the institutional medical research ethics committee of Shanghai Jiao Tong University School of Medicine.
Reagents
The Hsp60sp peptide (QMRPVSRVL), MOG35–55 peptide (MEVGWYRSPFSRVVHIYRNGK) and Qdm peptide (AMAPRTLLL) were synthesized by GL Biochem Ltd (Shanghai, China). All of the peptides were >98% pure. Mouse rGM-CSF and rIL-4 were purchased from Peprotech (Rocky Hill, NJ, USA). Heat-inactivated Mycobacterium tuberculosis H37Ra and incomplete Freund's adjuvant were purchased from BD Difco (Corpus Christi, TX, USA). Pertussis toxin was purchased from List Biological Laboratories Inc. (Campbell, CA, USA). Paraformaldehyde (PFA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fluorescence-conjugated monoclonal anti-CD4, anti-CD8, anti CD11c, anti-CD40, anti-CD86, anti-Qa-1b and anti-IAb antibodies were purchased from eBioscience (San Diego, CA, USA). The mouse CD8a (Ly-2) microBeads were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
BWR cell line
The BWR cell line expressing red fluorescent proteins was generated using the lentiviral plasmid vector pLV-CXIN-DRE2 generated by cloning of the red fluorescent protein DsRed-Express27 into the plasmid pLV-CXIN. The lentiviral vectors were packaged in the 293T cell line (ATCC, CRL-11268) and used for transduction of the thymoma BW5147.G.1.4.OUAR (a kind gift from Laurel A Eckhardt, Hunter College and Graduate Center of City University of New York). Stable cell lines expressing DsRed-Express2 were selected with G418 (BioVision, Milpitas, CA, USA) and designated as BWR.
Bone marrow DCs culture
DCs were generated from B6 mouse bone marrow cells as previously described.3 Briefly, the bone marrow was isolated from femurs and red blood cells were lysed. The bone marrow cells were incubated with GM-CSF and IL-4 for 6 days. On day 3, the suspension cells were aspirated and fresh culture medium was added. The cells were collected for use on day 6, and more than 80% of cells were immature DCs with a phenotype of CD11c+, major histocompatibility complex (MHC) class IIintermediate and CD86low.
DC manipulation
DCs were cultured with either Hsp60sp or the control peptide Qdm at 150 µM for 2 h at 37 °C.3 The cells were then washed once with cold phosphate-buffered saline (PBS). To stabilize the Qa-1/peptide complex, the cells were fixed using 2% (w/v) paraformaldehyde in PBS for 10 min at 4 °C, and then washed three times with PBS before use.5 In experiments testing complex stability, fixed DCs were stored at 4 °C for 72 h in PBS or at −80 °C in freezing medium (90% foetal bovine serum, 10% dimethyl sulfoxide) for 1 week.
Flow cytometric analysis
Fluorescence-conjugated antibodies were used for staining cell surface molecules according to the manufacturer's instructions. Analyses were performed with a FACSCalibur (BD Biosciences, San Jose, CA, USA) and FlowJo software (Treestar, Ashland, OR, USA).
Localization of DCs
To locate the transferred DCs in peripheral lymphoid tissues, DCs from GFP-Tg mice with or without fixation were injected intravenously into wild-type (WT) naive mice. After 3 h, the spleen and draining lymph nodes were collected and dissociated into single-cell suspensions. GFP + cells were counted by fluorescence-activated cell sorting (FACS).
Induction of Hsp60sp-specific CD8+ T cell in vitro
Spleen cells (SPCs) from FDC/sp- or DC/sp-immunized mice (WT- or Qa-1-KO) were re-stimulated with FDC/sp or DC/sp in six-well plates with culture medium containing IL-2 and IL-15. On day 6, CD8+ T cells were purified by MACS according to the manufacturer's instructions and used as Hsp60sp-specific CD8+ T-cell lines.
