Highlights
-
•
Pearl millet drought tolerance QTL on linkage group 2 is also associated with salt tolerance at reproductive growth stage.
-
•
Drought sensitive parent H77/833-2 accumulated higher root Na+ compared to tolerant PRLT 2/89-33 under short term salinity.
-
•
Rate of Na+ accumulation was faster in roots of H77/833-2, whereas it was gradual in PRLT2/89-33 and the two QTL-NILs.
-
•
Na+ ions accumulated preferentially in the older leaves of PRLT2/89-33 and two QTL-NILs; no trend observed in H77/833-2.
Keywords: Pearl millet, Drought and salt tolerance, Terminal drought tolerance, QTL-NILs, Ionic accumulation, Ionic compartmentation
Abbreviations: ABA, abscisic acid; DAS, days after sowing; DT-QTL, drought tolerance QTL; DT-QTL-NILs, DT-QTL-near isogenic lines; ECiw, electrical conductivity of the irrigation water; LG 2, linkage group 2
Abstract
Earlier, we established that a major drought tolerance QTL on linkage group 2 of pearl millet is also associated with reduced salt uptake and enhanced growth under salt stress. Present study was undertaken to re-assess the performance of drought tolerant (PRLT 2/89-33) and drought sensitive (H 77/833-2) parents along with two QTL-NILs (ICMR 01029 and ICMR 01040), under salinity stress specifically imposed during post-flowering growth stages when plants had developed their ion sinks in full. Time course changes in ionic accumulation and their compartmentalization in different plant parts was studied, specifically to monitor and capture changes conferred by the two alleles at this QTL, at small intervals. Amongst different plant parts, higher accumulation of toxic ion Na+ was recorded in roots. Further, the Na+ concentration in roots of the testcross hybrid of the drought-sensitive parent (H 77/833-2) reached its maximum at ECiw 15 dS m−1 within 24 h after salinity imposition, whereas it continued to increase with time in the testcross hybrids of the drought tolerant parent PRLT 2/89-33 as well as those of its QTL-NILs (ICMR 01029 and ICMR 01004) and reached at its maximum at 120 h stage. Comparison of differential distribution of toxic ions in individual leaves revealed that Na+ ions were not uniformly distributed in the leaves of the drought-tolerant parent and drought-tolerant QTL-NILs; but accumulated preferentially in the older leaves, whereas the hybrid of the drought-sensitive parent showed significantly higher Na+ concentration in all main stem leaves irrespective of their age. Dynamics of chlorophyll and proline concentration variation studied under salt stress at late flowering stages revealed a greater reduction, almost twice, in both leaf chlorophyll and proline concentrations in younger leaves in the hybrids of the sensitive parent as compared to the tolerant parent and QTL NILs. Imposition of salinity stress even at flowering stage affected the yield performance in pearl millet, wherein higher yield was recorded in drought tolerant parent and the two QTL-NILs compared to drought sensitive parent.
1. Introduction
Pearl millet [Pennisetum glaucum (L). Br.] is grown as a grain and stover crop by the poorest farmers in the harshest cropping environments of the arid and semi-arid tropical regions of sub-Saharan Africa and Asia. In these regions, it is predominantly grown as a rainfed crop and severe droughts occur due to scanty and untimely rains during the cropping season. The crop particularly experiences drought stress during post-flowering growth. Apart from drought, areas where pearl millet is grown are often characterised by saline underground waters. In times of severe drought, such brackish ground water is typically the only irrigation option that can save the dying crop.
Genetic mapping studies in pearl millet have identified a major quantitative trait locus (QTL) associated with maintenance of components of grain and stover yield under terminal drought stress conditions (Yadav et al., 2002, 2004; Bidinger et al., 2007), and evidence of constitutive differences in leaf abscisic acid concentration resulting in reduced transpiration rates in genotypes having the drought tolerance alleles at this QTL has been reported (Kholová et al., 2010a,b). Recently, we have established that the drought-tolerance allele at this QTL also contributes to better performance under salinity and alkalinity stress conditions (Sharma et al., 2011). Testcross hybrids of the QTL donor parent (drought-tolerant PRLT 2/89-33), QTL recipient parent (drought-sensitive H 77/833-2), and a set of six near-isogenic lines (NILs) derived by marker-assisted backcross introgression into H 77/833-2 background of donor parent alleles in the vicinity of the terminal drought tolerance (DT) QTL (QTL-NILs) were evaluated at germination, vegetative and maturity stages under three salinity and alkalinity levels in that study (Sharma et al., 2011). It was revealed that the DT-QTL alleles contributed by donor PRLT 2/89-33 exerted favourable effects on growth and productivity traits under conditions of salt stress right from the seedling stage through to maturity, by better compartmentalization of Na+ in nodes and internodes besides limiting Na+ accumulation in leaves.
During germination and emergence, tolerance is based on percent survival, while during the later developmental stages, tolerance results from a complex interaction of multitude of adaptation strategies involving compartmentation of toxic ions, accumulation of osmolytes and conservation of water. Therefore, the present study was undertaken to gain deeper insights of the effects of alleles of this DT QTL under salt stress specifically when the stress is applied at flowering and post-flowering growth stages and to better understand the mechanism of saline tolerance conferred by this major QTL at late flowering stages. Drought-tolerant and -sensitive parents, and the two QTL-NILs (ICMR 01029 and ICMR 01040) differing for the drought tolerance QTL (Yadav et al., 2002, 2011), were evaluated under post-flowering salt stress conditions by imposing salinity treatments beginning at 45 days after crop emergence, when flowering has almost completed and the plant sink is fully developed. Ionic accumulation and compartmentalization was quantified in different plant parts as a function of time after imposition of the stress, specifically to monitor and capture changes at small intervals during post-flowering growth conferred by two alleles at this QTL in fully developed ion sinks.
The synthesis and accumulation of compatible osmolytes such as proline has been reported widely as a metabolic response to maintain osmotic pressure under salt and water stress in many plant species (Ramanjulu and Sudhakar, 2000; deLacerda et al., 2003; Demiral and Türkan, 2005; Desingh and Kanagaraj, 2007; Koca et al., 2007; Sneha et al., 2013). In addition to its role as a cytosolic osmolyte, proline also serves as a sink for energy to regulate redox potentials, as a hydroxyl radical scavenger, and as a solute that protects macromolecules against denaturation (Blum and Ebercon, 1976; Simiroff and Cumbes, 1989; Venkamp et al., 1989). Therefore, besides ionic analysis, proline and chlorophyll concentrations were also assessed in individual leaves of the experimental materials used in this study to investigate whether the concomitant changes, if any, occurring in their levels under salinity stress is associated with salinity tolerance.
2. Materials and methods
2.1. Plant material
Plant material chosen was essentially the same as described in Sharma et al. (2011) and Kholová et al. (2010a,b). Testcross hybrids of two parental genotypes, PRLT 2/89-33 (drought-tolerant, donor parent for drought tolerance QTL) and H 77/833-2 (drought-sensitive, recipient parent for drought tolerance QTL), and two QTL-NILs (ICMR 01029 and ICMR 01040) were used in the detailed investigations reported in this study.
