Abstract
Recent studies have revealed that human sodium taurocholate cotransporting polypeptide (SLC10A1 or NTCP) is a functional cellular receptor for hepatitis B virus (HBV). However, whether human NTCP can support HBV infection in mouse hepatocyte cell lines has not been clarified. Because an HBV-permissible mouse model would be helpful for the study of HBV pathogenesis, it is necessary to investigate whether human NTCP supports the susceptibility of mouse hepatocyte cell lines to HBV. The results show that exogenous human NTCP expression can render non-susceptible HepG2 (human), Huh7 (human), Hepa1–6 (mouse), AML-12 (mouse) cell lines and primary mouse hepatocyte (PMH) cells susceptible to hepatitis D virus (HDV) which employs HBV envelope proteins. However, human NTCP could only introduce HBV susceptibility in human-derived HepG2 and Huh7 cells, but not in mouse-derived Hepa1–6, AML-12 or PMH cells. These data suggest that although human NTCP is a functional receptor that mediates HBV infection in human cells, it cannot support HBV infection in mouse hepatocytes. Our study indicated that the restriction of HBV in mouse hepatocytes likely occurs after viral entry but prior to viral transcription. We have excluded the role of mouse hepatocyte nuclear factors in the restriction of the HBV life cycle and showed that knockdown or inhibition of Sting, TBK1, IRF3 or IRF7, the components of the anti-viral signaling pathways, had no effect on HBV infection in mouse hepatocytes. Therefore, murine restriction factors that limit HBV infection need to be identified before a HBV-permissible mouse line can be created.
Keywords: HBV, HDV, NTCP
Introduction
Hepatitis B virus (HBV) is an enveloped virus containing a 3.2-kb genome of partially double-stranded DNA encoding four overlapping reading frames. The HBV envelope consists of the small (S), middle (M) and large (L) envelope proteins. These multiple transmembrane proteins share the same C-terminal domain corresponding to the S protein, but differ at their N-terminal domains.1 HBV infection constitutes a serious threat to public health worldwide because more than 350 million people are chronically infected with HBV. Chronic HBV infection causes serious consequences, including chronic hepatitis, cirrhosis and hepatocellular carcinoma, and is responsible for more than one million deaths each year.2 Hepatitis D virus (HDV) is a small satellite RNA virus of HBV. It carries all the three HBV envelope proteins and propagates only when coexisting with HBV. People co-infected with HBV and HDV are at a greater risk for severe liver disease. Despite its medical and social relevance, progress in HBV and HDV research has been impeded by the lack of highly HBV-susceptible cell lines that permit HBV entry in vitro.3
Two determinants of viral infectivity have been identified in the HBV envelope proteins: (i) the myristoylated N-terminal of pre-S1 region in the large surface protein, which has been demonstrated to play a key role in HBV-specific attachment and entry into hepatocytes;4,5,6,7,8 and (ii) the antigenic loop, which is structurally related to the antigenic ‘a' determinant of the small surface protein and may mediate initial virus attachment to cell-surface heparan sulfate proteoglycans.9,10,11,12 The infection occurs through viral attachment to cell-surface heparan sulfate proteoglycans before pre-S1 engages a specific receptor for uptake.
Studies that were performed over the past decades have suggested a variety of specific receptors for HBV infection in human hepatocytes, but none have been convincingly proved. Only recently, sodium taurocholate cotransporting polypeptide (SLC10A1 or NTCP) was demonstrated as a functional, specific receptor for HBV and HDV. NTCP can specifically bind to the myristoylated N-terminal of pre-S1, but is absent from the surface of commonly used hepatocyte cell lines, such as HepG2 and Huh7.13 Myristoylated pre-S1 domain binds to differentiated hepatocytes of both HBV-susceptible and non-susceptible species and specifically targets liver tissue.14,15 Yan et al. demonstrated that mouse NTCP also binds to the myristoylated pre-S1 domain but can only mediate viral entry when residues 84–87 are replaced by human counterparts,16 suggesting that homologs of human NTCP (hNTCP) in non-susceptible species are also able to bind HBV, but are unable to mediate entry. The identification of hNTCP as a functional receptor for HBV is an important milestone in the field of HBV research. It enables the establishment of in vitro HBV infection systems, and more importantly, provides the possibility to develop genetically modified mice that are susceptible to HBV infection. Because a mouse model would dramatically facilitate basic research into HBV and the development of more effective antiviral therapeutics, it is necessary to investigate HBV infection in a hNTCP-reconstituted mouse hepatocyte cell line and to provide a concept supporting or refuting the use of hNTCP to generate a mouse model line.
Here, we investigated the permissiveness to HBV and HDV in several human- or mouse-derived hepatocytes expressing exogenous hNTCP. In the presence of exogenous hNTCP expression, two widely used human hepatocellular carcinoma cell lines (HepG2 and Huh7) were observed to be permissive to HBV and HDV infection, which confirmed previous findings. Just as in human cells, the hNCTP-expressing mouse hepatocyte cell lines also support HDV infection. However, none of the mouse hepatocyte cell lines or primary cells can be converted by hNTCP to support HBV infection. Our study shows that HBV gene can be equally expressed in both human and mouse hepatocyte cell lines when introduced by an adenoviral delivery vector. Because HDV can infect both human and mouse hepatocyte cell lines that express hNTCP, the restriction step likely occurs after viral entry and prior to viral transcription. Because either expressing human hepatocyte nuclear factors or attenuating Sting-TBK1 signaling in mouse hepatocyte cell lines fails to affect HBV infection in mouse hepatocyte, this restriction should not be attributed to the possible involvement of mouse hepatocyte nuclear factors in supporting HBV replication or anti-viral signaling toward HBV in mouse hepatocyte cell lines. Further investigations of the HBV restriction factors in mouse cells would be a prerequisite for generating HBV-permissible mouse models.