CD8+ T-cell functional assay
CD8+ T cells induced by FDC/sp and DC/sp in vitro were tested for their ability to specifically kill cells expressing the target structure Qa-1/Hsp60sp. Naive GFP-Tg B6 SPCs were used as target cells and loaded with Hsp60sp or control peptide Qdm at 150 µM for 2 h at 37 °C in complete RPMI-1640 medium. CD8+ T cells and target SPCs were cocultured in 96-well round-bottom plates with an effector/target ratio ranging from 0 to 1∶1 at 37 °C with 5% CO2 for 20 h. The corresponding peptides were present at 150 µM throughout the culture process. At the end of the incubation, an equal number of red fluorescence-expressing BWR cells were added to each well as an internal reference for FACS analysis. Hsp60sp-specific killing was evaluated by comparing the ratio of the ‘green' target cells and ‘red' internal reference cells between wells with graded numbers of CD8+ T cells and control wells without the CD8+ T cells. The percentage of specific killing was calculated as: ((the ratio of target/internal cells in control cultures (without CD8+ T cells)−the ratio in experimental cultures (with CD8+ T cells))/the ratio in control cultures)×100%.3,5 Samples were analyzed using a FACSCalibur flow cytometer and FlowJo software.
EAE induction and assessment
Six- to eight-week-old male B6 mice were injected s.c. in the back with 50 µg of MOG35–55 peptide emulsified in incomplete Freund's adjuvant supplemented with heat-inactivated Mycobacterium tuberculosis H37RA. Pertussis toxin (200 ng per mouse) dissolved in PBS was administered i.v. on the day of immunization and 48 h later. The disease symptoms were assessed daily according to the following scoring criteria: 0, no clinical signs; 1, flaccid tail; 2, hind limb weakness; 3, complete hind limb paralysis of both legs; 4, paraplegia with forelimb weakness or paralysis; and 5, moribund or dead.8
Vaccinating mice with peptide-loaded DCs
Freshly prepared FDC/sp and DC/sp were administered immediately to naïve B6 mice intravenously at 3×106 cells in 200 µl PBS/mouse 1 week before EAE induction. Fixed Qdm-loaded immature dendritic cells (FDC/Qdm) were used as controls. The operating time is crucial for DC/sp vaccination due to the instability of the Qa-1/Hsp60sp complex present on the DCs surface. Therefore, the procedure time must be as short as possible.
Statistical analysis
The data are shown as the mean±s.d. One-way ANOVA was used to compare the clinical score of EAE, and the Chi-squared test was performed to compare disease incidence. A P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS 12.0 software.
Results
PFA fixation does not alter the immunophenotype of DC/sp
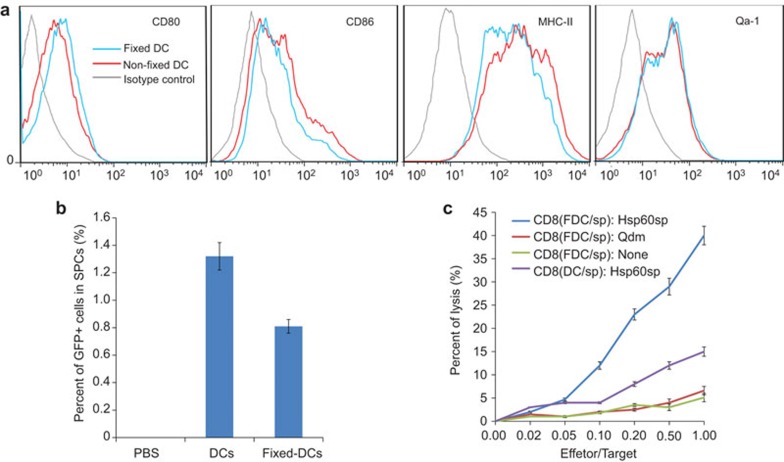
Maintaining a specific immunophenotype is critical for the function of DC/sp. To evaluate whether fixation alters the molecular/cellular characteristics of DC/sp, day 6 DC/sp were collected for testing. The expression of Qa-1, CD80, CD86 and major histocompatibility complex class II molecules was assessed by cell-surface staining (Figure 1a). There were no significant differences between the surface expression of the above molecules on FDC/sp and non-fixed DC/sp. These results indicate that fixation did not change the immunophenotype of the DC/sp.
Figure 1.