2.2. Plant growth and salinity stress treatments
Ten replicates of each genotype were sown directly in 20 kg capacity ceramic pots filled with sand. Initially, 15 seeds were sown in each pot. Emerged seedlings were thinned to three seedlings per pot 15 days after sowing (DAS). These plants were allowed to grow under normal conditions up to 45 DAS in a net house at the Central Soil Salinity Research Institute (CSSRI), Karnal, Haryana, India, from June to September, 2009, when natural growing conditions (temperature 25–35 °C, relative humidity 60–82%) are generally favourable for pearl millet growth and development. During this period, plants received irrigation daily with ¼ strength Hoagland nutrient solution.
At 45 DAS, salinity stress was initiated to study salt relations of these genotypes as a function of time at a stage when they have fully developed their sink capacity under non-stress conditions. This would permit study of salt uptake by the plants as well as of their compartmentalization ability of the toxic ions in the already developed and fully-expanded tissues/sink over time when confronted with salinity stress. At this stage, pots were divided into three sets: one set of plants received irrigation of ECiw 2.0 dS m−1, while the other two received irrigation of ECiw 10.0 and 15.0 dS m−1, respectively. The saline water for irrigation was prepared by adding NaCl, Na2SO4 and CaCl2, keeping Na:Ca and Cl:SO4 ratios of 4:1 in ¼ strength Hoagland nutrient solution as described in Sharma et al. (2011). The pots were irrigated daily so as to maintain the respective salinity level in the root zone. Plants were sampled destructively at the time of initiation of salinity stress treatments (zero hour) and thereafter at 24, 48 and 120 h following initial imposition of the salinity stress treatments.
2.3. Measurement of chlorophyll, proline, ion concentrations and yield related parameters
At each sampling, the plants were separated into stem, root and the individual leaves. For the sake of uniformity, eight leaves were sampled from each plant with basal leaf labelled as 1 and topmost leaf on the main stem as 8. The individual leaves were excised from the main stem to record chlorophyll a, chlorophyll b and total chlorophyll by extraction in 80% ethanol. For measuring chlorophyll, the extract absorbance was measured at 642 and 665 nm on a spectrophotometer and chlorophyll concentrations were calculated according to Arnon (1949). Required amounts of 300 mM glacial acetic acid were subsequently added to the same solution to make final concentrations 100 mM. The tissue was re-extracted for 2 h at 90 °C for the determination of Na+ and K+ concentrations of the individual leaves as described in Yeo and Flowers (1983). The ionic concentrations were also determined from main stem and root samples following wet digestion, using flame spectrophotometery as described by Sharma et al. (2011). Four replications were used for measuring ion concentrations, which were calculated as mmol g−1 fresh weight in case of leaves and as mmol g−1 dry weight in case of stem and root samples. The salinity-induced changes in proline concentrations were also recorded in different leaves following Bates et al. (1973).
Saline irrigation continued in the remaining six replications until harvest of the crop for recording yield and yield-related parameters. At maturity, three plants per pot were harvested and air dried prior to recording their biomass, stover and grain yields as described in Yadav et al. (2002, 2004).
2.4. Analysis of the data
Statistical analyses, including analysis of variance (ANOVA), were conducted using the statistical programme package Windostat ver. 8.5. Data were analysed using salinity and genotypes as main effects. Genotype × salinity × time-course treatment interactions were also analysed.
3. Results
3.1. Ionic accumulation in different plant parts
Significant genotypic differences in ion accumulations were evident amongst the two parental genotypes and two QTL-NILs in all the three plant parts (viz. main stem, roots and leaves) in the salinity treatments and the time-course of salinity treatments applied in this study (Fig. 1).
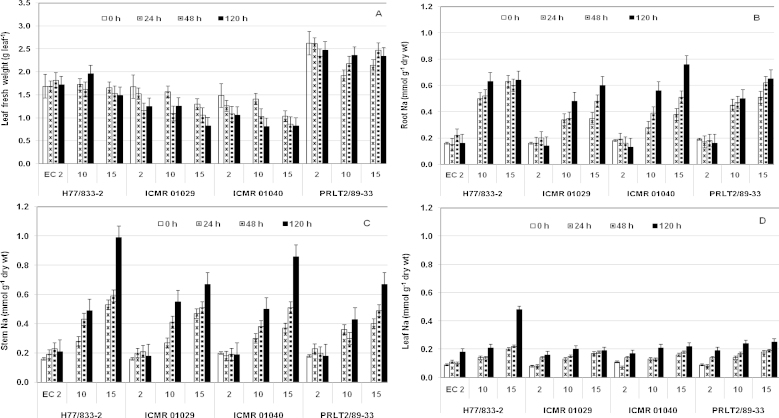
Fig. 1.
Time course changes in leaf fresh weight (g leaf−1, mean of 8 leaves per plant) (A) and Na+ concentration (mmol g−1) in root (B), main stem (C) and leaves (D) of pearl millet parental genotypes (H 77/833-2, PRLT 2/89-33) and QTL-NILs (ICMR 01029, ICMR 01040) differing for a terminal drought tolerance QTL, at three salinity levels (ECiw 2, 10 and 15 dS m−1 imposed at 45 days after sowing). Na+ concentration expressed on dry weight basis in stem and root and on fresh weight basis in leaves.
Twenty-four hour after imposition of salinity stress, maximum Na+ accumulation was recorded in roots (Fig. 1B) followed by the main stem (Fig. 1C) and leaves (Fig. 1D). Na+ accumulation increased continuously in these plant parts with the time-course of salinity treatments, from imposition until 120 h, both in parental genotypes as well as the two QTL-NILs. The salinity-sensitive parent H 77/833-2 recorded highest concentration of Na+ in roots as quickly as 24 h of salinity stress imposition of ECiw 15 dS m−1 and this Na+ level did not increase significantly thereafter (Fig. 1B). On the other hand, Na+ concentration in roots of the tolerant parent PRLT 2/89-33 and the QTL-NILs (ICMR 01029 and ICMR 01040) reached a lower level 24 h after imposition of the salinity stress treatments and then continued to increase throughout the 120 h time-course.
Na+ concentrations in main stems increased both with time and salinity levels and a marked contrast was notable between testcross genotypes at 120 h, in the ECiw 15 dS m−1 treatment (Fig. 1C). At this salinity level, H 77/833-2 accumulated significantly higher Na+ in its stems (0.99 mmol g−1 dry wt) compared to PRLT 2/89-33 (0.67 mmol g−1 dry wt). Compared to levels at the initiation of salinity stress (0.16 and 0.18 mmol g−1 dry wt in sensitive and tolerant parents, respectively), Na+ levels in stems increased by more than 6-fold in the sensitive parent and around 3.7-fold in the tolerant parent. Similar to PRLT 2/89-33, the two QTL-NILs also accumulated lower amounts of Na+ in their main stems than H 77/833-2 (Fig. 1C). At 120 h after salinity treatment imposition at ECiw 15 dS m−1, the mean Na+ concentration in stems of H 77/833-2 was 32% higher than PRLT 2/89-33. Further, the Na/K ratio in stems of H 77/833-2, 120 h after imposition of the salinity treatment of 15 dS m−1, was 6.7 times of the value at zero h; whereas this ratio increased by 3.6 and 4.9 times, in PRLT 2/89-33 and QTL-NILs, respectively.
