Abstract
The combination of immunotherapy and chemotherapy is regarded as a promising approach for the treatment of certain types of cancer. However, the underlying mechanisms need to be fully investigated to guide the design of more efficient protocols for cancer chemoimmunotherapy. It is well known that danger-associated molecular patterns (DAMPs) can activate immune cells, including dendritic cells (DCs), via Toll-like receptors (TLRs); however, the role of DAMPs released from chemical drug-treated tumor cells in the activation of the immune response needs to be further elucidated. Here, we found that colorectal cancer (CRC) cells treated with oxaliplatin (OXA) and/or 5-fluorouracil (5-Fu) released high levels of high-mobility group box 1 (HMGB1) and heat shock protein 70 (HSP70). After OXA/5-Fu therapy, the sera of CRC patients also exhibited increased levels of HMGB1 and HSP70, both of which are well-known DAMPs. The supernatants of dying CRC cells treated with OXA/5-Fu promoted mouse and human DC maturation, with upregulation of HLA-DR, CD80 and CD86 expression and enhancement of IL-1β, TNF-α, MIP-1α, MIP-1β, RANTES and IP-10 production. Vaccines composed of DCs pulsed with the supernatants of chemically stressed CRC cells induced a more significant IFN-γ-producing Th1 response both in vitro and in vivo. However, the supernatants of chemically stressed CRC cells failed to induce phenotypic maturation and cytokine production in TLR4-deficient DCs, indicating an essential role of TLR4 in DAMP-induced DC maturation and activation. Furthermore, pulsing with the supernatants of chemically stressed CRC cells did not efficiently induce an IFN-γ-producing Th1 response in TLR4-deficient DCs. Collectively, these results demonstrate that DAMPs released from chemically stressed cancer cells can activate DCs via TLR4 and enhance the induction of an anti-tumor T-cell immune response, delineating a clinically relevant immuno-adjuvant pathway triggered by DAMPs.
Keywords: DAMPs, dendritic cells, chemotherapy, immunotherapy, TLR4
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer deaths in developed countries. Oxaliplatin (OXA)- and 5-fluorouracil (5-Fu)-based FOLFOX chemotherapy is the first-line therapeutic regimen in the treatment of patients with advanced CRC.1,2 Chemical drugs mainly kill tumor cells through cytotoxic effects and also kill normal cells at the same time, which causes lymphopenia and suppresses the immune system's function. Immunotherapy is now becoming a promising approach for CRC treatment, and much attention has been paid to the combination of chemotherapy and immunotherapy. Efficiently killing tumor cells with chemical drugs while enhancing induction of the anti-tumor immune response is becoming increasingly important. CRC cells treated with OXA and 5-Fu may release danger-associated molecular patterns (DAMPs), which play important roles in immunogenic tumor cell death. Through experiments performed in vivo and in vitro, DAMPs induced by chemical drugs have been demonstrated to stimulate dendritic cells (DCs) to induce an anti-cancer immune response.3,4,5 However, DAMPs also play a putative role in the promotion of tumor progression by attracting immature myeloid cells and triggering tumor cell proliferation.6,7,8 Thus, how to more efficiently trigger an anti-cancer immune response mediated by DAMPs is worthy of attention.
DCs are characterized by their ability to efficiently present antigens and are uniquely equipped to stimulate T-cell responses. DCs have a strong ability to activate CD8+ cytotoxic T lymphocytes and CD4+ Th1 immunity and therefore play an essential role in the anti-tumor immune response.9,10,11,12,13 DCs in the tumor environment are mainly immature and are ideally equipped to engulf and process dying cells. However, tumor-derived factors maintain DC immaturity, trapping the cells within the tumor and hence impairing effective presentation of ingested antigens to T cells. Moreover, these immature DCs have immunosuppressive rather than immunostimulatory properties.14,15 Therefore, improving the immunogenicity of tumor cells and the efficiency of stimulated DCs in inducing potent anti-tumor immunity is an important way to enhance the efficacy of cancer therapy.
Toll-like receptors (TLRs) play important roles in the initiation of innate and adaptive immune responses. Increasing numbers of DAMPs are being reported as candidate agonists of TLRs, and particularly TLR4, including heat shock proteins (HSPs) (HSP70, HSP90),16,17,18 high-mobility group box 1 (HMGB1)19,20 and uric acid crystals.21 These molecules can bind to TLR4, thereby causing inflammatory responses and providing DCs with danger signals, which can be translated into the promotion of an anti-tumor T-cell response. Supporting these findings, patients with breast cancer who have lost TLR4 function have been shown to relapse more quickly after chemotherapy than patients with normal TLR4 expression.22
Here, to improve the efficacy of DC vaccines in the induction of an anti-tumor immune response, we investigated whether DCs pulsed with DAMPs from dying CRC cells could efficiently induce an anti-tumor T-cell response. We showed that OXA and 5-Fu induced the release of DAMPs from CRC cells and that DAMPs from CRC cells activated DCs to more significantly induce anti-tumor immune responses than did the supernatants of untreated CRC cells. More importantly, we found that TLR4 in DCs was essential for CRC cell-derived DAMPs to activate DCs and induce an anti-tumor immune response. Thus, our data demonstrate that TLR4 is essential for DC activation and anti-tumor T-cell response enhancement by DAMPs from chemically stressed cancer cells, providing a molecular mechanism for the synergy of immunotherapy and chemotherapy in the induction of an anti-tumor effect.