PFA fixation does not alter the phenotype or biological activity of DC/sp. (a) FDC/sp and DC/sp were assayed and compared for the surface expression of I-Ab (MHC-II), CD80, CD86 and Qa-1b by FACS. (b) DC/sp from GFP-Tg mice with/without fixation were injected into WT naive mice via the tail vein. Three hours later, GFP+ cells in the spleen were counted using a flow cytometer (n=3 mice/group). (c) CD8+ T cells activated by FDC/sp or DC/sp were tested and compared for their ability to specifically lyse target cells loaded with Hsp60sp. Target cells loaded with no peptide or Qdm peptide served as control. The data are representative of three independent experiments. DC/sp, Hsp60sp-loaded immature dendritic cells; FACS, fluorescence-activated cell sorting; FDC/sp, fixed Hsp60sp-loaded immature dendritic cells; MHC, major histocompatibility complex; PFA, paraformaldehyde; WT, wild-type.
PFA-fixed DC/sp reach lymphoid organs
It is known that DC can present antigens to T cells in the lymphoid organs. To determine whether FDC/sp can be transferred and migrate to lymphoid organs, PFA-fixed DC/sp from GFP-Tg mice were injected intravenously and unfixed DC/sp were injected as a control. After 3 h, the spleen and lymph nodes were harvested and tested for GFP + cells using a flow cytometer (Figure 1b). SPCs collected from mice that were treated with non-fixed DC/sp comprised 1.32%±0.11% GFP+ cells, and SPCs collected from mice administered fixed DCs comprised 0.81%±0.05% GFP+ cells. GFP+ cells were not found in the lymph nodes in either group. Our results indicated that despite being present in smaller numbers than the non-fixed DC/sp, the FDC/sp could still migrate to the mouse spleen. In the spleen, FDC/sp could activate the Hsp60sp-specific CD8+ Treg cells and control autoimmunity in vivo.
Induction of Hsp60sp-specific CD8+ T in vitro by the FDC/sp
To investigate the function of the FDC/sp, Hsp60sp-specific CD8+ T-cell lines were established by coculturing SPCs from FDC/sp- or DC/sp-immunized mice in vitro with FDC/sp or DC/sp, respectively, for 7 days. Purified CD8+ T cells were then tested for their ability to specifically kill cells expressing the target Qa-1/Hsp60sp complex. In the killing assay, naive GFP-Tg B6 SPCs loaded with Hsp60sp were used as target cells and GFP-Tg B6 SPCs loaded with Qdm served as controls. Red fluorescent BWR cells served as internal reference cells for FACS analysis. As shown in Figure 1c, CD8+ T cells stimulated by the FDC/sp more effectively lysed target cells than the DC/sp-induced CD8+ T cells. This result indicated that the PFA fixation significantly enhanced their ability to activate the Hsp60sp-specific CD8+ Treg cells. Furthermore, FDC/sp-induced CD8+ T cells only lysed target cells loaded with Hsp60sp and not cells loaded with control peptides. This observation confirmed that PFA fixation did not alter the capacity of the DC/sp to induce the Qa-1-restricted CD8+ T cells that specifically recognize the Qa-1/Hsp60sp complex expressed on target cells. Furthermore, CD8+ T-cell lines generated by the same procedure from Qa-1 deficient mice did not show obvious target cell inhibition compared with the Hsp60sp-specific CD8+ T-cell lines generated from the WT mice. This result confirms that the cell killing observed is Qa-1-restricted (Supplementary Figure 1 and Supplementary Table 1). Collectively, these results indicated that FDC/sp could be used in vivo to more effectively control autoimmune diseases.
