Abstract
Pseudorabies virus (PRV) is a swine alphaherpesvirus that is closely related to human herpes simplex virus (HSV). Both PRV and HSV express a variety of viral envelope glycoproteins in the plasma membranes of infected cells. Here we show that at least four major PRV glycoproteins (gB, gC, gD, and gE) in the plasma membrane of infected swine kidney cells and monocytes seem to be linked, since monospecific antibody-induced patching of any one of these proteins results in copatching of the others. Further, for all four PRV glycoproteins, monospecific antibody-induced patches were enriched in GM1, a typical marker of lipid raft microdomains, but were excluded for transferrin receptor, a nonraft marker, suggesting that these viral proteins may associate with lipid rafts. However, only gB and, to a lesser extent, gE were found in lipid raft fractions by using detergent floatation assays, indicating that gC and gD do not show strong lipid raft association. Addition of methyl-β-cyclodextrin (MCD), a cholesterol-depleting agent that is commonly used to disrupt lipid rafts, only slightly reduced copatching efficiency between the different viral proteins, indicating that other factors, perhaps tegument-glycoprotein interactions, may be important for the observed copatching events. On the other hand, MCD strongly reduced polarization of the antibody-induced viral glycoprotein patches to a cap structure, a gE-dependent process that has been described for specific PRV- and HSV-infected cells. Therefore, we hypothesize that efficient gE-mediated capping of antibody-antigen patches may require the lipid raft-associated signal transduction machinery.
Pseudorabies virus (PRV) is a swine alphaherpesvirus that is closely related to the prototypical herpes simplex virus (HSV). Like that of HSV, the PRV envelope contains at least 10 different viral envelope glycoproteins, designated glycoprotein B (gB), gC, gD, gE, gH, gI, gK, gL, gM, and gN. Upon infection of a susceptible cell, newly synthesized viral envelope glycoproteins travel through the Golgi network and are subsequently incorporated in the plasma membrane, rendering the cells recognizable for virus-specific antibodies.
Earlier, we and others have shown that the interaction between PRV or HSV polyclonal serum antibodies and the viral proteins on the cell surface initiates intriguing, cell type-dependent redistribution processes of the antibody-antigen complexes (8, 10, 39). Addition of polyclonal serum immunoglobulin G (IgG) to PRV-infected swine kidney (SK) cells or HSV-infected human embryonic lung fibroblasts (HEL cells) and human larynx epidermoid carcinoma (Hep-2) cells leads to aggregation of the viral cell surface proteins into patches, which subsequently polarize to one side of the cell to form a cap (10, 39), sometimes followed by shedding of the caps (10). In PRV-infected blood monocytes, on the other hand, patches of viral cell surface proteins are rapidly internalized and do not cap (8). The exact function of these processes is not fully understood, although there are strong indications that they may be important for alphaherpesviruses to enhance virus survival in the face of an antibody response. First, the internalization of patches of viral cell surface proteins in PRV-infected monocytes has been shown to interfere with efficient antibody-dependent lysis of the infected monocytes (45). Further, capping of antibody-antigen patches has been suggested to be related to antibody-dependent enhancement of cell-to-cell spread of HSV (39). Also, it has been shown for PRV-infected monocytes that the interaction with virus-specific antibodies ultimately leads to suppression of intracellular viral protein levels, perhaps even leading to a quiescent, persistent form of infection (11).
Both types of redistribution of the antibody-induced patches of viral cell surface proteins, capping and internalization, have been shown to be initiated by specific viral cell surface proteins. In both PRV- and HSV-infected cells, efficient capping has been shown to depend on the presence of viral protein gE, whereas internalization has been demonstrated to be initiated mainly by viral proteins gB and gD and to a lesser extent gE (8, 10, 39). Exactly how these viral proteins initiate the redistribution processes is not fully understood, but it has been shown that tyrosine-based amino acid motifs in the cytoplasmic tails of PRV gB and gE are of crucial importance for internalization and capping, respectively (7, 9). In addition, PRV gE-mediated capping has been suggested to require specific tyrosine kinase signaling (9).
Interestingly, the addition of PRV- or HSV-specific serum antibodies does not result in exclusive capping or internalization of the viral proteins that initiate these processes but also results in that of the other viral cell surface proteins that are recognized by the immune serum (8, 10, 39). One explanation for this massive change in surface viral glycoprotein distribution initiated by single or a few viral proteins may be that (some of) the major viral envelope proteins that are present on the surfaces of PRV- or HSV-infected cells are somehow linked, allowing one or a few viral proteins to initiate lateral movement of many, if not all, of the viral proteins in the cell surface.