Materials and methods
Antibodies and plasmids
Anti-Flag polyclonal antibody was raised by immunizing rabbits with flag peptide. Anti-hNTCP polyclonal antibody was raised by immunizing rabbits with hNTCP protein. Anti-mSting antibody is from Cell Signaling Technology locates at Beverly, Massachusetts, USA (3337) and the anti-mTBK1 is from Cell Signaling (3504S). Full-length cDNA of hNTCP, mouse NTCP, GFP, HNF1A/B, HNF4A, HNF6, TBK1-KD, IRF3-DN, IRF7-DN, mSting sh-1 and mTBK1 sh-5 were subcloned into lentiviral vectors for gene delivery. HNTCP and GFP were also subcloned into adenoviral vectors for gene delivery.
Cell lines
Human hepatocellular carcinoma cells (HepG2) and mouse hepatocellular carcinoma cells (AML12, Hepa1–6) were obtained from the American Type Culture Collection. The Huh7 cell line was obtained from the Japanese Collection of Research Bioresources. Huh7, HepG2 and Hepa1–6 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, United States of America) containing 10% fetal bovine serum (Invitrogen). The AML-12 cell line was cultured in Dulbecco's modified Eagle's medium/Ham's F-12 (1∶1) medium (Gibco, Carlsbad, CA, United States of America) containing 10% fetal bovine serum (Invitrogen).
Isolation and culture of primary mouse hepatocytes (PMHs)
Specific pathogen-free B6 mice were housed in the animal facility at the School of Life Science, Xiamen University. All studies were performed in accordance with institutionally approved protocols and adhered to guidelines of the School of Life Science Guide for the care and use of laboratory animals. PMH cells were obtained from anesthetized mice (6–8 weeks old, female) with a two-step perfusion method as previously described. Cell suspensions after perfusion were filtered through a 100-µm cell strainer and centrifuged at 50g for 3 min. The cell pellet containing PMH was resuspended in Williams E medium supplemented with 10% fetal bovine serum, 3 µg/ml insulin, 2 mM L-glutamine, 18 µg/ml hydrocortisone, 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were then plated on collagen-coated cell culture dishes or plates. Eight hours after plating, unattached cells were washed away and fresh media were added.
HDV and HBV production
A plasmid containing a head-to-tail dimer of 1.0× HDV cDNA of a genotype I virus under the control of a cytomegalovirus (CMV) promoter was constructed with de novo synthesized HDV cDNA for the production of HDV ribonucleoproteins (RNP). A pUC18 plasmid containing nucleotides 2431–1990 of HBV (Genotype D, GenBank accession no.: U95551.1) was used for expressing HBV envelope proteins under the control of an endogenous HBV promoter. HDV virions were produced by transfection of the plasmids in Huh-7 cells as previously described.8 HBV were produced from a stably transfected hepatoblastoma cell line that inducibly expresses HBV.17 Briefly, HepaAD38 cells were induced to produce HBV particles by withdrawing tetracycline. Four days after the withdrawal, the culture supernatants were collected and refreshed every 2 days. The supernatants were then concentrated and resuspended in Dulbecco's modified Eagle's medium.
Determination of hepatitis B e antigen (HBeAg) and, hepatitis Delta antigen (HDAg)
Extracellular HBeAg and intracellular HDAg were determined by chemiluminescence using commercial assay kits (Wantai, Beijing, China). The relative level of each antigen was expressed as a relative light unit) value on a linear range from 102 to 107 for two assays. For analysis of HBeAg in culture media, 100 µl of the supernatant was used for detection. For the intracellular HDAg assay, the infected cells were treated with a suitable lysis buffer (20 mM HEPES, 1 mM EGTA, 100 mM NaCl, 5 mM Mg2Cl, 0.4% n-dodecyl b-D-maltoside and 10% Glycerol) at room temperature for 30 min, and the supernatants were separated through centrifugation and used for assay.
HDV and HBV infection and antibody-mediated neutralization assays
Viral infections of HDV and HBV were conducted at multiplicities of genome equivalents (mge) of 100 and 500, respectively. When inoculated with HDV at 100 mge, approximately 10%–20% of the cells can be infected in the hNTCP-Huh7, hNTCP-HepaG2, hNTCP-Hepa1–6 and hNTCP-AML12 cell lines (based on HDAg staining). Normally, HDV and HBV were inoculated in the presence or absence of neutralizing antibodies (2C1 and 4D11) incubated for 16–20 h. Cells were then washed with media three times and maintained in culture media. For HDV and HBV infection, 4% PEG 8000 was present to increase infection efficiency during the viral inoculation period. The culture media used contained Williams E medium supplemented with 5 µg/ml transferrin, 10 ng/ml epidermal growth factor (EGF), 3 µg/ml insulin, 2 mM L-glutamine, 18 µg/ml hydrocortisone, 40 ng/ml dexamethasone, 5 ng/ml sodium selenite, 2% dimethyl sulfoxide (DMSO), 100 U/ml penicillin and 100 µg/ml streptomycin.
Data processing and analysis
All experiments were repeated several times with duplicate or triplicate samples for each condition. A representative result of multiple independent experiments is present in each of the figures. Data are expressed as means±s.d. A paired Student's t-test was used to compare the differences between the treated groups and their paired controls. Differences were considered to be statistically significant for P values ≤0.05.