In strong contrast to roots (Fig. 1B) and main stems (Fig. 1C), leaves recorded lower Na+ concentrations (Fig. 1D). Significant differences in Na+ accumulation started to appear in leaves of the parental genotypes and the QTL-NILs at 24 and 48 h into the time-course at ECiw 10 dS m−1. However, at ECiw 15 dS m−1, non-significant differences were observed in Na+ concentration across all genotypes at 24 and 48 h. It was only after 120 h that Na+ accumulation started to differ significantly between the two parents as H 77/833-2 and PRLT 2/89-33 had accumulated 2.2 and 1.3 times more Na+ than at the 24 and 48 h harvests, respectively. The two QTL-NILs, however, did not show any significant differences in Na+ concentrations when harvested 24, 48 and 120 h after salinity imposition at ECiw 15 dS m−1. Further, H 77/833-2 had accumulated almost double Na+ than PRLT 2/89-33 and more than two times the QTL NILs ICMR 01029 and ICMR 01040 at 120 h after salinity imposition of EC 15 dS m−1. Further, at the same stage and salinity level, Na/K ratio in leaves of the sensitive parent increased to 1.9 times that of the tolerant parent and of the two QTL-NILs.
3.2. Ionic accumulation in individual leaves
Significant differences in leaf Na+ concentration were evident across leaves, genotypes and salinity levels (Fig. 2). At 24 h after initial imposition of stress, H 77/833-2 accumulated 11% higher Na+ concentration than PRLT 2/89-33, and the difference further increased to 29% by 120 h after salinity treatment imposition. Further, the two QTL-NILs, ICMR 01029 and ICMR 01040, accumulated 15% and 20% lower Na+ concentrations, respectively, than H 77/833-2 at 24 h following salinity imposition; this difference further increased with time.
Fig. 2.
Time course changes in Na+ concentration (mmol g−1 leaf fresh weight) in different leaves of pearl millet parental genotypes H 77/833-2 (A) and PRLT 2/89-33 (B), and QTL-NILs ICMR 01029 (C) and ICMR 01040 (D) differing for a terminal drought tolerance QTL, at three salinity levels (ECiw 2, 10 and 15 dS m−1 imposed at 45 days after sowing). Leaf 1 is the basal leaf and leaf 8 is the topmost leaf on the main stem.
Upon imposition of salinity stress, Na+ started accumulating differentially in leaves with the passage of time; with higher Na+ concentrations in basal leaves and lower Na+ concentrations in leaves towards top of the main stem. The sensitive parent H 77/833-2 accumulated higher Na+ levels, regardless of leaf position, (Fig. 2A) than PRLT 2/89-33 (Fig. 2B) with increasing intensities and durations of salinity stress. All the leaves of the two QTL-NILs showed lower Na+ concentrations than did comparable leaves of either of the two parental genotypes (Fig. 2). The same trend of Na+ accumulation continued in different leaves 48 h after salinity imposition in the two parental genotypes as well as in the two QTL-NILs. The most significant observation at ECiw 10 dS m−1 was that the oldest leaf (leaf position 1) of PRLT 2/89-33 started to show increased accumulation of Na+ after 48 h (0.32 mM) as compared to the oldest leaf of H 77/833-2 (0.23 mM). Under the same salinity level (ECiw 10 dS m−1) at 120 h harvest, significant differences in Na+ accumulation were observed amongst the different genotypes studied. The Na+ concentration of leaves continued to increase with time through 120 h at ECiw 15 dS m−1 in all four genotypes evaluated. Na+ concentration in the lowermost leaf was higher in PRLT 2/89-33, 24 and 48 h after salinity stress imposition than in the comparable leaf of H 77/833-2; however, no significant differences were observed in the remaining leaves. Further, significant differences between leaf Na+ concentrations of the four genotypes were observed 120 h after imposition of ECiw 15 dS m−1 salinity stress. The leaves at positions 1 to 7 in H 77/833-2 (Fig. 2A) showed two times greater Na+ concentrations than did comparable leaves of PRLT 2/89-33 (Fig. 2B). Comparison of older and younger leaves in these genotypes revealed that the lowermost three leaves (leaves at position 1, 2, 3) of PRLT 2/89-33 and the QTL-NILs showed significantly higher Na+ concentrations than the uppermost five leaves (leaves at position 4, 5, 6, 7, 8), whereas H 77/833-2 consistently showed significantly higher Na+ concentrations in all leaves, irrespective of their age or position (Fig. 2).
Rate of Na+ accumulation in different leaves also differed amongst tolerant and sensitive parents as well as the QTL-NILs. Na+ accumulation was more rapid during the first 24 h following salinity treatment imposition and slowed thereafter in all genotypes, at ECiw 10 and 15 dS m−1 salinity levels. Genotypes H 77/833-2 and PRLT 2/89-33 did not differ in their rate of Na+ accumulation in different leaves at ECiw 10 dS m−1. However, such differences appeared with increase in salinity level to ECiw 15 dS m−1. During the first 24 h of salinity stress at ECiw15 dS m−1, both H 77/833-2 and PRLT 2/89-33 accumulated Na+ in leaves at rates of 7-11 mmol g−1 leaf fresh weight per hour in the basal leaves and 2-5 mmol g−1 leaf fresh weight per hour in topmost leaves. Though this rate of Na+ accumulation slowed a bit in H 77/833-2 beyond 24 h, a drastic reduction was recorded in PRLT 2/89-33. In the basal leaves of H 77/833-2, the rate of Na+ accumulation reduced from 11 mmol g−1 leaf fresh weight per hour (0–24 h) to 8 mmol g−1 leaf fresh weight per hour during 48–120 h following salinity stress imposition, respectively. The comparable decline in PRLT 2/89-33 was from 11 to 1 mmol g−1 leaf fresh weight per hour during these same intervals. Rate of Na+ accumulation in different leaves of the two QTL-NILs fell between the ranges of their donor and recurrent parents.
The Na/K ratio behaved in a similar fashion in different genotypes with respect to salinity levels, time intervals and leaf positions. This ratio declined with increase in leaf position from base to top. In H 77/833-2, the basal leaf showed 79% higher Na/K than the topmost leaf 120 h after imposition of salinity stress at ECiw 15 dS m−1, whereas, the similar increase in PRLT 2/89-33 and the two QTL-NILs was around 56% and 69% respectively (Table 1). Amongst different leaves, H 77/833-2 recorded around 30% higher Na/K ratios than PRLT 2/89-33 from basal leaf (position 1) to the fifth leaf (position 5), but the difference slightly declined thereafter towards the top.