The aim of the present study was therefore (i) to study whether such a putative link between several of the major viral cell surface proteins indeed does exist, by examining whether patching of single viral proteins on the cell surface by the addition of monospecific antibodies to PRV-infected cells leads to copatching of other viral proteins, and (ii) if so, to obtain indications about the nature of such a link between the different viral cell surface proteins.
MATERIALS AND METHODS
Antibodies and reagents.
Mouse monoclonal antibodies directed against gB (1C11), gD (13D12), and gE (18E8) were all described earlier (34) and were used at a dilution of 1/30 (1C11) or 1/100 (13D12 and 18E8). Mouse monoclonal anti-gC antibody was kindly provided by A. Brun and used at a dilution of 1/100. Polyclonal monospecific antibodies were used at a dilution of 1/50 and were kindly provided by S. Brockmeier (swine anti-gD) and K. Bienkowska-Szewczyk (rabbit anti-gE). Mouse anti-transferrin receptor (anti-TfR) was purchased from Zymed Laboratories, Inc. (San Francisco, Calif.) and diluted 1/100. Biotinylated cholera toxin B subunit (Sigma Chemical Co., St. Louis, Mo.) was used at a dilution of 1/100. Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse antibodies (used at a dilution of 1/100) and Texas red-labeled goat anti-mouse and anti-rabbit antibodies and streptavidin (all used at a dilution of 1/50) were all purchased from Molecular Probes (Eugene, Oreg.). Texas red-labeled goat anti-swine antibodies (Jackson Immunologicals, West Grove, Pa.) were used at a dilution of 1/50. Biotinylated sheep anti-mouse antibodies (Amersham Biosciences, Buckinghamshire, United Kingdom) were used at a dilution of 1/100, and peroxidase-conjugated streptavidin-biotin complex (Amersham Biosciences) was used at a dilution of 1/200. Methyl-β-cyclodextrin (MCD) and Triton X-100 were purchased from Sigma.
Copatching experiments.
Isolation of porcine blood monocytes, in vitro cultivation of SK cells and monocytes, and in vitro PRV inoculation of SK cells and monocytes were carried out as described before (8, 10). PRV strains 89V87, Kaplan, Kaplan gE-gI null, Kaplan ICP18.5 null, and Kaplan UL49 null were used, and all were described before (13, 21, 29, 30, 33). At 13 h postinfection (p.i.), cells were washed three times in phosphate-buffered saline (PBS), followed by incubation for 30 min at 37°C with primary antibodies (monoclonal anti-gB, -gC, -gD, and/or -gE antibodies or polyclonal rabbit anti-gE or swine anti-gD antibodies as indicated). Afterwards, cells were washed twice in PBS, and incubated with the appropriate secondary antibodies (FITC-labeled anti-mouse or anti-rabbit antibodies) for 30 min at 37°C. The cells were then either paraformaldehyde fixed (2% paraformaldehyde, 10 min, room temperature) or, for live-cell imaging, washed in ice-cold PBS. Cell were then washed in ice-cold PBS and incubated with polyclonal swine anti-gD antibodies, polyclonal rabbit anti-gE antibodies, mouse monoclonal anti-TfR antibody, or biotinylated cholera toxin B subunit for 1 h on ice. Thereafter, cells were washed twice in ice-cold PBS and incubated for 1 h on ice with Texas red-labeled antibodies (anti-swine, anti-rabbit, or anti-mouse) or Texas red-labeled streptavidin. Afterwards, cells were washed twice in ice-cold PBS and resuspended in 40 μl of ice-cold PBS. For fixed cells, cells were mounted in a glycerin-PBS solution (0.9:0.1, vol/vol) with 2.5% 1,4-diazabicyclo(2.2.2)octane (Janssen Chimica, Beerse, Belgium) on a microscope slide and analyzed by confocal microscopy. For the analysis of live cells, two coverslips were mounted on a 3-aminopropyltriethoxysilane-coated microscope slide, leaving a 0.5-cm gap between the two coverslips. The gap was filled with 15 μl of cell suspension and covered with a third coverslip. Cells were immediately analyzed by confocal microscopy.
Confocal microscopy.
Cells were analyzed by using a TCS SP2 laser scanning spectrum confocal system (Leica Microsystems GmbH, Heidelberg, Germany), using an argon 488-nm laser line and a Gre/Ne 543-nm laser line to excite FITC and Texas red, respectively. To avoid signal overlap, FITC and Texas red images were taken separately, and images stained with FITC only and Texas red only served as controls. Images were merged by using Leica confocal and Confocal Assistant software.