Results
hNTCP, but not mouse NTCP, expression in human hepatocyte cell lines supports HDV/HBV infection
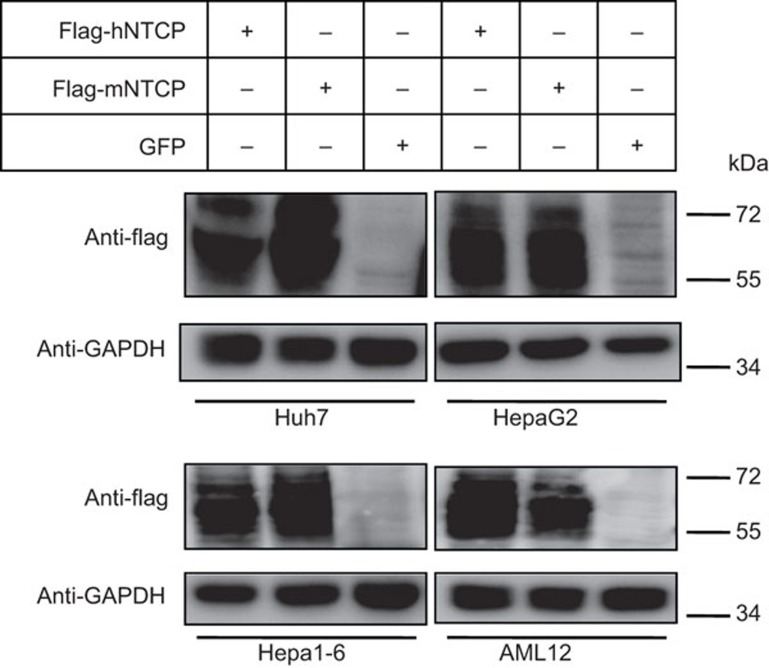
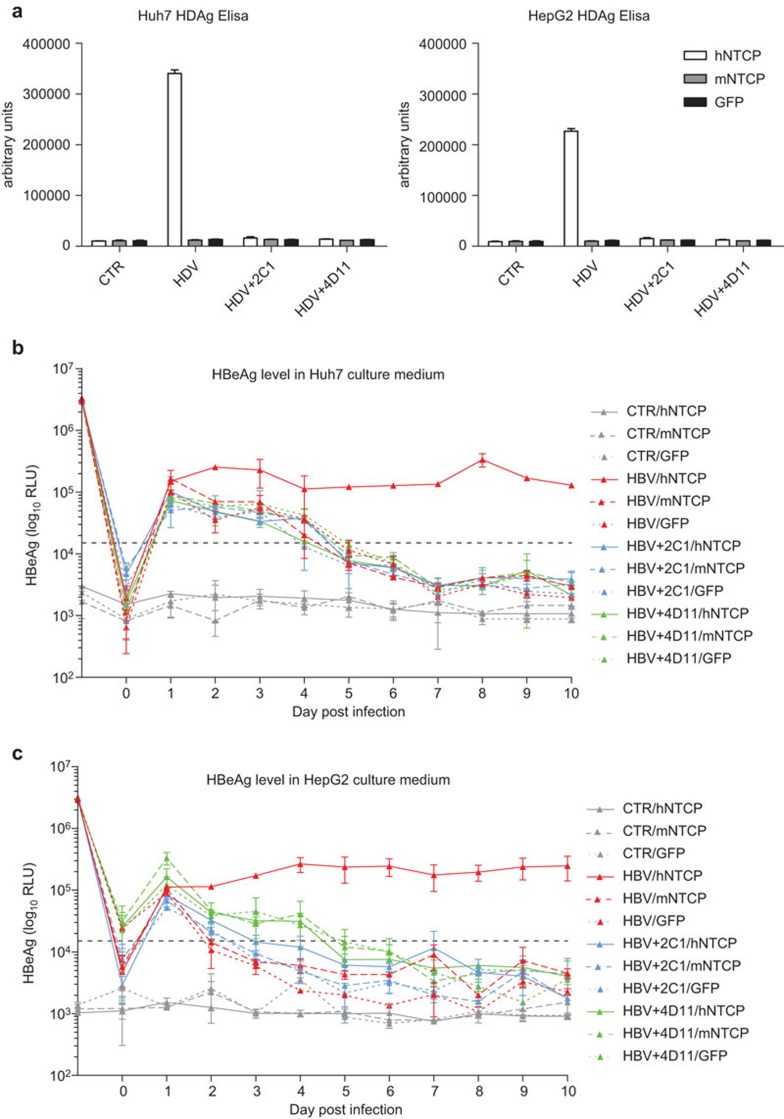
We stably expressed hNTCP, mouse NTCP and control GFP in the four hepatocyte cell lines (Huh7, HepG2, Hepa1–6 and AML-12) by lentiviral vectors. hNTCP and mouse NTCP were fused with a flag tag at the C-terminal. Western blotting, using the anti-flag antibody, shows that the protein levels of both hNTCP and mouse NTCP are comparable among all four of the cell lines (Figure 1). NTCP is a glycosylated membrane protein and has a western blot smear pattern between 55 kD and 72 kD. Published studies have reported that hNTCP expression confers HDV/HBV susceptibility to Huh7 and HepG2 cells, whereas mouse NTCP does not.13,16 To validate the stable cell lines generated for subsequent experiments, we repeated HDV and HBV infection in Huh7 and HepG2 cells. HDV and HBV were infected at 100 mge and 500 mge, respectively, either in the presence or absence of neutralizing antibodies. The monoclonal antibody 2C1 is a neutralizing antibody against the HBV ‘a' determinant18 and the monoclonal antibody 4D1119 is a neutralizing antibody against amino acid 21–47 in the pre-S1 region of the large envelope protein. hNTCP expression significantly increases the level of HDV viral antigen HDAg in the lysates of HDV-infected Huh7 and HepG2 cells (Figure 2a). Similarly, expression of human but not mouse NTCP in Huh7 and HepG2 cells significantly increased viral antigen HBeAg levels in the culture media (Figure 2b and c). Both 2C1 and 4D11 block HDV/HBV infection effectively. This is consistent with the current knowledge that pre-S1 region and ‘a' determinant are two critical regions required for HBV infection. Our data confirm previous studies that hNTCP, but not mouse NTCP, can support both HDV and HBV infection in human hepatocyte cell lines.