Table 1.
Na/K ratio 120 h after salinity treatment imposition in main stem leaves 1 (basal) to 8 (top) of two pearl millet parental genotypes and their QTL-NILs, at three salinity levels. Means that do not have a common letter within a column are significantly different by LSD0.05 test.
| Genotypes | Salinity (dS m−1) | Leaf position |
Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 843A × H 77/833-2 | 2 | 0.30ab | 0.28ab | 0.25abc | 0.22a | 0.23a | 0.17abc | 0.14ab | 0.16abc | 0.22 |
| 10 | 0.33ab | 0.29b | 0.23ab | 0.19a | 0.23a | 0.27d | 0.20c | 0.14a | 0.23 | |
| 15 | 1.12f | 0.72d | 0.49d | 0.42b | 0.40b | 0.29d | 0.21c | 0.25d | 0.49 | |
| 843A × ICMR 01029 | 2 | 0.28a | 0.24ab | 0.19ab | 0.20a | 0.22a | 0.18bc | 0.19c | 0.13ab | 0.20 |
| 10 | 0.30ab | 0.25ab | 0.26bc | 0.19a | 0.17a | 0.18bc | 0.17abc | 0.17ac | 0.21 | |
| 15 | 0.59d | 0.28ab | 0.26bc | 0.16a | 0.17a | 0.12a | 0.18bc | 0.21d | 0.25 | |
| 843A × ICMR 01040 | 2 | 0.28a | 0.24ab | 0.20ab | 0.20a | 0.19a | 0.16abc | 0.21c | 0.16abc | 0.20 |
| 10 | 0.40bc | 0.25ab | 0.25abc | 0.19a | 0.21a | 0.19bc | 0.21c | 0.21d | 0.24 | |
| 15 | 0.72e | 0.41c | 0.31c | 0.22a | 0.21a | 0.15ab | 0.19c | 0.23d | 0.30 | |
| 843A × PRLT 2/89-33 | 2 | 0.27a | 0.17a | 0.17a | 0.20a | 0.17a | 0.18bc | 0.22c | 0.17ac | 0.19 |
| 10 | 0.45c | 0.28ab | 0.19ab | 0.20a | 0.19a | 0.21c | 0.13a | 0.17ac | 0.23 | |
| 15 | 0.55d | 0.35bc | 0.32c | 0.23a | 0.18a | 0.17abc | 0.17abc | 0.21d | 0.25 | |
| Mean | 0.47 | 0.31 | 0.26 | 0.22 | 0.21 | 0.19 | 0.19 | 0.18 | 0.25 | |
| CD (P < 0.05) | Genotypes (G) | 0.01*** | G × S | 0.02*** |
| Salinity (S) | 0.01*** | G × L | 0.04*** | |
| Leaf position (L) | 0.02*** | S × L | 0.03*** |
3.3. Chlorophyll concentration in leaves
Significant differences in total chlorophyll concentration were observed with respect to genotypes, salinity levels, leaf position and their interactions 24, 48 and 120 h after initial salinity stress imposition (Fig. 3). The sensitive parent recorded higher total chlorophyll (mg g−1 fresh weight) concentration value (1.60) than the tolerant parent (1.48) on an overall mean basis (means of salinity level, time period and individual leaves), whereas the two QTL-NILs recorded total chlorophyll concentration values of 1.22 (ICMR 01029) and 1.13 (ICMR 01040), respectively. At the start of salinity stress imposition (0 h), all leaves at different positions recorded higher total chlorophyll concentration values in H 77/833-2 (+47% basal leaf and +10% top leaf) (Fig. 3A) than PRLT 2/89-33 (Fig. 3B) or those of the QTL-NILs (+58% basal leaf and +53% top leaf) (Fig. 3C, 3D). However, there was greater reduction of total chlorophyll concentration in leaves of H 77/833-2 under salinity stress (ECiw 15 dS m−1) 24 h later, as chlorophyll concentration values were then similar in the two parental genotypes. Further, at ECiw 15 dS m−1, leaf chlorophyll concentrations were much lower in H 77/833-2 (−75% in basal leaf and −40% on top leaf) than in QTL-NIL ICMR 01029. Similarly, the QTL-NIL ICMR 01040 also showed higher chlorophyll than H 77/833-2 at the higher salinity level (+21% for basal leaf and +19% for top most leaf). At ECiw 15 dS m−1, the mean chlorophyll concentrations declined by 28 and 10% in H 77/833-2 and PRLT 2/89-33 respectively, compared to their controls. In contrast, it increased by 30 (ICMR 01040) to 39% (ICMR 01029) in the QTL-NILs. Further, the total chlorophyll concentrations increased in different leaves from base to top in all the genotypes (Fig. 3).
Fig. 3.
Time course of changes in total chlorophyll concentration (mg g−1 leaf fresh weight) in different leaves of pearl millet parental genotypes H 77/833-2 (A) and PRLT 2/89-33 (B), and QTL-NILs ICMR 01029 (C) and ICMR 01040 (D) differing for a terminal drought tolerance QTL, at ECiw 15 dS m−1 imposed at 45 days after sowing. Leaf 1 is the basal leaf and leaf 8 is the topmost leaf on the main stem.
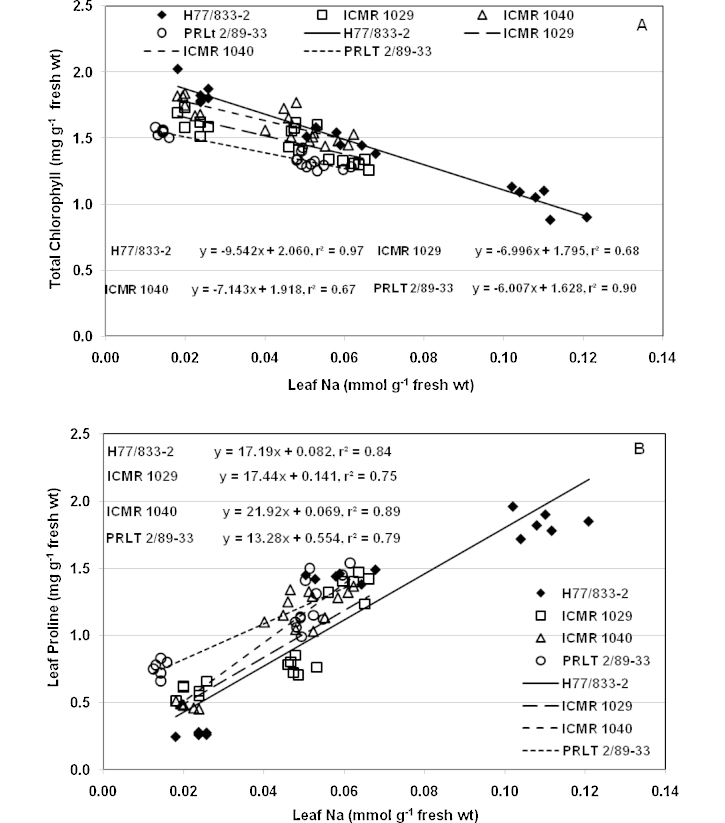
Accumulation of Na+ also affected chlorophyll concentration in leaves. Tissue tolerance (measured as the leaf Na+ concentration at which 50% loss of chlorophyll occurred) varied amongst genotypes and across time intervals after salinity stress imposition. At ECiw 15 dS m−1, 50% loss of chlorophyll occurred at Na+ concentration of 0.34 mmol g−1 fresh weight in leaves of H 77/833-2, whereas similar loss occurred at Na+ concentration of 0.40 mmol g−1 fresh weight in PRLT 2/89-33 after 24 h of salinity stress (data not shown). The same trend was also observed after 48 and 120 h of salinity stress. The tissue tolerance values were 0.38 and 0.54 mmol g−1 fresh weight in H 77/833-2 and PRLT 2/89-33 respectively, after 48 h of stress imposition. The tissue tolerance values were also computed from earlier experiment (Sharma et al., 2011) under long-term salt stress, in which plants were raised under salinity stress from germination stage onwards. In leaves of H 77/833-2, Na+ concentration required for 50% loss of chlorophyll was 0.12 mmol g−1 fresh weight, whereas similar loss in chlorophyll required 0.16 mmol Na+ g−1 fresh weight in PRLT 2/89-33 (Fig. 4A). Similarly, the two QTL-NILs required 0.14 (ICMR 01029) and 0.15 (ICMR 01040) mmol g−1 fresh weight leaf Na+, to cause 50% chlorophyll loss.
