Inflammasomes are large multiprotein platforms that translate recognition of immune ‘danger' into activation of pro-caspase-1. Caspase-1 in turn proteolytically activates the precursors of the pro-inflammatory cytokines IL-1β and IL-18, which are secreted to support the inflammatory response. The recruitment of pro-caspase-1 to both NLRP3- and absent in melanoma 2 (AIM2)-containing inflammasome complexes is mediated by the apoptosis-associated speck-like protein containing a caspase-recruitment domain (CARD) (ASC). Upon NLRP3 and AIND activation, ASC proteins self-aggregate to form complex structures known as ASC specks, which then recruit pro-caspase-1 through CARD–CARD interactions.1,2
In a recent study,3 Hara et al. observed that, upon NLRP3 and AIM2 inflammasome activation, the tyrosine kinases Syk and Jnk regulate ASC speck formation. Phosphorylation of ASC molecules is a crucial step for their aggregation, consequent pro-caspase-1 recruitment and cytokines release (Figure 1). In particular, the authors observed that IL-18 release by lipopolysaccharide-primed peritoneal or bone marrow-derived macrophages in response to nigericin and alum (NLRP3 activators), or poly(dA:dT) (an AIM2 activator) was severely impaired by Syk and Jnk inhibitors. Similar results were obtained after infection of macrophages with Mycobacterium tuberculosis and Lysteria monocytogenes (which trigger NLRP3 and AIM2 inflammasomes, respectively). Moreover, IL-1β and IL-18 release from Syk, Mapk8 and Mapk9 knockout macrophages was significantly reduced. These data reveal a crucial role for Syk and Jnk kinases in regulating the activation of NLRP3 and AIM2 inflammasomes: treatment of cells with Syk or Jnk inhibitors, or knockdown of Syk or Jnk gene expression abolished caspase-1 activation, illustrating their importance for the activation of caspase-1 via NLRP3 and AIM2 inflammasomes. Hara and colleagues went on to dissect the mechanisms mediated by Syk and Jnk that led to inflammasome activation. While inflammasome-activating signals induced phosphorylation of both Syk and Jnk, intriguingly, it seems that the two kinases acted independently upon inflammasome complexes.
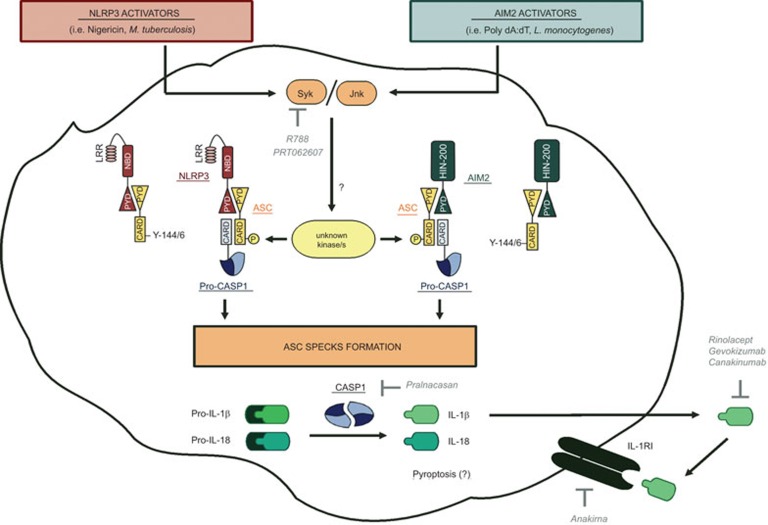
Figure 1.
Post-translational regulation of inflammasome activation by ASC. NLRP3 (nigericine, Mycobacterium tuberculosis) and AIM2 (poly dA:dT, Listeria monocytogenes) activators induce phosphorylation of ASC (Y144 in murine ASC and Y146 in human ASC) through a mechanism that is Syk- and/or Jnk-dependent. The full spectrum of kinase/s involved in the phosphorylation of ASC remains unknown. This mechanism is essential for the recruitment of pro-caspase-1 and the formation of ASC specks, inducing the activation of caspase-1. Activated caspase-1 drives pyroptosis and the cleavage of pro-inflammatory cytokines, such as IL-1 β and IL-18, which are then released and can signal through their receptors. The compounds targeting this pathway that have undergone, or are undergoing, clinical trials are shown in italics. AIM2, absent in melanoma 2; ASC, apoptosis-associated speck-like protein; CARD, caspase-recruitment domain; CASP, caspase; HIN200, hematopoietic interferon-inducible nuclear antigens with 200 amino-acid repeat; IL, interleukin; IL-1RI, interleukin-1 receptor type I; Jnk, c-Jun N-terminal kinase; LRR, leucine rich repeat; NBD, nucleotide binding domain; NLRP3, Nod-like receptor family, pyrin domain-containing-3; PYD, pyrin domain; Syk, splenic tyrosine kinase; Y, tyrosine.
Since ASC is a necessary adaptor molecule for pro-caspase-1 recruitment to NLRP3 and AIM2 inflammasomes, the authors asked whether Syk and Jnk kinases were involved in the NLRP3/ASC or AIM2/ASC interaction. Using an in situ proximity ligation assay, they found that pretreatment of cells with Syk or Jnk inhibitors did not affect ASC/NLRP3 interaction following stimulation with nigericin. However, Syk and Jnk signaling was required for formation of the ASC specks and pro-caspase-1 recruitment in macrophages stimulated with nigericin and poly(dA:dT). Following inflammasome activation, ASC phosphorylation was Syk- and Jnk-dependent, and the authors identified Tyr144 as a putative site of ASC phosphorylation by these kinases (Figure 1). Further experiments will reveal the mechanisms of ASC phosphorylation since there is no clear evidence that Syk or Jnk can phosphorylate ASC directly.