Lipid raft floatation assay.
Isolation of porcine blood monocytes, in vitro cultivation of SK cells and monocytes, and in vitro PRV inoculation (PRV strain 89V87) of SK cells and monocytes were carried out as described before (8, 10). At 13 h p.i., cells were washed three times in ice-cold TNE (25 mM Tris HCl, 150 mM NaCl, 5 mM EDTA [pH 6.7]), and subsequently 107 cells were lysed for 30 min on ice, with regular shaking, in 1 ml of lysis buffer (1% Triton X-100 in TNE supplemented with complete protease inhibitor cocktail [Roche Diagnostics GmbH, Mannheim, Germany]). Afterwards, the lysate was homogenized by being passed 20 times through an 18-gauge needle on a 1-ml syringe and subsequently mixed with 2 ml of ice-cold 60% Optiprep (Nycomed-Pharam, Oslo, Norway). This mixture was put at the bottom of a Beckman SW41 ultracentrifuge tube (Beckman, Munich, Germany), overlaid with 5 ml of ice-cold 35% Optiprep in TNE and 3 ml of ice-cold 5% Optiprep in TNE, and centrifuged at 200,000 × g at 4°C for 20 h. Ten fractions from the top to the bottom of the tube were collected and diluted 1:2 in 2× concentrated nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. Fifteen microliters of each diluted fraction was then subjected to SDS-PAGE and Western blotting. The blots were blocked in PBS with 0.1% Tween 20 (Sigma) (PBS-T) supplemented with 5% membrane blocking agent (Amersham Biosciences) and washed twice in PBS-T. Afterwards, blots were incubated with different monoclonal antibodies (anti-gB, -gC, -gD, -gE, or -TfR) or biotinylated cholera toxin subunit B in PBS-T for 1 h, washed three times in PBS-T, and incubated with biotinylated sheep anti-mouse antibodies and/or peroxidase-conjugated streptavidin-biotin complex for 1 h, with three washing steps between each incubation. Finally, blots were washed three times in PBS-T and revealed with 3,3′-diaminobenzidine (Sigma).
RESULTS
Copatching of PRV glycoproteins gB, gC, gD, and gE on the surfaces of infected cells.
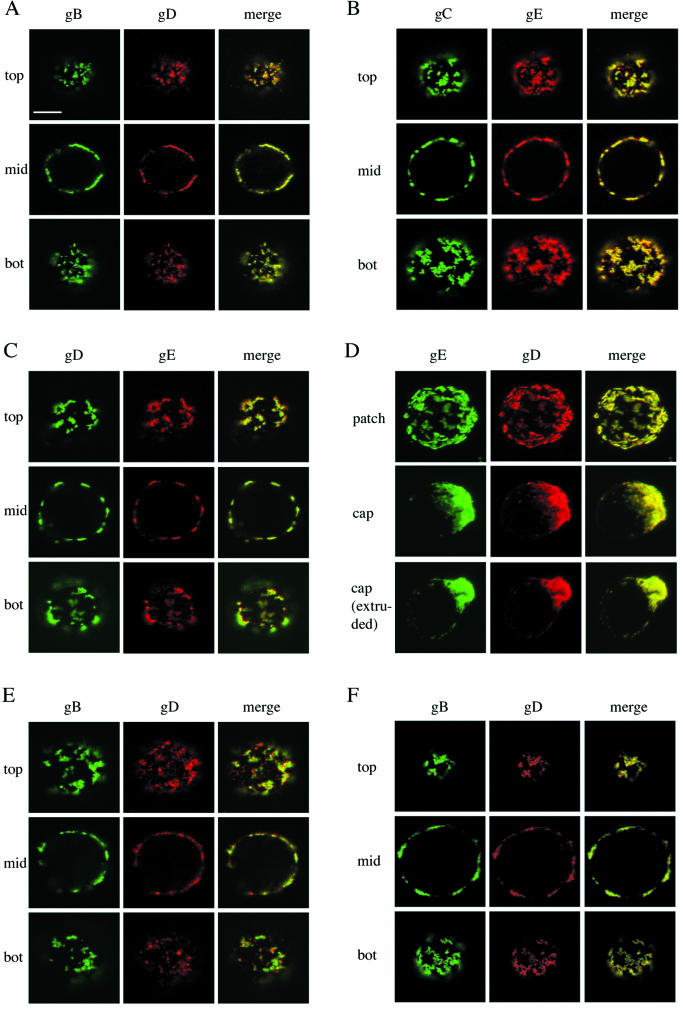
To determine whether different viral envelope proteins that are present on the surfaces of PRV-infected SK cells are linked, we aggregated single viral cell surface proteins into patches by incubating the PRV-infected SK cells with monoclonal antibodies directed against either gB, gC, gD, or gE and fluorochrome-labeled secondary antibodies for 30 min at 37°C. Afterwards, cells were paraformaldehyde fixed and stained for either gD or gE by using monospecific polyclonal antibodies and secondary antibodies conjugated with another fluorochrome. As exemplified in Fig. 1A, B, and C, patching of any one of the four tested viral cell surface proteins (gB, gC, gD, or gE) resulted in copatching of the other tested viral cell surface proteins. Capping of the monospecific antibody-induced glycoprotein patches was observed in approximately 30% of the infected SK cells irrespective of to which viral protein the primary antibody was directed and was also accompanied by cocapping of the other viral proteins, as illustrated in Fig. 1D. Similarly, patching of either gB, gC, gD, or gE on PRV-infected monocytes also resulted in copatching of the other viral cell surface proteins (Fig. 1E and data not shown).