Materials and methods
Serum samples
Sera were obtained from patients with CRC stage III/IV who had received FOLFOX (5-Fu+OXA) chemotherapy at Shanghai Changzheng Hospital (Shanghai, China). All patients gave written informed consent, and the protocol was approved by local institutional review boards. Blood was collected before and after the first chemotherapy treatment (any day among the third to seventh days after the treatment).
Animals and cell lines
Wild-type (WT) C57BL/6 and Balb/c mice, 6–8 weeks of age, were obtained from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China). TLR4-deficient mice (TLR4−/−, C57BL/6 strain) were kindly provided by Dr S Akira (Research Institute for Microbial Diseases, Osaka, Japan).23 All mice were housed in a specific pathogen-free facility during all experiments. The human CRC cell line SW480 (HLA-A2.1+) and the mouse CRC cell line CT-26 were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured according to the provided instructions.
Preparation of supernatants from chemically stressed cancer cells
SW480 cells and CT-26 cells were cultured in cell culture flasks (1×106/ml) in the presence of 10 mg/ml 5-Fu (Sigma, St Louis, MO, USA) and/or 0.5 mg/ml OXA (Sanofi, Paris, France). Supernatants were collected after 0, 6, 12, 18, 24, 30, 36 and 42 h, and HSP70 and HMGB1 levels were then measured using ELISA kits (Stressgen, Cedar Creek, TX, USA). To remove the chemical drugs, the supernatants collected after 30 h were dialyzed in phosphate-buffered saline at 4 °C for 48 h and concentrated by centrifugation in 10000 MWCO centrifuge tubes at 2000g for 15 min at 4 °C. Untreated sup-CTR describes the supernatants of untreated SW480/CT26 cells (the control group); OXA-sup, the supernatants of SW480/CT26 cells treated with OXA; 5-Fu-sup, the supernatants of SW480/CT26 cells treated with 5-Fu; and OXA+5-Fu-sup, the supernatants of SW480/CT26 cells treated with OXA and 5-Fu.
Generation of DCs
Human peripheral blood monocytes were isolated from healthy volunteers (HLA-A2.1+), and monocyte-derived DCs were prepared as described previously.24 Mouse bone marrow-derived DCs (BMDCs) were prepared as previously described.25
Assays for cytokine and chemokine production
Human DCs and BMDCs were cultured for 5 days and adjusted to a final concentration of 5×105 cells/ml in 24-well plates. Next, 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup and OXA+5-Fu-sup and 100 ng/ml lipopolysaccharide (LPS) were added to separate wells of the 24-well plates. Supernatants from designated wells were harvested after 24 h for quantification of cytokines, such as IL-6, IL-1β and TNF-α, and of chemokines, including MIP-1α, MIP-1β and RANTES, using ELISA kits (R&D Systems, Minneapolis, MN, USA).
Flow cytometry
After 5 days of culture, human DCs and BMDCs were stimulated with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup or 100 ng/ml LPS for 24 h. The DCs were then collected; washed with phosphate-buffered saline; and stained with phycoerythrin-conjugated anti-CD40 or anti-CD86 or fluorescein isothiocyanate-conjugated anti-HLA-DR (I-Ab) monoclonal antibody (PharMingen, San Diego, CA, USA) for analysis with a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA) and CellQuest software (Becton Dickinson).
Generation of T-cell response
Human DCs and BMDCs (from WT or TLR4−/− C57BL/6 mice) were cultured for 5 days; harvested; stimulated with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup for 24 h; and then washed twice in serum-free RPMI 1640 medium. In the TLR4-antagonist group, 30 µg/ml HTA125 (HBT, Uden, The Netherlands), an anti-TLR4 monoclonal antibody, was administered before stimulation with untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup.26 Human DCs (2×105) and autologous peripheral blood lymphocytes (2×106) or BMDCs (2×105) and autologous splenocytes (2×106) from WT or TLR4−/− C57BL/6 mice were cocultured in 1 ml RPMI 1640 medium supplemented with 10% fetal calf serum in 24-well plates. The human peripheral blood lymphocytes or mouse splenocytes were restimulated with autologous fresh supernatant-pulsed DCs every 7 day for three times respectively. On the third day after the second stimulation, 20 IU/ml rhIL-2 (Sigma) was added. Half of the medium was removed every 3 days and replaced with fresh medium containing rhIL-2 (20 IU/ml). On the seventh day after the final stimulation, the cells were harvested and prepared for ELISPOT analysis.
ELISPOT assay
Human lymphocytes were re-stimulated with 50 µg/ml SW480 tumor cell lysate antigen (prepared by repeatedly freezing and thawing SW480 cells) for 72 h, as described above, and then collected and used as effector cells. SW480 (1×105) tumor cells, serving as stimulator cells, were cocultured with 2×105 effector cells and seeded into 96-well polyvinylidene difluoride-backed microplates coated with anti-human IFN-γ mAb. After incubation at 37 °C for 24 h, the cells were removed, and the plates were processed following the manufacturer's protocol (R&D Systems).
Splenocytes from WT or TLR4−/− C57BL/6 mice were re-stimulated with 50 µg/ml CT-26 tumor cell lysate antigen for 72 h and then collected and used as effector cells. BMDCs (1×105) pulsed with CT-26 tumor lysate antigen, serving as stimulator cells, were cocultured with 2×105 effector cells and seeded into 96-well polyvinylidene difluoride-backed microplates coated with anti-mouse IFN-γ mAb. After incubation at 37 °C for 24 h, the cells were removed, and the plates were processed following the manufacturer's protocol for the ELISPOT kit (R&D Systems). The resulting spots were counted using an ImmunoSpot Analyzer (Cellular Technology Ltd, Cleveland, OH, USA).