The requirement for Syk and Jnk for NLRP3 inflammasome activation was also assessed in vivo in a mouse model of MSU- and alum-induced peritonitis. Infiltration of the inflammatory cells into the peritoneal cavity was severely impaired in mice lacking Syk (Syk−/− chimeras) or Jnk (Mapk8−/− and Mapk9−/− chimeras) in the immune compartment after MSU and alum challenge. Similarly, infiltration of inflammatory cells was reduced in wild-type mice treated with Jnk inhibitors. Thus, the expression of Syk and Jnk kinases in the hematopoietic compartment was crucial for NLRP3-associated ASC phosphorylation, leading to ASC speck formation which supports MSU- and alum-driven recruitment of inflammatory cells to the peritoneal cavity.
Overall, this paper provides novel insight into a new molecular mechanism of inflammasome activation, in which Syk and Jnk kinases regulate inflammasome complex formation through the phosphorylation of ASC molecules, leading to ASC oligomerization and caspase-1 activation (Figure 1). The findings of Hara et al. form a solid base from which to explore regulation of inflammasome activity. It would be interesting, for example, to investigate whether ligands that trigger inflammasome activation through Dectin receptors and Syk-dependent production of reactive oxygen species (ROS) similarly induce the phophorylation of ASC by Syk and Jnk kinases: furthermore, which of these pathways predominate in vivo, and under what conditions, will make for fascinating research.
More broadly speaking, these findings, and the questions arising as a result of the current study, have direct implications for clinical research. For example, an additional consequence of inflammasome activation by intracellular pathogens can be a type of caspase-1-dependent inflammatory cell death called pyroptosis.4 Excessive pyroptosis is a feature of pathological conditions, including endotoxic shock, inflammatory bowel disease, cerebral ischemia and myocardial infarction.5 It would be interesting to establish whether, as for caspase-1-mediated cytokines production, pyroptosis depends on Syk and Jnk phosphorylation of ASC. This would open the possibility of using Syk and Jnk inhibitors for the treatment of pyroptosis-associated disorders in the clinic.
Similarly, excessive inflammasome-mediated IL-1 production is linked to several autoinflammatory diseases including cryopyrin-associated periodic syndromes (CAPS) and deficiency of the IL-1-receptor antagonist. Drugs to treat these conditions currently rely upon interference with IL-1 signaling;6 for example, Anakinra (Kineret), is an IL-1 receptor antagonist used to treat CAPS and rheumatoid arthritis, while Rinolacept is an IL-1-blocking fusion protein. However, it has been proposed that strategies targeting the processes upstream of IL-1 production might be more effective. Pralnacasan (VX-740; Vertex Pharmaceuticals Inc., Cambridge, MA) is aspecific caspase-1 inhibitor, which showed promise in animal models of osteoarthritis but was too toxic for clinical development.7 Hence, the search goes on.
The findings of Hara and colleagues support a growing body of evidence indicating the importance of Syk and Jnk in the inflammatory response. Accordingly, several drugs targeting these kinases have recently undergone clinical testing in patients suffering from severe auto-inflammation.8 While some promising candidates remain under evaluation (e.g., PRT062607; Biogen Idec, Cambridge, MA) earlier Syk inhibitors suffered from low specificity and high toxicity, which forced their development to be abandoned (AstraZeneca's R-788, for example).9,10 Thus, Hara et al. have revealed that specific inhibition of phosphorylated ASC could be an alternative means to enable suppression of caspase-1 activation and the associated pathological consequences. This approach might enable more selective blocking of IL-1 maturation and release, without the toxicity associated with kinase inhibition.
In conclusion, inflammasome activation and signaling is a multistep process involving numerous molecular players and complex mechanisms that combine to trigger and maintain the inflammatory response. Thus, the interrogation of this pathway will certainly offer, in the near future, new candidates that can be targeted in order to limit pathological dysregulation of the inflammatory response.
References
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activatio of inflammatory caspases and processing of proIL-1beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Zambetti LP, Laudisi F, Licandro G, Ricciardi-Castagnoli P, Mortellaro A. The rhapsody of NLRPs: master players of inflammation…and a lot more. Immunol Res. 2012;53:78–90. doi: 10.1007/s12026-012-8272-z. [DOI] [PubMed] [Google Scholar]
- Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, et al. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, van der Meer JW.Treating inflammation by blocking interleukin-1 in humans Semin Immunol 2013. in press. [DOI] [PMC free article] [PubMed]
- Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W. Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage. 2003;11:738–746. doi: 10.1016/s1063-4584(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov. 2010;9:956–970. doi: 10.1038/nrd3297. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Kavanaugh A, Weinblatt ME, Peterfy C, DiCarlo J, White ML, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum. 2011;63:337–345. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- Flight MH. Deal watch: high hopes for oral SYK inhibitor in rheumatoid arthritis. Nat Rev Drug Discov. 2012;11:10. doi: 10.1038/nrd3631. [DOI] [PubMed] [Google Scholar]