FIG.1.
Copatching of four major PRV glycoproteins (gB, gC, gD, and gE) on the surfaces of PRV-infected SK cells and monocytes. Aggregation of one of these four viral cell surface proteins (patching) by using monospecific antibodies leads to coaggregation of the others (copatching). (A, B, C, and D) SK cells, at 13 h p.i., were incubated with monoclonal antibodies against gB (A), gC (B), gD (C), or gE (D) for 30 min at 37°C and subsequently for 30 min at 37°C with FITC-labeled goat anti-mouse antibodies, leading to patching of gB, gC, gD, or gE. Afterwards, cells were paraformaldehyde fixed and incubated with swine polyclonal anti-gD (A and D) or rabbit polyclonal anti-gE (B and C) antibodies followed by goat anti-swine-Texas red or goat anti-rabbit-Texas red, respectively. The left columns (green) show patched antigen, the middle columns show copatched antigen (red), and the right columns (yellow) show merged image of the former two. In panels A, B, and C, sections through the top, middle (mid), and bottom (bot) of a cell are shown. Panel D shows extended-focus images of cells with patched, capped, and extruded antibody-antigen complexes. (E) Same as panel A but with PRV-infected monocytes instead of SK cells. (F) Same as panel A but without paraformaldehyde fixation. Bar, 5 μm.
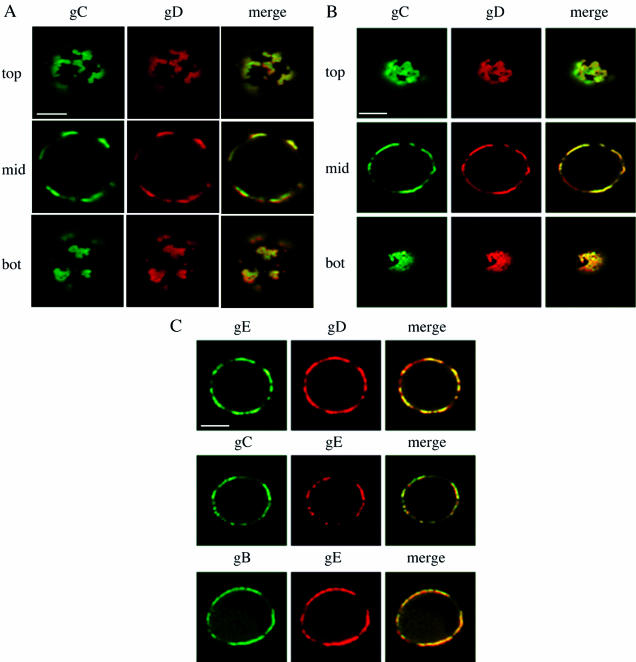
To ensure that the observed copatching could not be attributed to fixation artifacts, patching and copatching experiments were repeated without fixation. After monoclonal antibody-induced patching of single viral cell surface proteins, PRV-infected SK cells were cooled on ice and subsequently incubated with monospecific polyclonal antibodies and fluorochrome-labeled secondary antibodies without fixation. Afterwards, live cells were mounted on a microscope slide and immediately analyzed by confocal laser scanning microscopy. As illustrated in Fig. 1F, copatching between different viral cell surface proteins was also observed in living cells and cannot be attributed to fixation. Further, copatching of the different viral cell surface proteins could not be attributed to antibody-bipolar bridging mediated by the gE-gI Fc receptor (12), since copatching of gC and gD in SK cells infected with a gE-gI null virus was as efficient as that in cells infected with a wild-type virus (Fig. 2A). In addition, copatching of gB and gD in SK cells infected with an ICP18.5 null PRV strain, which is impaired in virus particle formation but still shows cell surface expression of viral glycoproteins (29), was as efficient as that in cells infected with wild-type virus, indicating that antibody-induced patches consisted mainly of cell surface-anchored viral glycoproteins rather than released and cell surface-associated virus particles (Fig. 2B).