DC vaccination
BMDCs generated from Balb/c mice were harvested on the fifth day; plated at a cell concentration of 2×106/ml; pulsed with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup for 24 h; and then washed three times with phosphate-buffered saline. Balb/c mice were subcutaneously immunized three times at an interval of 1 week with supernatant-pulsed BMDCs (1×106 per mouse).
Tumor challenge
Five days after the final immunization, the C57BL/6 mice were subcutaneously challenged with 2×105 CT-26 tumor cells in the flank area. Tumor growth was monitored by measuring the diameter of the tumor with a caliper every 2 days and was recorded as the average of two perpendicular diameter measurements. The survival time following tumor challenge was also recorded.
Statistical analysis
Differences in the growth of the CT-26 tumors, as indicated by the tumor diameters within each group, were compared using the Mann-Whitney U test. To compare mouse survival between the treatment groups and the control group, a Kaplan–Meier statistical analysis was performed. All other statistical analyses were based on the Student's t-test. P<0.05 was considered as a statistically significant difference.
Results
DAMPs release from cancer cells induced by chemical drugs
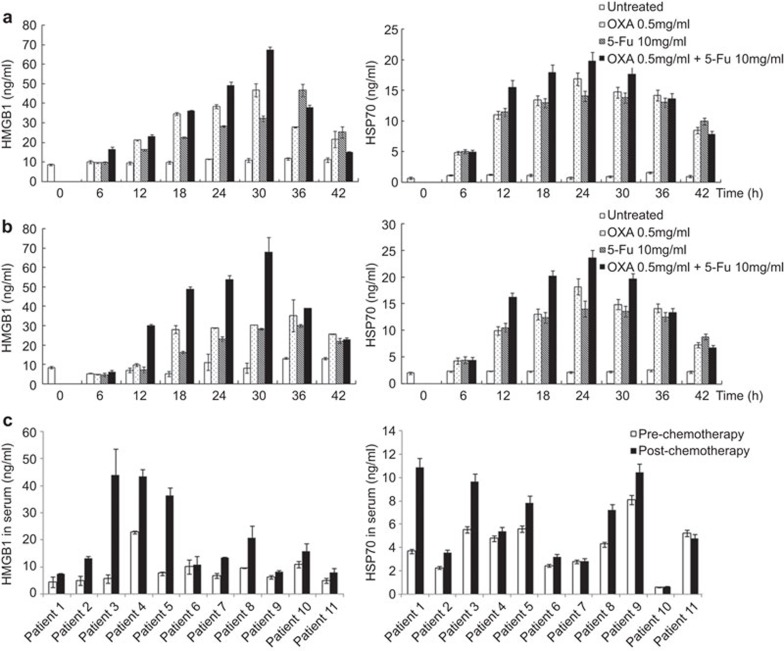
HMGB1 and HSP70 are prototypical DAMPs that are hallmarks of cancer and are released following chemotherapy.3,4,27 The release of HMGB1 and HSP70 was detected in the supernatants of the mouse CRC cell line CT-26 (Figure 1a) and the human CRC cell line SW480 (Figure 1b) after treatment with the chemical drugs OXA and/or 5-Fu. We found that HSP70 and HMGB1 release into the cell supernatants 6 h after treatment with OXA and/or 5-Fu was significantly increased compared with release in the untreated control group (P<0.05). Additionally, the HSP70 and HMGB1 concentrations in the cell supernatants 18, 24 and 30 h after treatment with the combination of OXA and 5-Fu were even higher than following treatment with OXA or 5-Fu alone (Figure 1a and b).
Figure 1.
HMGB1 and HSP70 release from chemically stressed CRC cells and in the sera of CRC patients after chemotherapy. (a) CT26 cells or (b) SW480 cells (3×105/ml) were treated with 0.5 mg/ml OXA, 10 mg/ml 5-Fu or a combination of 0.5 mg/ml OXA and 10 mg/ml 5-Fu. Supernatants were collected after 0, 6, 12, 18, 24, 30, 36 and 42 h of culture. HMGB1 and HSP70 levels in the supernatants were measured by ELISA. (c) HMGB1 and HSP70 levels in the sera of patients with advanced CRC were measured by ELISA before and after FOLFOX chemotherapy. The data are presented as the mean±s.e.m. of three independent experiments. CRC, colorectal cancer; 5-Fu, 5-fluorouracil; HMGB1, high-mobility group box 1; HSP, heat shock protein; OXA, oxaliplatin.
DAMPs in sera from cancer patients induced by chemotherapy
Next, we investigated changes in the expression of HMGB1 and HSP70 in the sera of 11 patients with advanced CRC who had received FOLFOX chemotherapy (5-Fu+OXA). We found that the serological concentrations of HSP70 and HMGB1 in the patients who had received chemotherapy increased markedly after chemotherapy (Figure 1c). The results indicated that chemotherapy can increase DAMPs release in cancer patients.