Figure 1.
Expression of human NTCP, mouse NTCP or GFP by lentiviral infection in Huh7, HepG2, Hepa1–6 and AML-12 cells. Huh7, HepaG2, Hepa1–6 and AML12 cells were infected with lentivirus expressing human and mouse NTCP with a flag taq at the C terminal, together with GFP as a negative control. Extracts were collected 48 h after infection. Immunoblotting was conducted with anti-flag antibodies to detect NTCP protein levels from the four cell lines. NTCP, sodium taurocholate cotransporting polypeptide.
Figure 2.
Human NTCP, but not mouse NTCP, expression in Huh7 and HepG2 cells supports HDV/HBV infection. (a) Huh7 and HepG2 cells were infected with lentivirus expressing human NTCP, mouse NTCP or GFP. Forty-eight hours after infection, the cells were inoculated with or without HDV at 100 mge in the presence or absence of 2C1 and 4D11. Seven days post inoculation, the cells were lysed and HDAg was detected using an ELISA kit. Huh7 (b) and HepG2 (c) cells expressing human NTCP, mouse NTCP or GFP were inoculated with or without HBV at 500 mge in the presence or absence of 2C1 and 4D11. Culture media were changed every day, the supernatants were collected and HBeAg was detected using an ELISA kit. The HBeAg level at day 1 represents the level in the inoculums. The HBeAg level at day 0 represents the level in the fresh media. Data are presented as means±s.d. HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HDAg, hepatitis D antigen; HDV, hepatitis B virus; mge, multiplicities of genome equivalents; NTCP, sodium taurocholate cotransporting polypeptide.
hNTCP expression in mouse hepatocytes supports HDV infection, but not HBV infection
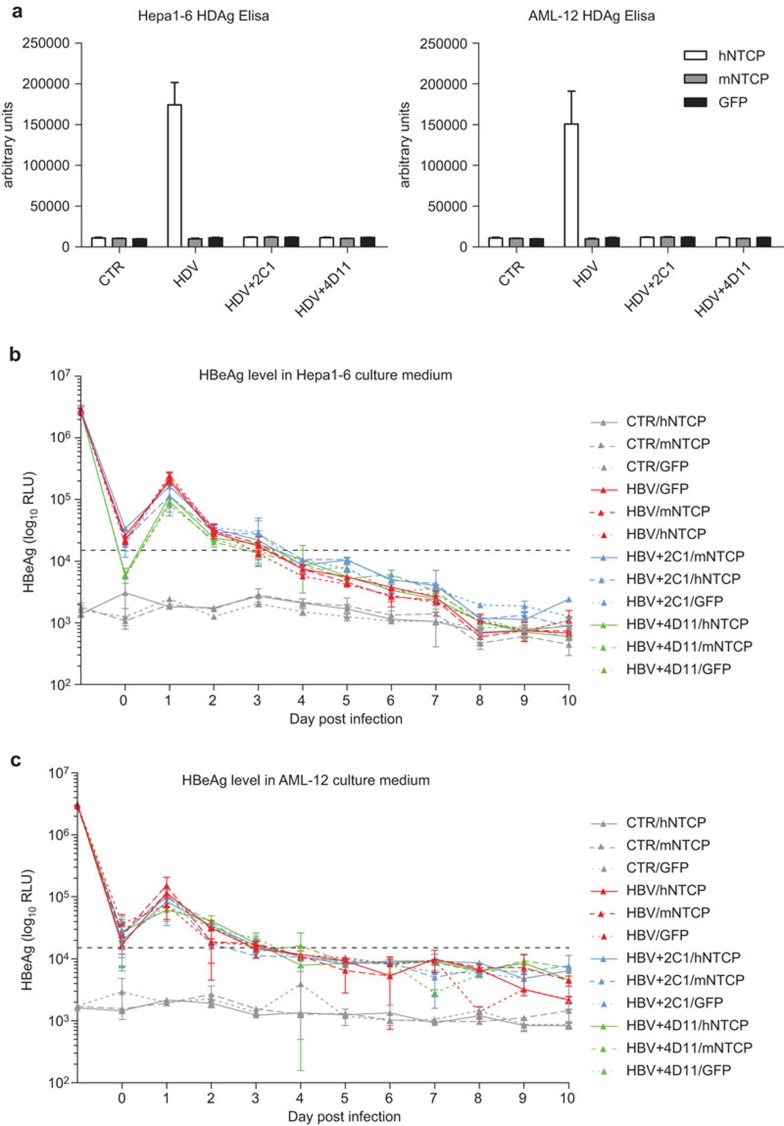
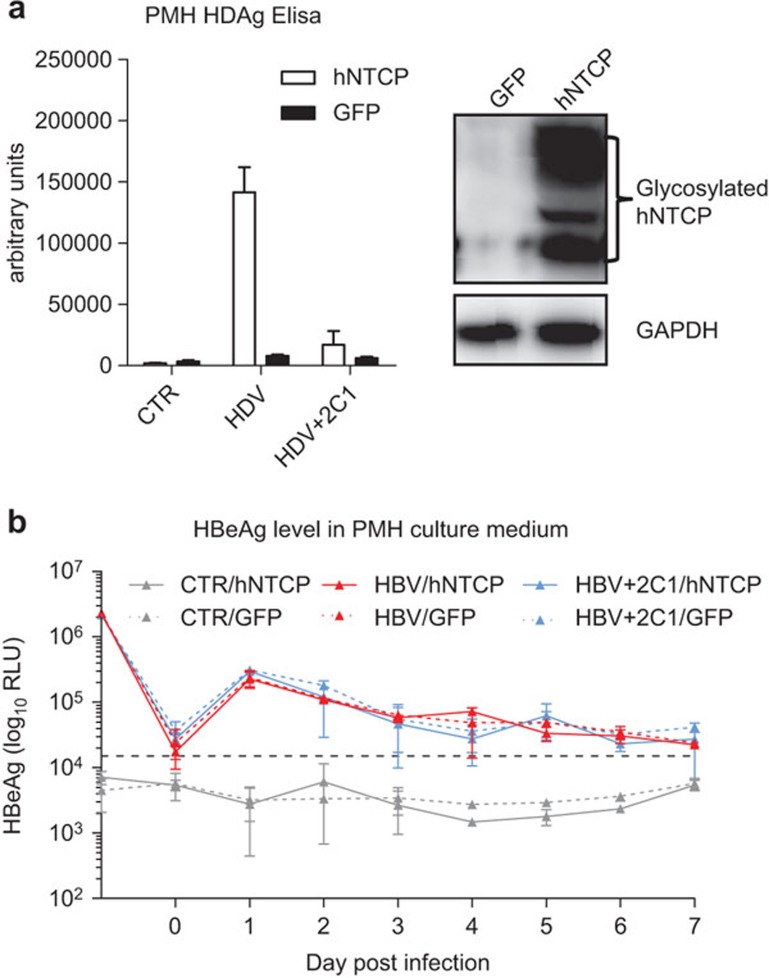
We investigated whether exogenous hNTCP expression can render mouse hepatocyte cell lines permissive to HDV and HBV infection. HDV inoculation of hNTCP-expressing Hepa1–6 and AML-12 cells results in significantly higher levels of HDAg compared with mouse NTCP or GFP-expressing cells (Figure 3a). The increase in HDAg levels can also be blocked by 2C1 and 4D11 (Figure 3a). In contrast to HDV infection, the dynamic levels of secreted HBeAg in the supernatants of HBV-infected hNTCP, mouse NTCP or GFP-expressing Hepa1–6 and AML-12 cells are all the same and are indistinguishable from the neutralizing antibodies-treated groups (Figure 3b and c), indicating that hNTCP expression is not sufficient to support HBV infection in mouse hepatocyte cell lines. To test the possibility that the unsuccessful HBV infection in hNTCP-expressing mouse hepatocyte cell lines is attributed to defects within the cell lines used, we repeated this experiment using PMH cells. We infected PMH cells with adenovirus expressing hNTCP or GFP