Fig. 4.
Relationship between decline in leaf chlorophyll concentration (A) and increase in leaf proline content (B) with increasing leaf Na+ content (linear regression fit to the data) in pearl millet parental genotypes (H 77/833-2 and PRLT 2/89-33), and of QTL-NILs (ICMR 01029 and ICMR 01040) differing for a terminal drought tolerance QTL.
3.4. Proline concentrations in leaves
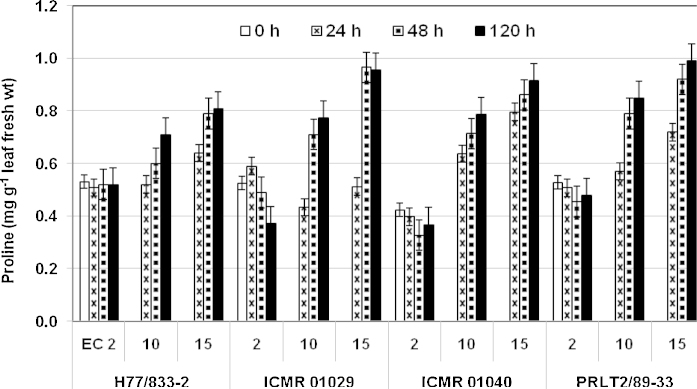
Leaf proline concentration increased with increase in salinity level and duration of salinity stress in all the genotypes evaluated. Significant differences in proline concentrations were recorded for the four genotypes in harvests made at zero and 24 h after salinity imposition, whereas at 48 and 120 h, non-significant differences were recorded (Fig. 5). Further, significant differences were observed for both salinity treatments (ECiw 10 and 15 dS m−1) at harvests taken 24, 48 and 120 h after salinity imposition. Compared to the zero h harvest, after 120 h of salinity stress at ECiw 15 dS m−1, the mean proline concentration (mean of individual leaves) had increased by 52% in H 77/833-2, whereas the comparable increase was 87% in PRLT 2/89-33. Further, PRLT 2/89-33 showed 22% higher proline concentration in different leaves (on mean basis) than H 77/833-2 120 h after imposition of the ECiw 15 dS m−1 salinity treatment. Similarly, the two QTL-NILs recorded 19 (ICMR 01029) and 14% (ICMR 01040) higher leaf proline concentrations than H 77/833-2 120 h after imposition of this treatment. Comparison of proline concentrations in individual leaves revealed higher proline levels in younger leaves than in older leaves as salinity stress increased. Genotype PRLT 2/89-33 showed almost twice the proline concentration in younger leaves at 48 and 120 h after imposition of salinity stress than H 77/833-2, at both salinity levels (ECiw 10 and 15 dS m−1, data not shown). The QTL-NILs, however, did not show any discernible patterns for proline distribution in individual leaves.
Fig. 5.
Mean proline concentration (mg g−1 leaf fresh weight, mean of 8 leaves) in pearl millet parental genotypes (H 77/833-2 and PRLT 2/89-33) and of QTL-NILs (ICMR 01029 and ICMR 01040) differing for a terminal drought tolerance QTL, at three salinity levels (ECiw 2, 10 and 15 dS m−1 imposed at 45 days after sowing).
3.5. Stover and seed yield
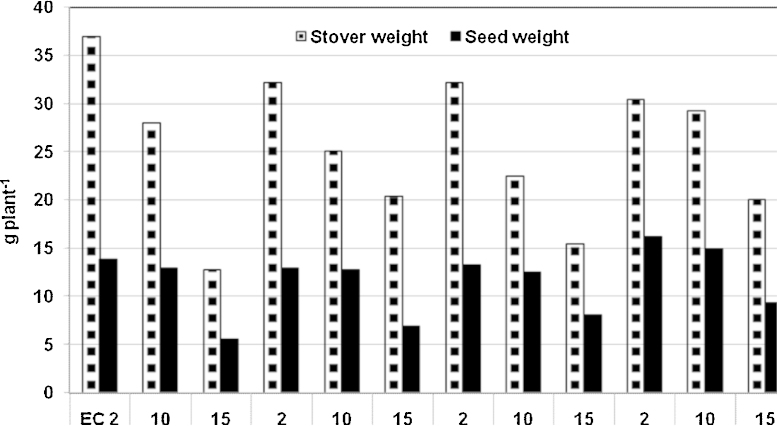
Interestingly, the imposition of salinity even at flowering stage (45 DAS) affected the pearl millet yield performance. Under control conditions (ECiw 2 dS m−1), H 77/833-2 recorded highest stover yield (Fig. 6). Significant genotypic differences were evident for both stover and grain yield under salinity stress on per plant as well as on per pot basis. Compared to the control (ECiw 2 dS m−1), stover yield at ECiw 15 dS m−1 declined by 65% in H 77/833-2 and by 44% in PRLT 2/89-33. This decline in the two QTL-NILs was 37% (ICMR 01029) and 52% (ICMR 01040), respectively. Compared to H 77/833-2, PRLT 2/89-33 recorded 57% higher stover yield at ECiw 15 dS m−1. Further, the two QTL-NILs showed 60% (ICMR 01029) and 21% (ICMR 01040) higher stover yield than H 77/833-2 at ECiw 15 dS m−1. Similar trend was also observed for grain yield. Genotype PRLT 2/89-33 showed 32% higher grain yield (per plant) than H 77/833-2 at ECiw 15 dS m−1 (Fig. 6). Similarly, the two QTL-NILs (ICMR 01029 and ICMR 01040) recorded 32 and 33% higher grain yield, respectively, than H 77/833-2 at ECiw 15 dS m−1.