Supernatants from chemically stressed human cancer cells induce maturation of human DCs in TLR4-dependent manner
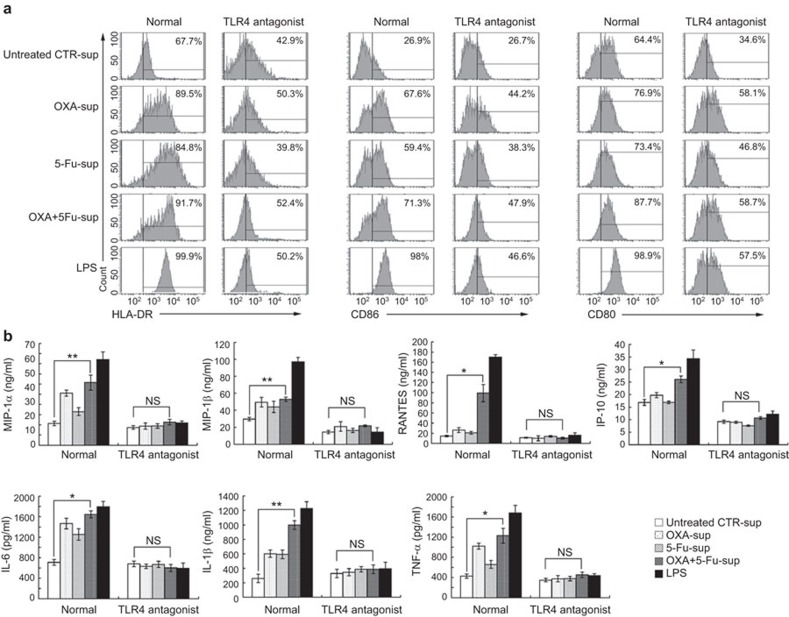
DAMPs are shown to be candidate TLR4 agonists and to promote the maturation and activation of DCs.4,18,19 To investigate the role of TLR4 in mediating DC maturation by supernatants from chemical drug-treated SW480 cells, we observed the phenotypic changes in DCs stimulated with supernatants from chemically stressed SW480 cells. We found that stimulation with OXA-sup, 5-Fu-sup or OXA+5-Fu-sup induced upregulation of HLA-DR, CD80 and CD86 expression on human DCs compared with stimulation with untreated sup-CTR. Stimulation with OXA+5-Fu-sup particularly induced greater phenotypic changes than did stimulation with OXA-sup or 5-Fu-sup alone. When we used the mAb HTA125, a TLR4 antagonist, to block the interaction of TLR4 with DAMPs in the supernatants of SW480 cells treated with chemical drugs, the upregulation of HLA-DR, CD80 and CD86 expression on DCs was reversed (Figure 2a), indicating that TLR4 on the DCs mediated the enhancing effect.
Figure 2.
Phenotypic and functional maturation of human DCs induced by the supernatants of chemically stressed SW480 cells in a TLR4-dependent manner. (a) Human DCs (5×105/ml) cultured on day 5 were treated with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup or 100 ng/ml LPS for 24 h and then collected for flow cytometry analysis of HLA-DR, CD80 and CD86 expression. (b) Cytokine production by DCs stimulated with the supernatants of chemically stressed SW480 cells. Normal human DCs (normal group) and DCs blocked with the TLR4 antagonist HTA125 (TLR4 antagonist group) were cultured for 5 days and then stimulated with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup or 100 ng/ml LPS for 24 h. The levels of IL-6, IL-1β, TNF-α, MIP-1α, MIP-1β, RANTES and IP-10 in the supernatants were measured by ELISA. The results are presented as the mean±s.d. of triplicate samples. **P<0.01; *P<0.05. DC, dendritic cell; 5-Fu, 5-fluorouracil; 5-Fu-sup, supernatants from SW480 cells treated with 5-Fu; LPS, lipopolysaccharide; NS, no significant difference; OXA, oxaliplatin; OXA+5-Fu-sup, supernatants from SW480 cells treated with OXA and 5-Fu; OXA-sup, supernatants from SW480 cells treated with OXA; TLR, Toll-like receptor; untreated sup-CTR, supernatants from untreated SW480 cells.
Next, we investigated whether TLR4 was essential for the induction of pro-inflammatory cytokine and chemokine secretion by DCs stimulated with supernatants from chemically stressed SW480 cells. We found an increase in pro-inflammatory cytokine and chemokine secretion by DCs stimulated with OXA-sup, 5-Fu-sup or OXA+5-Fu-sup, especially when stimulated with OXA+5-Fu-sup (P<0.05), but not DCs blocked with the TLR4 antagonist (Figure 2b). These results indicated that TLR4 was the crucial receptor mediating the DC maturation induced by DAMPs from SW480 cells treated with chemical drugs.
Supernatants from chemically stressed mouse cancer cells induce maturation of mouse DCs in TLR4-dependent manner
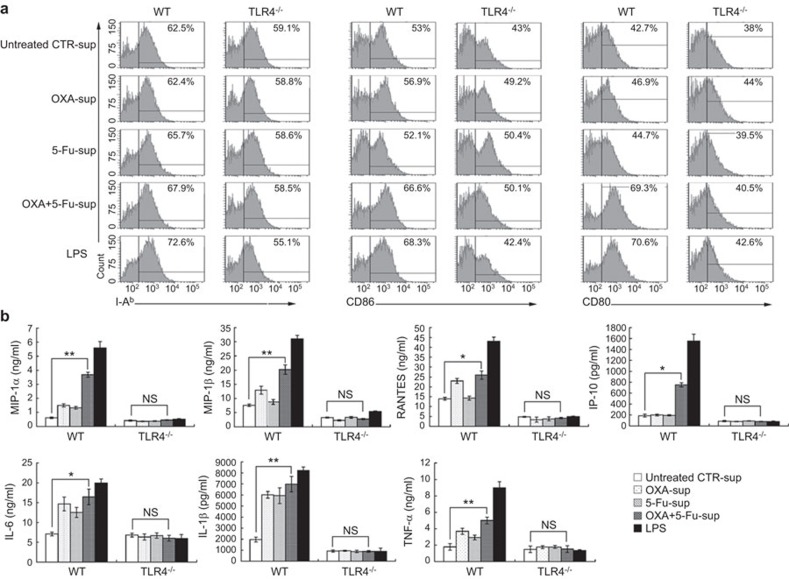
Next, we prepared DCs from WT and TLR4−/− C57BL/6 mice and observed the phenotypic changes in these DCs when stimulated with supernatants from chemically stressed CT-26 cells. We found that stimulation with OXA-sup, 5-Fu-sup or OXA+5-Fu-sup induced upregulation of I-ab, CD80 and CD86 expression on WT DCs, but not TLR4−/− DCs (Figure 3a). The production of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) and chemokines (MIP-1α, MIP-1β, RANTES and IP-10) was significantly increased among WT mouse DCs stimulated with OXA+5-Fu-sup. However, there was no significant increase in the production of these cytokines and chemokines by TLR4−/− DCs stimulated with OXA+5-Fu-sup (Figure 3b).