FDC/sp pre-treatment and disease control
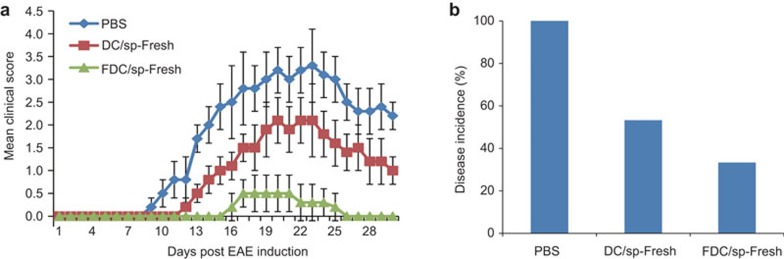
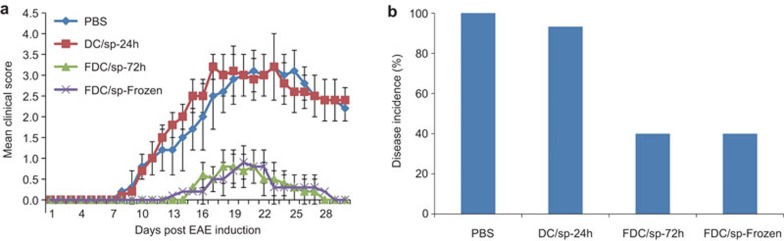
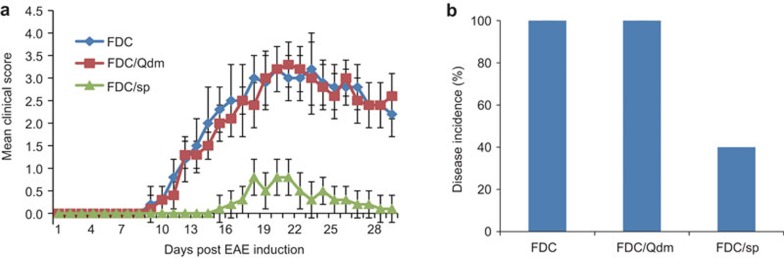
To test the in vivo efficacy of the FDC/sp vaccination in control of EAE, freshly prepared FDC/sp and DC/sp were injected intravenously into naive B6 mice at 3×106 cells/mouse 1 week before EAE induction. As shown in Figure 2, although the DC/sp were able to reduce the severity of EAE in mice, the FDC/sp showed an enhanced capacity to protect the mice from the development of EAE. To evaluate the stability of the FDC/sp, B6 mice were also injected with FDC/sp stored at 4 °C for 72 h or cells stored at −80 °C for 1 week before injection. Both the 72 h-stored and cryopreserved FDC/sp showed a strong capacity to prevent the development of EAE (Figure 3). In contrast, pre-treatment of mice with 24 h-stored non-fixed DC/sp did not provide any protection from EAE (Figure 3). These results are consistent with the in vitro observations and confirmed that PFA fixation could significantly enhance their ability to activate the Hsp60sp-specific CD8+ Treg cells. We hypothesize the effect is mediated by improving the stability of the Qa-1/Hsp60sp complex expressed on DC/sp. Importantly, the protection was FDC/sp-specific because FDC/Qdm or FDC vaccination did not have any protective effects on EAE induction (Figure 4).
Figure 2.
Compared with freshly prepared DC/sp, FDC/sp more effectively protect B6 mice from EAE when used as a vaccine in mice. Fresh FDC/sp and DC/sp were injected into naive B6 mice immediately after preparation. Mice were injected intravenously with 3×106 cells per mouse 1 week before EAE induction as described in the section on ‘Materials and methods'. The clinical scores and incidence of disease were compared. The clinical scores for the FDC/sp and DC/sp groups were significantly different, P<0.01. The disease incidence in the FDC/sp and DC/sp groups were significantly different, P<0.05. The data are representative of three independent experiments with five mice/group/experiment. DC/sp, Hsp60sp-loaded immature dendritic cells; EAE, experimental allergic encephalomyelitis; FDC/sp, fixed Hsp60sp-loaded immature dendritic cells.
Figure 3.
Long-term storage of FDC/sp did not impair the capacity to prevent EAE when used as a vaccine. One week before EAE induction, B6 mice were injected with FDC/sp stored at 4 °C for 72 h in PBS or stored at −80 °C in freezing medium (90% FBS, 10% DMSO) for 1 week before injection, as described in the section on ‘Materials and methods'. The clinical scores of the FDC/sp-72 h or cryopreserved FDC/sp vs. DC/sp-24 h were significantly different, P<0.01. The disease incidences for the FDC/sp-72 h or cryopreserved FDC/sp groups vs. DC/sp-24 h were significantly different, P<0.01. The data are representative of three independent experiments with five mice/group/experiment. DC/sp, Hsp60sp-loaded immature dendritic cells; DMSO, dimethyl sulfoxide; EAE, experimental allergic encephalomyelitis; FBS, foetal bovine serum; FDC/sp, fixed Hsp60sp-loaded immature dendritic cells; PBS, phosphate-buffered saline.
Figure 4.