Fig. 6.
Stover and seed weight (g plant−1) in pearl millet parental genotypes (H 77/833-2 and PRLT 2/89-33), and QTL-NILs (ICMR 01029 and ICMR 01040) differing for a terminal drought tolerance QTL, at three salinity levels (ECiw 2, 10 and 15 dS m−1 imposed at 45 days after sowing).
4. Discussion
We have shown previously that the major terminal drought tolerance (DT) QTL on pearl millet linkage group 2 (LG 2) exerts favourable effects on growth of drought-tolerant parent PRLT 2/89-33 and its QTL-NILs (introgressed with the LG 2 DT-QTL from drought-tolerant parent PRLT 2/89-33) right from germination and seedling emergence through to maturity under varying salinity and alkalinity stress conditions as well, by limiting Na+ accumulation in leaves and by partitioning these into nodes and internodes of the stem (Sharma et al., 2011). Further, it has been demonstrated that the drought-tolerant parent allele of this QTL is associated with constitutively increased leaf ABA concentration (Kholová et al., 2010a) and reduced transpiration rates (Kholová et al., 2010b). The focus of our present study was to improve our understanding of how the tolerant parent allele cope with high salt stress, particularly when applied at reproductive growth stages. For this, we have imposed more severe salt stress (ECiw 15 dS m−1) than before (up to ECiw 12 dS m−1, Sharma et al., 2011), during late flowering and post-flowering growth stages following development of sink strength on main stem panicles, and studied the time course of changes in ion accumulation in different ion sinks (individual leaves, main stem and roots), to assess the differences in ion uptake and distribution in different plant parts and on yield parameters. We also focused on understanding the dynamics of chlorophyll and proline concentration variation, if any, associated with this DT-QTL under salt stress at late flowering stages.
The three most significant observations made in the present study were as follows: first, the roots accumulated high Na+ concentrations (means across salinity levels and time periods) in the present study (0.40 mmol g−1 dry weight), which is approximately at par with the Na+ accumulated in the main stem (0.39 mmol g−1 dry weight). It is in marked contrast to our previous results, where main stems accumulated 24% higher Na+ concentrations than roots on a mean basis (Sharma et al., 2011). In the present study of short-term salinity stress applied during late flowering and post-flowering growth stages, the drought-sensitive parent H 77/833-2 accumulated 9% higher Na+ concentration in its roots than the drought-tolerant parent PRLT 2/89-33, whereas in the previous study involving long-term salinity application throughout the plant growth cycle, this trend was exactly reversed. These differences in the present and previous results suggest different mechanisms involved in counteracting salinity stress applied at different growth stages. The reduced transpiration rates reported in drought-tolerant parent and the two QTL-NILs, as a result of constitutively enhanced foliar ABA levels (Kholová et al., 2010a,b), could explain the differences observed in both the short-term and long-term salinity stress treatments.
The second noteworthy observation made was that Na+ concentration in roots of H 77/833-2 reached its maximum at ECiw 15 dS m−1 within 24 h after salinity imposition. However, root Na+ continued to increase with time in PRLT 2/89-33 and QTL-NILs (ICMR 01029 and ICMR 01004) from 24 to 120 h and reached at its maximum at 120 h stage (Fig. 1B). Conclusively, though both parents accumulated similar levels of Na+ in their roots after 120 h at ECiw 15 dS m−1, higher rate of Na+ accumulation in roots of H 77/833-2 results in greater negative impact on its growth and productivity compared to PRLT 2/89-33 and two QTL-NILs. Further, after 120 h at ECiw 15 dS m−1, the Na/K ratio was 8.5 times that of the initial value in roots of H 77/833-2 while similar increase in PRLT 2/89-33 and two QTL-NILs was 6.5 and 7 times, respectively. Such clear differences in the roots of parental genotypes were not observed previously (Sharma et al., 2011), mainly because the root growth occurred under normal conditions until 45 DAS in the present study, providing a higher root capacity to accumulate these toxic ions.
Differential accumulation of Na+ was clearly evident in the main stem and individual leaves of the two parental genotypes as well the QTL NILs. Na+ accumulation was 6 times higher in the main stem of H 77/833-2 120 h after imposition of the ECiw 15 dS m−1 salinity stress, whereas it increased by 3.5 times in PRLT 2/89-33. Similar increases in the two QTL-NILs were 3.5- and 4.5-fold respectively. These observations could be a simple function of the drought-tolerant parent and those of the QTL-NILs having lower transpiration rates than the drought-sensitive parent [as reported by Kholová et al. (2010a,b), but not measured in the present study], and so drawing in less salt while taking up soil water to support transpiration.
Comparison of Na+ concentrations amongst different leaves on main stems revealed differential distribution of ions between old and young leaves. The oldest leaf (leaf position 1) of PRLT 2/89-33 (Fig. 2B) started to show increased Na+ concentration at ECiw 10 dS m−1 and stored Na+ at twice the concentration as found in the oldest leaves of H 77/833-2 48 h after imposition of salinity stress (Fig. 2A). When compared with the drought-sensitive parent at 120 h, Na+ concentration in the lowermost leaf (leaf position 1) in the drought-tolerant parent and the two QTL-NILs was two-fold greater. Most significantly, comparison of older and younger leaves in the genotypes at ECiw 15 dS m−1 revealed that the lowermost three leaves (leaf positions 1, 2, 3) on the main stem of the drought-tolerant parent and the two QTL-NILs showed significantly higher Na+ concentrations than the uppermost five younger leaves (leaf positions 4, 5, 6, 7, 8) on the same main stems 120 h after salinity stress imposition. These results indicated that Na+ ions were not uniformly distributed in the leaves of the drought-tolerant parent and drought-tolerant QTL-NILs; but accumulated preferentially in the older leaves, whereas the drought-sensitive parent showed significantly higher Na+ concentration in all main stem leaves irrespective of their age (Fig. 2).
We observed therefore that with the increase in imposed salinity level from ECiw 10 to 15 dS m−1, the drought-tolerant parent showed consistency in its control of the distribution of Na+ amongst its older and younger leaves, with successively younger leaves taking the least share of the salt stress. However, similar consistency was not shown by drought-sensitive parent. Storing Na+ preferentially in older leaves and limiting transmission of salt into younger leaves in drought-tolerant parent and the two QTL-NILs served as a protective mechanism. Controlled uptake and better compartmentalization of Na+ besides the ability to maintain low Na+/K+ have been described as important mechanisms of salt tolerance in plants (Munns and Tester, 2008; deVos et al., 2013). Similar differential distribution of Na+ ions between old (expanded) and young (expanding) leaves of plants grown under high-salinity conditions has been reported in other salt-tolerant crops (Greenway et al., 1965, 1966; Yeo and Flowers, 1986; Soliman and Dos, 1992; Nakamura et al., 1996; Ramanjulu and Sudhakar, 2001; Kumar et al., 2003).