Figure 3.
Phenotypic and functional maturation of mouse DCs induced by the supernatants of chemically stressed CT-26 cells in a TLR4-dependent manner. (a) DCs from wild-type C57BL/6 mice (WT) and TLR4-deficient C57BL/6 mice (TLR4−/−) were cultured for 5 days and then stimulated with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup or 100 ng/ml LPS for 24 h. The cells were then collected for flow cytometry analysis of I-ab, CD80 and CD86 expression. (b) Cytokine production by DCs stimulated with the supernatants of chemically stressed CT-26 cells. The levels of IL-6, IL-1β, TNF-α, MIP-1α, MIP-1β, RANTES and IP-10 in the supernatants were measured by ELISA. The results are presented as the mean ± SD of triplicate samples. **P<0.01; *P<0.05. DC, dendritic cell; 5-Fu, 5-fluorouracil; LPS, lipopolysaccharide; NS, no significant difference; OXA, oxaliplatin; TLR, Toll-like receptor.
Taken together, the results suggest that the supernatants of CRC cells treated with chemical drugs induced DC phenotypic maturation and production of chemokines and pro-inflammatory cytokines via TLR4, indicating that TLR4 was one of the functional receptors mediating DC activation by the supernatants of the chemically stressed CRC cells.
DCs pulsed with supernatants from chemically stressed cancer cells induce Th1 response in vitro in TLR4-dependent manner
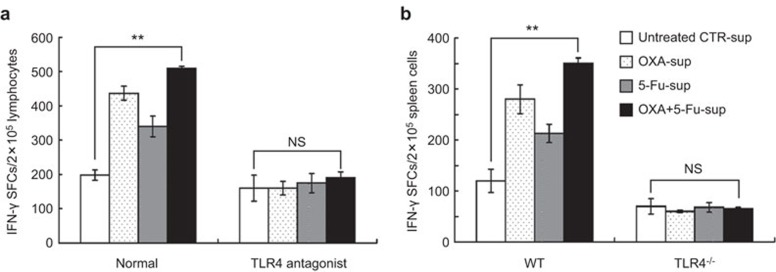
To assess whether TLR4 played an important role in the in vitro induction of a Th1 response by the DCs pulsed with DAMPs, we incubated peripheral blood lymphocytes from health donors and autologous DCs pulsed with the supernatants of chemically stressed SW480 cells in vitro. Alternatively, we incubated splenocytes from WT or TLR4−/− C57BL/6 mice and syngeneic DCs pulsed with the supernatants of chemically stressed CT-26 cells. As shown in Figure 4, the number of IFN-γ-producing cells induced by the DCs pulsed with OXA-sup, 5-Fu-sup or OXA+5-Fu-sup was significantly higher than the number in the untreated sup-CTR group (P<0.01). However, the number of IFN-γ-producing cells was decreased by the TLR4 antagonist HTA125 (Figure 4a), and an increase in the number of IFN-γ-producing cells was not observed in the TLR4−/− mice immunized with syngeneic DCs pulsed with supernatants from chemical drug-treated CT-26 cells (Figure 4b). These results further indicated that TLR4 functioned as the receptor by which DAMPs activated the DCs. The data suggested that the DCs were activated by the DAMP-containing supernatants of chemically stressed CRC cells via TLR4 and then triggered the Th1 response more potently.
Figure 4.
DCs pulsed with the supernatants of chemically stressed CRC cells induced an IFN-γ-producing Th1 cell response in vitro in a TLR4-dependent manner. (a) Human peripheral blood lymphocytes from health donors were stimulated with autologous DCs (normal group) pulsed with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup or with autologous DCs treated with the TLR4 antagonist HTA125 (TLR4 antagonist group) before pulsing. An IFN-γ ELISPOT was used to detect the specific Th1 immune response. (b) Mouse splenocytes from wild-type (WT) and TLR4-deficient (TLR4−/−) C57BL/6 mice were stimulated with syngeneic BMDCs pulsed with 100 µl/ml untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup. IFN-γ ELISPOT analysis of the splenocytes was then used to assay specific Th1 immune responses. The results are expressed as the number of positive SFCs/2×105 lymphocytes or spleen cells. The columns are the mean±s.e.m. of three independent experiments. **P<0.01. BMDC, bone marrow-derived dendritic cell; DC, dendritic cell; 5-Fu, 5-fluorouracil; OXA, oxaliplatin; SFCs, spot forming cells; TLR, Toll-like receptor.