and then inoculated the PMH cells with HDV and HBV, respectively (Figure 4). hNTCP-expressing PMHs are permissible to HDV infection, which can be blocked by the 2C1 neutralizing antibody (Figure 4a). However, hNTCP-expressing PMH cells are not permissive to HBV infection, as both hNTCP and GFP-expressing PMH cells show similar dynamic patterns of secreted HBeAg and no difference compared to the 2C1-treated groups (Figure 4b). Therefore, our results suggest that while hNTCP does support HDV infection in mouse hepatocytes, it is not sufficient to support HBV infection.
Figure 3.
Human NTCP expression in Hepa1–6 and AML-12 supports HDV infection but not HBV infection. (a) Hepa1–6 and AML-12 cells were infected with lentivirus expressing human NTCP, mouse NTCP or GFP. Forty-eight hours after infection, the cells were inoculated with or without HDV at 100 mge in the presence or absence of 2C1 and 4D11. Seven days post inoculation, the cells were lysed and HDAg was detected using an ELISA kit. Hepa1–6 (b) and AML-12 (c) cells expressing human NTCP, mouse NTCP or GFP were inoculated with or without HBV at 500 mge in the presence or absence of 2C1 and 4D11. Culture media were changed every day, the supernatants were collected and HBeAg was detected using an ELISA kit. The HBeAg level at day 1 represents the level in the inoculums. The HBeAg level at day 0 represents the level in the fresh media. Data are presented as means±s.d. HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HDAg, hepatitis D antigen; HDV, hepatitis B virus; mge, multiplicities of genome equivalents; NTCP, sodium taurocholate cotransporting polypeptide.
Figure 4.
Human NTCP expression in PMHs supports HDV infection, but not HBV infection. (a) PMHs were infected with adenovirus expressing human or GFP. Twenty-four hours after infection, the cells were inoculated with or without HDV at 100 mge in the presence or absence of 2C1. Seven days post inoculation, the cells were lysed and HDAg was detected using an ELISA kit. Rabbit anti-human NTCP polyclonal antibodies were used in the western blotting for detecting human NTCP expression. (b) PMH cells expressing human NTCP or GFP were inoculated with or without HBV at 500 mge in the presence or absence of 2C1. Culture media were changed every day, the supernatants were collected and HBeAg was detected using an ELISA kit. The HBeAg level at day 1 represents the level in the inoculums. The HBeAg level at day 0 represents the level in the fresh media. Data are presented as means±s.d. HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HDAg, hepatitis D antigen; HDV, hepatitis B virus; mge, multiplicities of genome equivalents; NTCP, sodium taurocholate cotransporting polypeptide; PMH, primary mouse hepatocyte.