PFA fixation does not affect the biological function or antigen specificity of the FDC/sp. FDC/sp and FDC/Qdm were administered to naive B6 mice intravenously 1 week before EAE was induced as described in the section on ‘Materials and methods'. The clinical scores and incidence of disease were compared. The clinical scores of group FDC/sp group vs. FDC/Qdm group were significantly different, P<0.01. The disease incidences of the FDC/sp group vs. FDC/Qdm group were significantly different, P<0.01. The data are representative of three independent experiments with five mice/group/experiment. DC/sp, Hsp60sp-loaded immature dendritic cells; EAE, experimental allergic encephalomyelitis; FDC/Qdm, fixed Qdm-loaded immature dendritic cells; FDC/sp, fixed Hsp60sp-loaded immature dendritic cells; PFA, paraformaldehyde.
Discussion
In this study, we compared the efficacy of FDC/sp with that of non-fixed DC/sp as a vaccine to protect mice from EAE by activating Qa-1-restricted and Hsp60sp-specific CD8+ T cells. Our previous studies have shown that vaccinating animals with DCs loaded with a self-peptide Hsp60sp can activate Qa-1-restricted CD8+ T cells and provide protection from EAE.3 The Qa-1-restricted CD8+ Treg cells could specifically eliminate autoreactive T cells by recognizing the Qa-1/Hsp60sp complex preferentially expressed on the target cells.4,9 The DC/sp could also correct the defect of HLA-E-restricted CD8+ Treg cells in human type 1 diabetes patients when used to reactivate CD8+ T cells in vitro.10 These studies indicated a potential new approach to prevent and treat autoimmune diseases by DC/sp vaccination. However, the association between Qa-1 and its binding peptide is relatively weak, which results in an unstable Qa-1/peptide complex, even with its dominant peptide, Qdm.6 The short half-life of the association/dissociation between Qa-1 and its binding peptide6 inevitably led to an unstable Qa-1/peptide complex presented on the membrane of the DC/sp. The membrane stability is critical for the function of the DC/sp to activate Qa-1/HLA-E-restricted CD8+ T cells in vivo and control autoimmune diseases. This feature of the DC/sp could severely limit their capacity to be further studied and utilized as a potential new vaccine for the prevention and treatment of autoimmune diseases. Methods of stabilizing the surface expression of the complex of Hsp60sp associated with Qa-1/HLA-E molecules on DC/sp is an important issue that must be solved to allow continued studies of the potential clinical applications of the Qa-1/HLA-E-restricted CD8+ T cells.
PFA fixation by formation of protein–protein crosslinks is an economical and effective method that has been widely used in vaccines made with infectious agents.11,12,13 In addition, PFA fixation is known to stabilize cell surface molecules and prevent cell lysis and is used to treat FACS samples. Fixed cell samples can be stored for up to 1 week without affecting the flow cytometry results.14 Therefore, we chose to use the PFA fixation method to treat DC/sp in an attempt to stabilize the surface expression of the Hsp60sp complex associated with Qa-1/HLA-E molecules on DC/sp.
One of our major concerns was whether PFA fixation impaired the capacity of DCs to function as antigen-presenting cells and induce Qa-1-restricted CD8+ T cells in vivo. As shown in Figure 1, PFA fixation did not interfere with the expression of relevant molecules on the surface of FDC/sp (Figure 1a). In addition, although more than 90% of the cells are killed by PFA fixation, the remaining FDC/sp cells reached the spleen and retained biological function when injected i.v. (Figure 1b).
The ability of CD8+ T cells activated by FDC/sp to specifically kill target cells loaded with Hsp60sp was significantly increased compared with the CD8+ T cells activated by unfixed DC/sp (Figure 1c). These observations demonstrated that PFA fixation of DC/sp did not alter the phenotype or decrease the function of antigen presentation by DC/sp. PFA fixation of DC/sp significantly enhanced their ability to activate the Qa-1-restricted CD8+ T cells in vitro and increased target cell killing.