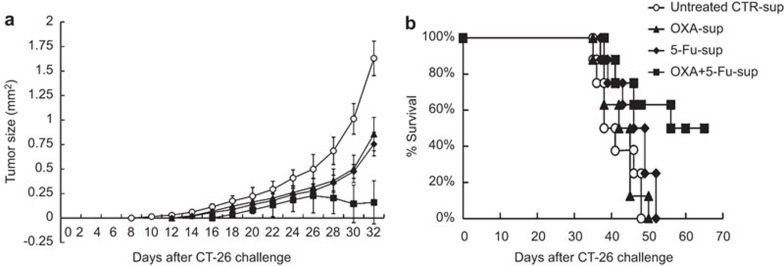
More efficient induction of anti-tumor effect in vivo by immunization with DCs pulsed with supernatants from chemically stressed cancer cells
Induction of the in vivo anti-tumor immune response by DAMPs was assessed in a murine model with CT-26 tumor challenge. Balb/c mice were subcutaneously immunized three times at an interval of 1 week with BMDCs that had been stimulated with the supernatants of chemically stressed CT-26 cells. On the seventh day after the last immunization, the mice were subcutaneously challenged with CT-26 cells, and the tumor size of the mice was measured every 2 days. Tumor growth was significantly suppressed in the OXA+5-Fu-sup group compared with the control group (P<0.05) (Figure 5a), and the survival time was markedly prolonged in the OXA+5-Fu-sup group compared with the untreated sup-CTR group (P<0.05) (Figure 5b). These data demonstrated that immunization of mice with DCs that had been pulsed with the DAMP-containing supernatants of chemically stressed CRC cells more efficiently induced an anti-tumor immune response.
Figure 5.
Immunization with DCs pulsed with the supernatants of chemically stressed CT-26 cells induced more potent anti-tumor immunity. Balb/c mice were subcutaneously immunized in the thigh with DCs that had been pulsed with untreated CTR-sup, OXA-sup, 5-Fu-sup or OXA+5-Fu-sup. Five days after the final immunization, the mice were subcutaneously challenged with 2×105 CT-26 tumor cells in the flank area. (a) Tumor growth curves. Following CT-26 tumor challenge, tumor growth was monitored by measuring the diameter of the tumor every 2 days and was recorded as the average tumor diameter. (b) Survival of the immunized mice after CT-26 tumor challenge. Each group contained 10 mice. DC, dendritic cell; 5-Fu, 5-fluorouracil; OXA, oxaliplatin.
Discussion
It has recently been shown that DAMPs are released from dying cells treated with certain chemical drugs, such as OXA, cyclophosphamide and doxorubicin.4,5,28,29 In our study, we found that the release of HSP70 and HMGB1 into CRC cell supernatants was significantly increased after OXA and/or 5-Fu treatment. Additionally, HSP70 and HMGB1 concentrations in the supernatants of cells treated with the combination of OXA and 5-Fu were even higher than after treatment with OXA or 5-Fu alone. Indeed, our data showed that the combination of OXA and 5-Fu treatment significantly increased DAMPs release, suggesting that the combination chemotherapy FOLFOX directly triggers more CRC cell death while more efficiently stimulating endogenous immune responses against the tumor. Timing is critical for effective anti-tumor immunotherapy. It has been reported that sequential events are associated with chemically stressed tumor cells. When tumor cells are treated with doxorubicin, intracellular calreticulin rapidly translocates to the cell surface (within 1 h). At 12 h after drug treatment, molecular chaperones, such as HSP70, may appear on the tumor cell surface. Finally, at 18 h, the release of HMGB1 from dying tumor cells can be observed.30,31 In contrast to the results for doxorubicin, our data showed that the levels of HSP70 and HMGB1 peaked at 30 h after exposure to OXA and 5-Fu and gradually decreased afterward. Thus, we evaluated the anti-tumor effect of adjuvant immunotherapy induced by a DC vaccine, including protocols for maturing DCs pulsed with 30-h supernatants. Consistent results were also collected from human patients, showing that the serum concentrations of HSP70 and HMGB1 in patients receiving FOLFOX chemotherapy increased markedly following chemotherapy.
DAMPs appear to play an alternate paradoxical role during tumor formation. It has been reported that DAMPs recognition can favor tumor progression by attracting immature myeloid cells and triggering the proliferation, migration and sprouting of endothelial cells in tumor beds.6,7,8 Moreover, in CRC, HMGB1 expression is increased at metastatic sites compared with primary tumor sites in non-metastatic patients.32 In addition to the putative role of HMGB1 in the promotion of tumor progression, certain preclinical and clinical data have revealed that HMGB1 seems to induce the functional maturation of DCs and to trigger protective anti-cancer T cell responses.4,5,16,20 The question of how DCs decode DAMPs needs to be further investigated. Recent studies have highlighted that after treatment with chemotherapeutic drugs, apoptotic tumor cells can release microparticles, which may contain DAMPs that stimulate DCs and can be used to effectively kill tumor cells.33,34 Therefore, when appropriate antigenic transfer to DCs occurs, the acute release of DAMPs may help to elicit immune responses in cancer-bearing patients. Maturation and activation of DCs is required for DC migration to T-cell regions within the lymph node.9,10,11
In this study, we found that DAMPs from CRC cells treated with OXA and 5-Fu promoted mouse and human DC maturation in vitro, activating the DCs to produce pro-inflammatory cytokines, such as IL-1β and TNF-α, whose autocrine or paracrine secretion can stimulate DC maturation.9,14,16 Moreover, in our study, increased MIP-1α, MIP-1β, RANTES and IP-10 secretion by DCs stimulated with DAMPs was also observed. These chemokines have been shown to play important roles in the immune response due to their involvement in the inflammatory response and their capacity to chemoattract leukocytes. Our previous studies have shown that chemokine gene transfer can enhance DC-mediated and T cell-dependent anti-tumor immunity and thus represents a potent anti-tumor immunotherapeutic approach.35,36,37 Therefore, increased chemokine secretion by DAMP-stimulated DCs is expected to exert strong chemoattractant effects on DCs and T cells, resulting in improved protective immunity and an enhanced T-cell response.