Both human and mouse hepatocyte cell lines secrete comparable levels of HBeAg when the HBV genome sequence is introduced into the cells by an adenoviral vector
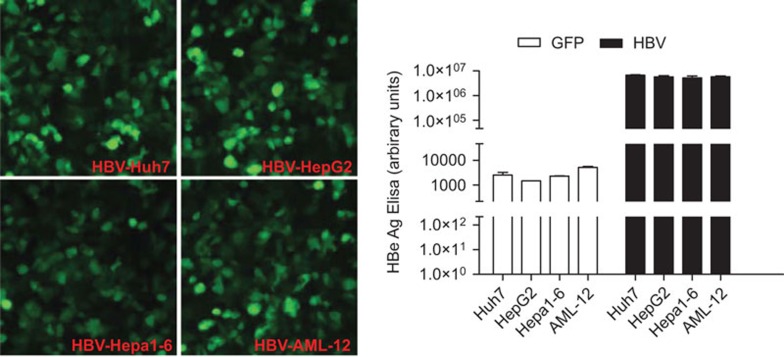
It is possible that mouse hepatocyte cell lines, compared with human hepatocyte cell lines, are less efficient in the expression or secretion of HBV viral proteins, thus resulting in undetectable levels of HBeAg in the culture media of the infected cells. To examine this possibility, we subcloned HBV 1.3 genomic DNA into an adenoviral vector and introduced it into four different cell lines, Huh7, HepG2, Hepa1–6 and AML-12. The adenovirus vector also contains a GFP gene, allowing us to evaluate the infection efficiency. By controlling the titer of the HBV genome-expressing adenovirus, we ensured that the four cell lines were approximately 80%–100% GFP-positive 36 h after infection (Figure 5, left panel). Sixty hours after infection, the cell culture media were collected and HBeAg levels were measured. The results showed that the four cell lines secreted HBeAg at comparable levels (Figure 5, right panel), indicating that the limitation of HBeAg expression in mouse hepatocyte cell lines is not due to the inefficient expression of HBeAg in the mouse cells.
Figure 5.
Huh7, HepG2, Hepa1–6 and AML-12 cells secrete comparable levels of HBeAg when infected with HBV genome-expressing adenovirus. Huh7, HepG2, Hepa1–6 and AML-12 cells were infected with adenovirus expressing the HBV genome or GFP as a control. The HBV-expressing adenovirus vector also contains a GFP gene allowing us to evaluate the infection efficiency. By titrating the titer of the HBV genome-expressing adenovirus, we ensure that the four cell lines are approximately 80%–100% GFP-positive at a similar intensity 36 h after infection. Sixty hours after infection, the supernatants were collected and measured for HBeAg. Data are presented as means±s.d. HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
Expressing human hepatocyte nuclear factors (HNFs) or attenuating anti-viral signaling in hNTCP-expressing Hepa1–6 fails to complete the HBV life cycle
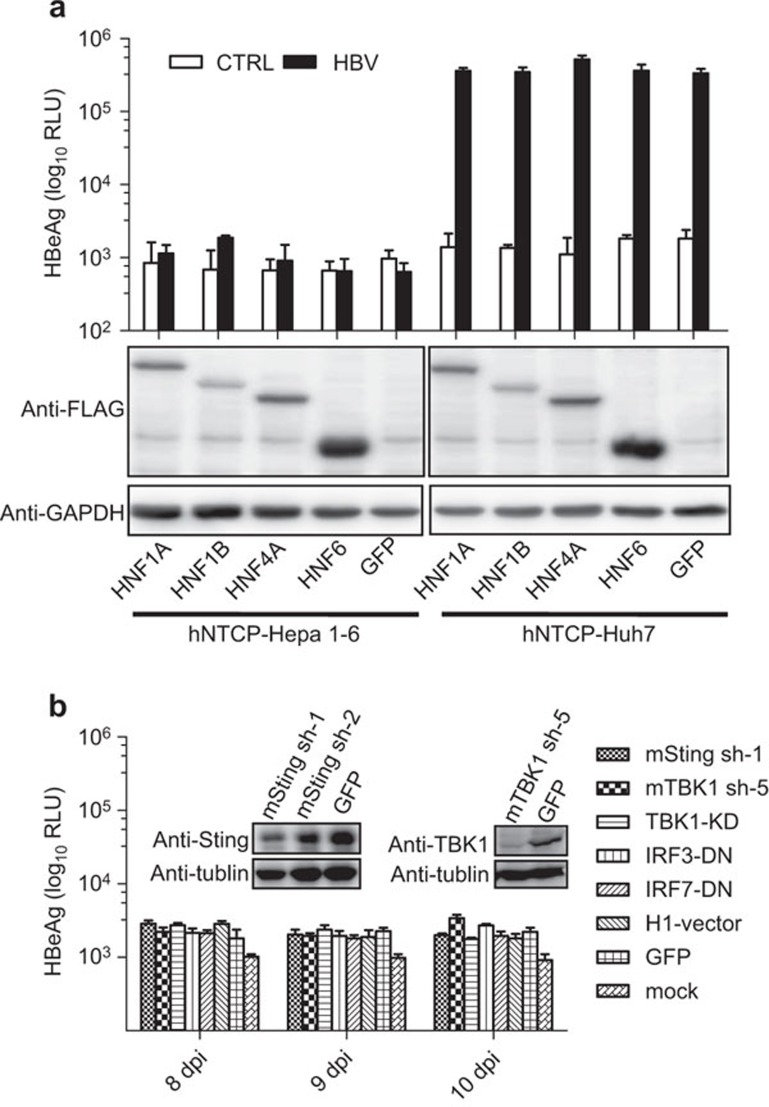
Previous studies show that human HNF4 alpha is required for HBV replication.20 Thus we tested the possibility that human HNFs may be required to complete HBV life cycle in mouse hepatocyte cell lines. We overexpressed HNF1 alpha (HNF1A), HNF1 beta (HNF1B), HNF4 alpha (HNF4A), HNF6 or GFP in hNTCP-expressing Hepa1–6 and Huh7 cells, respectively (Figure 6a). HNF4A slightly increases HBeAg levels in hNTCP-Huh7 by less than twofold, but the rest of the HNF proteins have no effect on the HBeAg levels compared with GFP (Figure 6a). None of the HNF proteins have an effect on the HBeAg levels in hNTCP-Hepa1–6 cells, as the HBeAg levels in the HBV-treated group are indistinguishable from the control group (Figure 6a).