Various studies have reported varietal differences with respect to the decline in leaf chlorophyll concentration under increasing salinity stress as well as under increased leaf Na+ concentration (Yeo and Flowers, 1983; Lutts et al., 1996; Netondo et al., 2004). The present results showed higher leaf chlorophyll concentration in the drought-sensitive parent H 77/833-2 than in the drought-tolerant parent PRLT 2/89-33 and two QTL-NILs at the start of salinity stress imposition (0 h). However, H 77/833-2 showed greater reduction in leaf chlorophyll concentration than PRLT 2/89-33 under short-term (present experiment) as well as under long-term salt stress (Sharma et al., 2011). The stability of leaf chlorophyll, being membrane-bound, is dependent upon membrane stability, which under saline conditions is seldom maintained. It was shown in rice that cell ultrastructure differs in its stability under varying leaf Na+ concentration, causing higher loss of chlorophyll in salt-sensitive varieties (Yeo and Flowers, 1986). There were also large varietal differences in the average tissue Na+ concentration associated with 50% leaf chlorophyll loss; higher leaf Na+ is required to bring about a 50% loss of chlorophyll in salt-tolerant varieties than in salt-sensitive rice varieties (Flowers et al., 1985; Yeo and Flowers, 1986; Lutts et al., 1996). Under short term salinity stress in pearl millet, higher leaf Na+ was required to cause 50% loss in chlorophyll in drought-tolerant parent than drought-sensitive parent, at 24 and 48 h after salinity imposition, whereas, the tissue tolerance values of the two QTL-NILs did not fall in between the values of the two parents. However, the tissue tolerance values computed from long term salt stress in pearl millet showed 50% loss of chlorophyll at leaf Na+ of 0.12 mmol g−1 fresh weight in H 77/833-2, and 0.16 mmol in PRLT 2/89-33 and the two QTL NILs. The short-term salinity stress did not show such trends in tissue tolerance mainly because the stress was imposed at a later growth stage (45 DAS) when the leaves were fully expanded. Chlorophyll concentration, however, is not necessarily an exhaustive index of tissue tolerance. Indeed, Yeo et al. (1985) demonstrated that in rice genotypes, photosynthesis was reduced by half at a leaf Na+ concentration that did not reduce chlorophyll levels.
Many investigators have demonstrated the osmo-protective role of proline at the whole-plant level and in cell cultures (Kishore et al., 1995; Misra and Gupta, 2005; Szabados and Savouré, 2010). Increased proline content in transgenic plants is associated with enhanced tolerance to various abiotic stresses (Kishore et al., 1995; Kasukawe et al., 2004). In the presence of low water potentials under salinity stress, the accumulation of compatible osmolytes involved in osmoregulation allows additional water to be taken up from the environment, thus buffering the immediate effect of water shortage within the plant. In the present study, at ECiw 15 dS m−1, the proline concentration had increased by 52% after 120 h of salinity stress in H 77/833-2, whereas the comparable increase was 87% in PRLT 2/89-33 (Fig. 5). The two QTL-NILs showed the pattern similar to the drought-tolerant parent.
The parallel increase in proline concentration with decreased Na+ concentration in younger leaves of PRLT 2/89-33 and the two QTL NILs suggests that this differential distribution of salt and proline together between young and old leaves (i.e., an increase in the level of salt in old leaves, and an accumulation of proline in young leaves) is allowing the younger leaves to support sufficient metabolic and physiological activity through osmotic adjustment for survival under high-salinity conditions. Leaf proline concentration also increased under increasing long-term root zone salt stress. However, the behaviour of the genotypes with this DT QTL allele in terms of proline accumulation was exactly opposite under long-term and short-term salt stress. Though the drought-sensitive parent showed higher proline concentrations than the drought-tolerant parent under long-term salt stress, the sensitive parent required higher leaf Na+ than the tolerant parent to produce the same level of proline, as shown in Fig. 4B. For example, the sensitive parent required 0.053 mmol Na+ g−1 leaf fresh weight to produce 1.00 mg proline, g−1 leaf fresh weight, whereas the similar level of proline was produced by 0.033 mmol Na+ g−1 leaf fresh weight in the tolerant parent. The results are in accordance with the studies where free proline increased appreciably in salt tolerant plants (Kumar et al., 2003; Demiral and Türkan, 2005; Misra and Gupta, 2005; Koca et al., 2007; Veeranagamallaiah et al., 2007) and also where salt-sensitive cultivars accumulated significantly higher levels of proline compared to tolerant ones (Lutts et al., 1999; deLacerda et al., 2003; Vaidyanathan et al., 2003) as the proline concentration differences between tolerant and sensitive genotypes depends up on the timing of onset, severity, and duration of the imposed stress.
5. Conclusions
In total, our results suggest that the hybrid of drought-tolerant parent PRLT 2/89-33 performed better under post-flowering salinity stress by differentially regulating Na+ accumulation in roots, better compartmentalization of accumulated Na+ in the main stem, limiting translocation of Na+ from older leaves to metabolically active younger leaves, and restriction of Na+ influx and its effective efflux in leaves through osmotic adjustment facilitated by adjusted accumulation of proline. These findings are compatible with the earlier observation that the LG 2 drought tolerance QTL allele of this parent is associated with constitutively elevated leaf ABA levels and reduced leaf transpirations rates.
Acknowledgements
The work reported in this study was conducted under “Collaborative Project with Scientists & Technologists of Indian Origin Abroad” (CP-STIO) award to P.C.S. and R.S.Y. by the Department of Science and Technology (DST), Government of India. Financial support provided by the DST via grant number DST/INT/CP-STIO/2006-07/60/2006 is gratefully acknowledged. Plant materials used in the study was generated in a separate project funded by the Biotechnology and Biological Sciences Research Council (BBSRC) and Department for International Development (DFID) to R.S.Y. via grant number BB/F004133/1.