To investigate the function of FDC/sp in vivo, we compared the efficacy of FDC/sp with DC/sp in an EAE model. We transplanted FDC/sp and DC/sp into naive B6 mice via the tail vein 1 week before EAE induction. As shown in Figure 2, although DC/sp vaccination reduced the severity of EAE in mice, FDC/sp showed a significantly enhanced capacity to protect mice from the development of EAE. To evaluate the stability of FDC/sp, 1 week before EAE induction, B6 mice were also treated with FDC/sp stored at 4 °C for 72 h in PBS, or stored at −80 °C in freezing medium for 1 week. As shown in Figure 3, both cells stored for 72 h and cryopreserved FDC/sp retained the capacity to protect mice from EAE. Conversely, 24 h-stored, non-fixed DC/sp completely lost their protective ability. These results indicated that PFA fixation could maintain or enhance the capacity of the DC/sp to activate Qa-1-restricted CD8+ T cells. As shown in Figure 4, PFA fixation of DC/sp did not alter the specific protection from EAE. Additionally, only vaccination with FDC/sp and not FDC/Qdm or the unloaded FDC protected the animals from EAE.
In summary, our study demonstrated that PFA fixation of DC/sp could significantly improve the capacity of DC/sp to activate Qa-1-restricted CD8+ T cells to control autoimmune EAE disease when used as a vaccine. The enhanced function of FDC/sp induced by PFA fixation was likely caused by an increased stabilization of the Qa-1/Hsp60sp complex on DC/sp without altering the surface phenotype. Our approach has addressed the problem of poor complex stability on DC/sp used in basic vaccine research and has demonstrated a potential clinical use. Our approach could also be used in other DC-based vaccines for autoimmunity, cancer therapies and infectious diseases to improve the effect of DC-based vaccinations. In addition, PFA fixation creates a sufficient time period for storage and transport of DC/sp that can be applied in the clinical use of FDC/sp vaccinations.
Acknowledgments
This work has been supported by the National Natural Science Foundation of China (NSF-30830093) and National Key Program (973) for Basic Research of China (2009CB522409) to HJ.
Footnotes
Supplementary Information accompanies the paper on Cellular &Molecular Immunology's website.
Supplementary Information
References
- Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403–1414. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Kalb S, Liang B, Aldrich CJ, Lemonnier FA, Jiang H, et al. A peptide from heat shock protein 60 is the dominant peptide bound to Qa-1 in the absence of the MHC class Ia leader sequence peptide Qdm. J Immunol. 2003;170:5027–5033. doi: 10.4049/jimmunol.170.10.5027. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang L, Liang B, Saenger Y, Li J, Chess L, et al. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci USA. 2007;104:20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zheng Z, Jiang Y, Chess L, Jiang H. The specificity of T cell regulation that enables self-nonself discrimination in the periphery. Proc Natl Acad Sci USA. 2009;106:534–539. doi: 10.1073/pnas.0811843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen B, Song JH, Zhen T, Wang BY, Li X, et al. Eriocalyxin B ameliorates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells. Proc Natl Acad Sci USA. 2013;110:2258–2263. doi: 10.1073/pnas.1222426110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Kraft-Leavy JR, Dauner JG, Sullivan BA, Laur O, Jensen PE. The nonclassical MHC class I molecule Qa-1 forms unstable peptide complexes. J Immunol. 2004;172:1661–1669. doi: 10.4049/jimmunol.172.3.1661. [DOI] [PubMed] [Google Scholar]
- Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS ONE. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. The Qa-1 dependent CD8+ T cell mediated regulatory pathway. Cell Mol Immunol. 2005;2:161–167. [PubMed] [Google Scholar]
- Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, et al. HLA-E-restricted regulatory CD8+ T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010;120:3641–3650. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian A, Araujo L, Naidu NN, Place DJ, Choudhury B, Demetriou M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J Biol Chem. 2011;286:40133–40141. doi: 10.1074/jbc.M111.277814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, et al. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386–2393. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Liu Y, Peng H, Beers R, Racke MK, Lovett-Racke AE. Comparison of a classical Th1 bacteria versus a Th17 bacteria as adjuvant in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2011;237:33–38. doi: 10.1016/j.jneuroim.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Nagarkatti PS, Nagarkatti M. CD44 reciprocally regulates the differentiation of encephalitogenic Th1/Th17 and Th2/regulatory T cells through epigenetic modulation involving DNA methylation of cytokine gene promoters, thereby controlling the development of experimental autoimmune encephalomyelitis. J Immunol. 2011;186:6955–6964. doi: 10.4049/jimmunol.1004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.