Figure 6.
Expressing human HNFs or attenuating the function of key components of anti-viral signaling pathway in human NTCP-expressing Hepa1–6 cannot eliminate the restriction of HBV in mouse hepatocytes. (a) hNTCP-Hepa1–6 and hNTCP-Huh7 cells were infected with lentiviruses expressing HNF1A, HNF1B, HNF4A, HNF6 or GFP. Then, those cells were infected with HBV at 100 mge. Ten days post inoculation, the supernatants were collected and measured for HBeAg levels. (b) hNTCP-Hepa1–6 was infected with lentiviruses expressing mouse Sting shRNA (mSting sh-1), mouse TBK1 shRNA (mTBK1 sh-5), dominant-negative mutants of TBK1 (TBK1-KD), IRF3 (IRF3-DN) and IRF7 (IRF7-DN), GFP and an empty vector. The cells were then infected with HBV at 100 mge. Supernatants at 8, 9 and 10 days post inoculation were collected and measured for HBeAg levels. The knockdown efficiencies of mSting and mTBK1 are shown. Data are presented as means±s.d. HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HNF, hepatocyte nuclear factor; mge, multiplicities of genome equivalents; NTCP, sodium taurocholate cotransporting polypeptide.
Recent studies show that transgenic mice, stably expressing human CD81 and OCLN, support hepatitis C virus (HCV) entry, but innate and adaptive immune responses restrict HCV infection in vivo.21 Blunting antiviral immunity in genetically humanized mice results in the production of measurable viremia over several weeks.21 Although host immune responses toward HCV and HBV are very different, it is worthwhile to evaluate whether the restriction of HBV can result from an anti-viral immune response toward HBV in mouse hepatocyte cell lines. We then tested whether attenuating anti-viral signaling in hNTCP-Hepa1–6 cells allows for successful HBV infection as in the case of HCV.21 Because Sting, TBK1 and IRF3/7 are several central mediators of anti-viral immunity in response to DNA viruses, we knocked down Sting or TBK1 or overexpressed dominant-negative mutants of TBK1 (TBK1-KD), IRF3 (IRF3-DN) and IRF7 (IRF7-DN) in hNTCP-Hepa1–6 cells (Figure 6b). Mouse Sting sh-RNA (sh-1) and mouse TBK1 sh-RND (sh-5) effectively knock down Sting and TBK1, respectively (Figure 6b). The effects of TBK1-KD, IRF3-DN and IRF7-DN on attenuating interferon production were determined by infecting those cells with herpes simplex virus-1 (data not shown), which induces robust immune response. As shown in Figure 6b, expression of mSting sh-1, mTBK1 sh-5, TBK1-KD, IRF3-DN or IRF7-DN did not increase the HBeAg levels in HBV-treated hNTCP-Hepa1–6 cells.
Discussion
NTCP was identified recently as a receptor of HBV in human hepatocytes. We were able to reproduce previous studies and show that expression of hNTCP in mouse hepatocytes is not sufficient to support HBV infection. Because hNTCP can render mouse hepatocytes permissible to HDV infection and the genomic DNA of HBV can effectively express HBeAg proteins, we believe that mouse cells very likely exert a restriction on HBV after HBV entry and before viral replication. Because either expressing human hepatocyte nuclear factors or attenuating anti-viral signaling in mouse hepatocyte cell lines fails to eliminate the restriction on HBV infection in mouse cells, this restriction should not be attributed to the assumed impotency of mouse HNFs in supporting HBV replication or anti-viral signaling toward HBV in mouse hepatocyte cell lines.