References
- Arnon D.I. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiology. 1949;24:1–14. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Bidinger F.R., Nepolean T., Hash C.T., Yadav R.S., Howarth C.J. Identification of QTLs for grain yield of pearl millet [Pennisetum glaucum (L.) R. Br.] in environments with variable moisture during grain filling. Crop Science. 2007;47:969–980. [Google Scholar]
- Blum A., Ebercon A. Genotypic responses in sorghum to drought stress. III. Free proline accumulation and drought resistance. Crop Science. 1976;16:428–431. [Google Scholar]
- deLacerda C.F., Cambraia J., Oliva M.A., Ruiz H.A., Prisco J.T. Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environmental and Experimental Botany. 2003;49:107–120. [Google Scholar]
- Demiral T., Türkan I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environmental and Experimental Botany. 2005;53:247–257. [Google Scholar]
- Desingh R., Kanagaraj G. Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. General and Applied Plant Physiology, 2007. 2007;33(3–4):221–234. [Google Scholar]
- deVos A.C., Broekman R., Guerra C.C.A., van Rijsselberghe M., Rozema J. Developing and testing new halophyte crops: a case study of salt tolerance of two species of the Brassicaceae, Diplotaxis tenuifolia and Cochlearia officilais. Environmental and Experimental Botany. 2013;92:154–164. [Google Scholar]
- Flowers T.J., Duque E., Hajibagheri M.A., McGonigle T.P., Yeo A.R. The effect of salinity on the ultrastructure and net photosynthesis on the two varieties of rice; further evidence for a cellular component of salt resistance. New Phytologist. 1985;100:37–43. [Google Scholar]
- Greenway H., Gunn A., Pitman M.G., Thomas O.A. Plant response to saline substrate. VI. Chlorine, sodium and potassium uptake and distribution within the plant during ontogenesis of Hordeum vulgare. Australian Journal of Biological Sciences. 1965;18:525–540. [Google Scholar]
- Greenway H., Gunn A., Thomas D.A. Plant response to saline substrate. VIII. Regulations of ion concentrations in salt-sensitive and halophilic species. Australian Journal of Biological Sciences. 1966;19:741–756. [Google Scholar]
- Kasukawe Y., He L., Nada K., Misawa S., Ihara I., Tachibana S. Over-expression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant and Cell Physiology. 2004;45:712–722. doi: 10.1093/pcp/pch083. [DOI] [PubMed] [Google Scholar]
- Kholová J., Hash C.T., Kakkera A., Kočová M., Vadez V. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.] Journal of Experimental Botany. 2010;61:369–377. doi: 10.1093/jxb/erp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholová J., Hash C.T., Lava Kumar P., Yadav R.S., Kočová M., Vadez V. Terminal drought tolerant pearl millets [Pennisetum glaucum (L.) R. Br.] have high leaf ABA and limit transpiration at high vapor pressure deficit. Journal of Experimental Botany. 2010;61:1431–1440. doi: 10.1093/jxb/erq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore P.B.K., Hong Z., Miao G.H., Hu C., Verma D.P.S. Over expression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiology. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koca H., Bor M., Özdimir F., Türkan I. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environmental and Experimental Botany. 2007;60:344–351. [Google Scholar]
- Kumar S.G., Reddy A.M., Sudhakar C. NaCl effects on proline metabolism in two high-yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Science. 2003;165:1245–1251. [Google Scholar]
- Lutts S., Kinet J.M., Bouharmont J. NaCl induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity. Annals of Botany. 1996;78:389–398. [Google Scholar]
- Lutts S., Majerus V., Kinet J.M. NaCl effects on proline metabolism in rice (Oryza sativa L.) seedlings. Physiologia Plantarum. 1999;105:450–458. [Google Scholar]
- Misra N., Gupta A.K. Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Science. 2005;169:331–339. [Google Scholar]
- Munns R., Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ishitani M., Harinasut P., Nomura M., Takabe T., Takabe T. Distribution of glycinebetaine in old and young leaf blades of salt stressed barley plants. Plant and Cell Physiology. 1996;37:873–877. [Google Scholar]
- Netondo G.W., Onyango J.C., Beck E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Science. 2004;44:806–811. [Google Scholar]
- Ramanjulu S., Sudhakar C. Proline metabolism during dehydration in two mulberry genotypes with contrasting drought tolerance. Journal of Plant Physiology. 2000;157:81–85. [Google Scholar]
- Ramanjulu S., Sudhakar C. Alleviation of NaCl salinity stress by calcium is partly related to the increased proline accumulation in mulberry (Morus alba L.) callus. Journal of Plant Biology. 2001;28:203–206. [Google Scholar]
- Sharma P.C., Sehgal D., Singh D., Singh G., Yadav R.S. A major terminal drought tolerance QTL of pearl millet is also associated with reduced salt uptake and enhanced growth under salt stress. Molecular Breeding. 2011;27:207–222. [Google Scholar]
- Simiroff N., Cumbes Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Sneha S., Rishi A., Dadhich A., Chandra S. Effect of salinity on seed germination, accumulation of proline and free amino acid in Pennisetum glaucum (L.) R. Br. Pakistan. Journal of Biological Sciences. 2013;16:877–881. doi: 10.3923/pjbs.2013.877.881. [DOI] [PubMed] [Google Scholar]
- Soliman M.S., Dos M. Salinity and mineral nutrition effects of growth and accumulation of organic ions in two cultivated tomato varieties. Journal of Plant Nutrition. 1992;15:2789–2799. [Google Scholar]
- Szabados L., Savouré A. Proline: a multifunctional amino acid. Trends in Plant Science. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan H., Sivakumar P., Chakraborty R., Thomas G. Scavenging of reactive oxygen species in NaCl stressed rice (Oryza sativa L.) differential responses and salt tolerant and sensitive varieties. Plant Science. 2003;165:1411–1418. [Google Scholar]
- Veeranagamallaiah G., Chandraobulreddy P., Jyothsnakumari G., Sudhakar C. Glutamine synthetase expression and pyrroline-5-carboxylate reductase activity influence proline accumulation in two cultivars of foxtail-millet (Setaria italica L.) with differential salt sensitivity. Environmental and Experimental Botany. 2007;60:239–244. [Google Scholar]
- Venkamp J.H., Lampe J.E.M., Koot J.T.M. Organic acids as sources of drought induced proline synthesis in field bean plants Vicia faba L. Journal of Plant Physiology. 1989;133:654–659. [Google Scholar]
- Yadav R.S., Hash C.T., Bidinger F.R., Cavan G.P., Howarth C.J. Quantitative trait loci associated with traits determining grain and stover yield in pearl millet under terminal drought stress conditions. Theoretical and Applied Genetics. 2002;104:67–83. doi: 10.1007/s001220200008. [DOI] [PubMed] [Google Scholar]
- Yadav R.S., Hash C.T., Bidinger F.R., Devos K.M., Howarth C.J. Genomic regions associated with grain yield and aspects of post-flowering drought tolerance in pearl millet across stress environments and tester background. Euphytica. 2004;136:265–277. [Google Scholar]
- Yadav R.S., Sehgal D., Vadez V. Using genetic mapping and genomics approaches in understanding and improving drought tolerance in pearl millet. Journal of Experimental Botany. 2011;62:397–408. doi: 10.1093/jxb/erq265. [DOI] [PubMed] [Google Scholar]
- Yeo A.R., Flowers T.J. Varietal differences in the toxicity of sodium ions in rice leaves. Physiologia Plantarum. 1983;59:189–195. [Google Scholar]
- Yeo A.R., Flowers T.J. Salinity resistance in rice (Oryza sativa L.) and a breeding approach to breeding varieties for salinity soils. Australian Journal of Plant Physiology. 1986;13:161–173. [Google Scholar]
- Yeo A.R., Capcorn S.J.M., Flowers T.J. The effect of salinity upon photosynthesis in rice (Oryza sativa L.): gas exchange by individual leaves in relation to their salt content. Journal of Experimental Botany. 1985;36:1240–1248. [Google Scholar]