It is strongly recommended that vaccination strategies aiming to induce immunity against cancer include a means to stimulate the maturation of the targeted DCs. However, tumor-derived factors maintain DC immaturity, trap these cells within the tumor and hence, impair the effective presentation of ingested antigens to T cells.38 The presence of HSPs from dying tumor cells promotes the formation of tumor antigen–HSP complexes, which are processed by DCs for T-cell cross-priming more efficiently than tumor antigens alone.9,11 In the current study, the release of HSP70 and HMGB1 into the cell supernatants was significantly increased after OXA and/or 5-Fu treatment. HSP70 could have chaperoned tumor antigen generated by dying cells, triggering tumor-specific T-cell responses and anti-tumor effects via cross-presentation by DCs. We also found that a vaccine generated by pulsing DCs with the supernatants of chemically stressed CRC cells induced a polarizing IFN-γ-producing Th1 response in vitro. Induction of a Th1 response could be a vital mechanism underlying anti-tumor immunotherapy. We have previously reported that gene modification of Th1 cytokines (e.g., IL-18 and IFN-γ) can efficiently induce a T cell response to treat immunological disorders, including cancer and chronic infection.12,13,39 Moreover, a CEA576–669-HSP70L1 fusion protein promotes the secretion of Th1 cytokines (such as IL-12 and IFN-γ) and stimulates an anti-tumor cytotoxic T lymphocyte response to a DC vaccine.40 Thus, certain types of chemotherapy can kill tumor cells through efficient cytotoxic effects while enhancing the induction of an anti-tumor immune response.
An increasing number of endogenous proteins are being reported as stimulating TLRs (in particular, TLR2 and TLR4), such as HMGB119,20 and HSPs, including HSP60, HSP70, endoplasmin and HSPB8.9,11,41,42 In this study, we found phenotypic maturation and significantly increased cytokine secretion among WT DCs, but not TLR4−/− DCs, after stimulation with the supernatants of chemically stressed CT-26 cells. Similarly, the supernatants of chemically stressed SW480 cells failed to promote the maturation and activation of human DCs when the mAb HTA125 was used to block TLR4 function in DCs. HMGB1 and HSP70 bind promiscuously to multiple proteins and are reported to bind to the receptor for advanced glycosylation endproducts,43,44 TLR241,42 and TLR4,16,19,20 all of which can be presented on the surfaces of DCs. Our data suggest that the effect of the supernatants of dying CRC cells on DCs is mainly TLR4 mediated. Therefore, our current study indicated that TLR4 on DCs is one of the most important members of the TLR family to target in order to enhance the anti-tumor immune response.
The activation of TLRs by their cognate ligands leads to inflammatory cytokine production and upregulation of costimulatory signals and MHC molecules among DCs, thereby linking innate recognition to adaptive T- and B-cell immune responses, as well as to memory responses after the immune system encounters any pathogen. Interestingly, TLR4 appears to be required for efficient DC-mediated induction of a T-cell response. Our studies have proven that DCs pulsed with the supernatants of chemically stressed CRC cells induce an IFN-γ-producing T cell response in a TLR4-dependent manner. Thus, during chemotherapy, DAMPs, which are released from chemically stressed tumor cells, can activate the TLR4 signaling pathway and induce anti-tumor T-cell immunity against tumor cells, delineating a clinically relevant immuno-adjuvant pathway triggered by DAMPs.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81273332, 81101657 and 81123006) and the National High Technology Research, Development Program of China (2012AA020902).
All authors declare no conflict of interest.