NTCP is a sodium-dependent transporter for taurocholic acid that belongs to a family of solute carrier proteins that consists of seven members (SCL10A1–A7).22,23 NTCP is expressed in hepatocytes at the basolateral membrane. The protein expression of NTCP is controlled by hepatocyte-specific transcription factors, including HNF3 and C/EBP. The expression pattern of NTCP is consistent with the observed tissue tropism of HBV.24,25 The identification of hNTCP as a functional receptor for HBV has resolved a fundamental question in HBV studies. Researchers in the HBV field can now utilize hNTCP to generate cell lines susceptible to HBV infection. This will facilitate the study of many poorly understood steps in the HBV life cycle, including additional host factors required for viral entry, endocytosis of HBV virus, transport of core particles into the nucleus and the cccDNA formation. However, to date, scientists in the HBV field are still limited by the lack of a mouse model to investigate HBV pathogenesis in vivo26 because mouse NTCP cannot function as an HBV receptor and expression of hNTCP does not support HBV infection in mouse hepatocytes.13,16
Our data demonstrated that expression of hNTCP does not support HBV infection in mouse hepatocytes, suggesting that transgenic mice expressing hNTCP cannot be used as a model system to study HBV infection. It has been shown in previous studies that copy number amplification of cccDNA does not occur in normal mouse hepatocytes of HBV transgenic mice. However, it can be detected in HBV transgenic mice that are deficient for HNF1A.27,28 Therefore, it would be interesting to investigate whether HNF1A-null mice expressing hNTCP in a liver-specific manner could support HBV infection.
Taken together, the observations with human hepatocyte cell lines and mouse hepatocyte cell lines indicate that a successful HBV infection requires both a functional cell surface receptor and additional intracellular host factors. Subsequent studies using gain-of-function or loss-of-function screening in hNTCP-expressing mouse hepatocyte cell lines may help identify the relevant restriction factors in mouse hepatocyte cell lines and will hopefully lead to the construction of an HBV-permissible mouse line.
Acknowledgments
This work was supported by National Scientific and Technological Major Project (2013ZX10002-002). We would like to thank Junzong Shao for editing our manuscript.
References
- Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85. doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- Gripon P, Le Seyec J, Rumin S, Guguen-Guillouzo C. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology. 1995;213:292–299. doi: 10.1006/viro.1995.0002. [DOI] [PubMed] [Google Scholar]
- Le Seyec J, Chouteau P, Cannie I, Guguen-Guillouzo C, Gripon P. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J Virol. 1999;73:2052–2057. doi: 10.1128/jvi.73.3.2052-2057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouteau P, Le Seyec J, Cannie I, Nassal M, Guguen-Guillouzo C, Gripon P. A short N-proximal region in the large envelope protein harbors a determinant that contributes to the species specificity of human hepatitis B virus. J Virol. 2001;75:11565–11572. doi: 10.1128/JVI.75.23.11565-11572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet M, Sureau C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J Virol. 2007;81:5841–5849. doi: 10.1128/JVI.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duff Y, Blanchet M, Sureau C. The pre-S1 and antigenic loop infectivity determinants of the hepatitis B virus envelope proteins are functionally independent. J Virol. 2009;83:12443–12451. doi: 10.1128/JVI.01594-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Jaoude G, Sureau C. Entry of hepatitis delta virus requires the conserved cysteine residues of the hepatitis B virus envelope protein antigenic loop and is blocked by inhibitors of thiol-disulfide exchange. J Virol. 2007;81:13057–13066. doi: 10.1128/JVI.01495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol. 2008;10:122–133. doi: 10.1111/j.1462-5822.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- Salisse J, Sureau C. A function essential to viral entry underlies the hepatitis B virus “a” determinant. J Virol. 2009;83:9321–9328. doi: 10.1128/JVI.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Mehrle S, Weiss TS, Mier W, Urban S. Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology. 2013;58:31–42. doi: 10.1002/hep.26181. [DOI] [PubMed] [Google Scholar]
- Schieck A, Schulze A, Gahler C, Muller T, Haberkorn U, Alexandrov A, et al. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology. 2013;58:43–53. doi: 10.1002/hep.26211. [DOI] [PubMed] [Google Scholar]
- Yan H, Peng B, He W, Zhong G, Qi Y, Ren B, et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol. 2013;87:7977–7991. doi: 10.1128/JVI.03540-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner SK, Otto MJ, Barker CS, Zaifert K, Wang GH, Guo JT, et al. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother. 1997;41:1715–1720. doi: 10.1128/aac.41.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, et al. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J Hepatol. 2012;57:720–729. doi: 10.1016/j.jhep.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Ge S, Xiong J, Yan Q, Li Z, Hao X, et al. A novel immunoassay for PreS1 and/or core-related antigens for detection of HBsAg variants. J Virol Methods. 2010;168:108–113. doi: 10.1016/j.jviromet.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Tang H, McLachlan A. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc Natl Acad Sci USA. 2001;98:1841–1846. doi: 10.1073/pnas.041479698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner M, Horwitz JA, Donovan BM, Labitt RN, Budell WC, Friling T, et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501:237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–1823. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- Shiao T, Iwahashi M, Fortune J, Quattrochi L, Bowman S, Wick M, et al. Structural and functional characterization of liver cell-specific activity of the human sodium/taurocholate cotransporter. Genomics. 2000;69:203–213. doi: 10.1006/geno.2000.6329. [DOI] [PubMed] [Google Scholar]
- Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol. 2004;286:G752–G761. doi: 10.1152/ajpgi.00456.2003. [DOI] [PubMed] [Google Scholar]
- Seeger C, Mason WS. Sodium-dependent taurocholic cotransporting polypeptide: a candidate receptor for human hepatitis B virus. Gut. 2013;62:1093–1095. doi: 10.1136/gutjnl-2013-304594. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney AK, Eggers CM, Kline EF, Guidotti LG, Pontoglio M, Yaniv M, et al. Nuclear covalently closed circular viral genomic DNA in the liver of hepatocyte nuclear factor 1 alpha-null hepatitis B virus transgenic mice. J Virol. 2001;75:2900–2911. doi: 10.1128/JVI.75.6.2900-2911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]