References
- Ruzzo A, Graziano F, Loupakis F, Rulli E, Canestrari E, Santini D, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol. 2007;25:1247–1254. doi: 10.1200/JCO.2006.08.1844. [DOI] [PubMed] [Google Scholar]
- Landry JC, Feng Y, Cohen SJ, Staley CA, 3rd, Whittington R, Sigurdson ER, et al. Phase 2 study of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: ECOG 3204. Cancer. 2013;119:1521–1527. doi: 10.1002/cncr.27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nace G, Evankovich J, Eid R, Tsung A. Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity. J Innate Immun. 2012;4:6–15. doi: 10.1159/000334245. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–636. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:628–631. doi: 10.1038/cddis.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zha J, Wang Y, Liu W, Yang X, Yu P. Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013;73:629–639. doi: 10.1158/0008-5472.CAN-12-2704. [DOI] [PubMed] [Google Scholar]
- Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72:3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Wu Y, Yan F, Wang N, Wang W, Cao X, et al. Efficient induction of a Her2-specific anti-tumor response by dendritic cells pulsed with a HSP70L1-Her2(341–456) fusion protein. Cell Mol Immunol. 2011;8:424–432. doi: 10.1038/cmi.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hee Lee J, Park MS, Hwang JE, Cho SH, Bae WK, Shim HJ, et al. Dendritic cell-based immunotherapy for colon cancer using an HLA-A*0201-restricted cytotoxic T-lymphocyte epitope from tumor-associated antigen 90K. Cell Mol Immunol. 2013;10:275–282. doi: 10.1038/cmi.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju DW, Tao Q, Lou G, Bai M, He L, Yang Y, et al. Interleukin 18 transfection enhances antitumor immunity induced by dendritic cell–tumor cell conjugates. Cancer Res. 2001;61:3735–3740. [PubMed] [Google Scholar]
- Zhang LH, Pan JP, Yao HP, Sun WJ, Xia DJ, Wang QQ, et al. Intrasplenic transplantation of IL-18 gene-modified hepatocytes: an effective approach to reverse hepatic fibrosis in schistosomiasis through induction of dominant Th1 response. Gene Ther. 2001;8:1333–1342. doi: 10.1038/sj.gt.3301524. [DOI] [PubMed] [Google Scholar]
- Shurin GV, Ma Y, Shurin MR. Immunosuppressive mechanisms of regulatory dendritic cells in cancer. Cancer Microenviron. 2013;6:159–167. doi: 10.1007/s12307-013-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- Fang H, Wu Y, Huang X, Wang W, Ang B, Cao X, et al. Toll-like receptor 4 (TLR4) is essential for HSP70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J Biol Chem. 2011;286:30393–30400. doi: 10.1074/jbc.M111.266528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer D, Hammann A, Benikhlef N, Fourmaux E, Bouchot A, Wettstein G, et al. Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells. J Biol Chem. 2011;286:3418–3428. doi: 10.1074/jbc.M110.154823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A, et al. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J Immunol. 2009;183:3092–3098. doi: 10.4049/jimmunol.0901235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Galluzzi L, Rousseau V, Rigoni A, Tesniere A, Delahaye N, et al. Loss-of-function alleles of P2RX7 and TLR4 fail to affect the response to chemotherapy in non-small cell lung cancer. Oncoimmunology. 2012;1:271–278. doi: 10.4161/onci.18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, et al. Novel heat shock protein HSP70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103:1747–1754. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhan Z, Li D, Xu L, Ma F, Zhang P, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredly H, Ersvær E, Gjertsen BT, Bruserud O. Immunogenic apoptosis in human acute myeloid leukemia (AML): primary human AML cells expose calreticulin and release heat shock protein (HSP) 70 and HSP90 during apoptosis. Oncol Rep. 2011;25:1549–1556. doi: 10.3892/or.2011.1229. [DOI] [PubMed] [Google Scholar]
- Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, et al. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Invest Dermatol. 2013;133:1610–1619. doi: 10.1038/jid.2012.444. [DOI] [PubMed] [Google Scholar]
- Tongu M, Harashima N, Yamada T, Harada T, Harada M. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer Immunol Immunother. 2010;59:769–777. doi: 10.1007/s00262-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28–33. doi: 10.1158/1078-0432.CCR-11-2701. [DOI] [PubMed] [Google Scholar]
- Fahmueller YN, Nagel D, Hoffmann RT, Tatsch K, Jakobs T, Stieber P, et al. Immunogenic cell death biomarkers HMGB1, RAGE, and DNAse indicate response to radioembolization therapy and prognosis in colorectal cancer patients. Int J Cancer. 2013;132:2349–2358. doi: 10.1002/ijc.27894. [DOI] [PubMed] [Google Scholar]
- Date K, Hall J, Greenman J, Maraveyas A, Madden LA. Tumour and microparticle tissue factor expression and cancer thrombosis. Thromb Res. 2013;131:109–115. doi: 10.1016/j.thromres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang M, Tang H, Cao X. Fas signal links innate and adaptive immunity by promoting dendritic-cell secretion of CC and CXC chemokines. Blood. 2005;106:2033–2041. doi: 10.1182/blood-2004-12-4831. [DOI] [PubMed] [Google Scholar]
- Guo J, Zhang M, Wang B, Yuan Z, Guo Z, Chen T, et al. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int J Cancer. 2003;103:212–220. doi: 10.1002/ijc.10816. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang B, Zhang M, Chen T, Yu Y, Regulier E, et al. Macrophage-derived chemokine gene transfer results in tumor regression in murine lung carcinoma model through efficient induction of antitumor immunity. Gene Ther. 2002;9:793–803. doi: 10.1038/sj.gt.3301688. [DOI] [PubMed] [Google Scholar]
- Li DY, Gu C, Min J, Chu ZH, Ou QJ. Maturation induction of human peripheral blood mononuclear cell-derived dendritic cells. Exp Ther Med. 2012;4:131–134. doi: 10.3892/etm.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Mi J, Yu Y, Yao H, Chen H, Li M, et al. IFN-gamma gene therapy by intrasplenic hepatocyte transplantation: a novel strategy for reversing hepatic fibrosis in Schistosoma japonicum-infected mice. Parasite Immunol. 2001;23:11–17. doi: 10.1046/j.1365-3024.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N, et al. HSP70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine. Cancer Res. 2005;65:4947–4954. doi: 10.1158/0008-5472.CAN-04-3912. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008;183:111–127. doi: 10.1007/978-3-540-72167-3_6. [DOI] [PubMed] [Google Scholar]
- Todorova J, Pasheva E. High mobility group B1 protein interacts with its receptor RAGE in tumor cells but not in normal tissues. Oncol Lett. 2012;3:214–218. doi: 10.3892/ol.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhiainen A, Kuja-Panula J, Tumova S, Rauvala H. RAGE-mediated cell signaling. Methods Mol Biol. 2013;963:239–263. doi: 10.1007/978-1-62703-230-8_15. [DOI] [PubMed] [Google Scholar]