FIG. 2.
Wild-type copatching efficiency of PRV cell surface proteins in SK cells infected with a gE-gI null (A), an ICP18.5 null (B), or a UL49 null (C) PRV strain. At 13 h p.i. with the different PRV strains, cells were washed and incubated with monospecific antibodies against different viral cell surface glycoproteins and FITC-labeled secondary antibodies to induce patching of the respective viral glycoprotein. Afterwards, cells were paraformaldehyde fixed and stained for another viral cell surface protein (Texas red staining). Panels A and B each show sections through the top, middle (mid), and bottom (bot) of a single PRV-infected cell, whereas panel C shows sections through the middle of different PRV-infected cells. The left columns show the patched viral cell surface protein, the middle columns show the localization of another viral cell surface protein (copatching), and the right columns show a merged image. Bar, 5 μm.
Together, these data show that at least four major PRV glycoproteins that are present on the surfaces of PRV-infected SK cells and monocytes may be linked.
PRV cell surface glycoproteins gB, gC, gD, and gE associate with lipid rafts.
The cellular plasma membrane has long been considered to be a homogenous sea of lipids with embedded proteins. However, the past years have seen the gradual realization that the plasma membranes of many, if not all, cells contain 20- to 200-nm microdomains, generally referred to as lipid rafts. Lipid rafts are composed mainly of cholesterol and sphingolipids and are relatively poor in polyunsaturated lipids (4). The rigid hexagonal rings of cholesterol can tightly pack against the saturated hydrocarbon chains of some membrane lipids, allowing these lipids to assemble into cohesive units that float on the sea of loosely packed polyunsaturated plasma membrane components (5). Lipid rafts are enriched in glycosyl-phosphatidylinositol-linked proteins, specific transmembrane proteins, signal transduction molecules, and the ganglioside GM1. Recently it has been shown that the HSV gB glycoprotein, as well as two HSV tegument-membrane proteins (vhs and UL56), are lipid raft associated in infected cells or become lipid raft associated during virus entry (2, 24, 26).
At least two factors may be involved in the copatching behaviors of different viral cell surface proteins that we observe here: (i) protein-protein interactions between viral tegument and cell surface proteins and (ii) association of viral cell surface proteins with lipid rafts. Indeed, if viral cell surface proteins show association with lipid rafts, then aggregation of one of these proteins is likely to lead to aggregation of the entire rafts with all other associated viral proteins.
Over the past few years, there have been increasing indications that a very complex network of protein-protein interactions exists between alphaherpesvirus tegument and membrane proteins (31). This complex network is not yet fully understood and is thought to contain many redundant interactions, which makes it, at this time, impossible to carefully assess the contributions of these protein-protein interactions in the copatching behaviors of PRV gB, gC, gD, and gE that we observe here. We did, however, find that a PRV mutant that does not express the UL49 protein, which has been shown to interact with the cytosolic domain of gE (13), still showed efficient copatching of gB, gC, and gD with gE (Fig. 2C). Again, because of the numerous redundancies that are believed to exist in the interactions between tegument and membrane proteins (31), this certainly does not exclude a possible crucial role of these interactions for the observed PRV glycoprotein copatching behavior and will need further investigation.
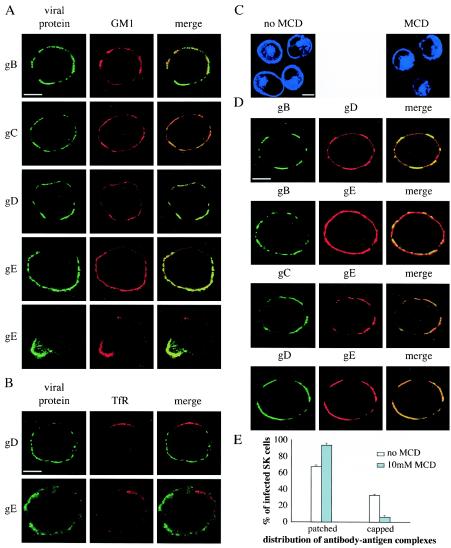
To evaluate whether lipid rafts are involved in the observed copatching behaviors of PRV gB, gC, gD, and gE, we first examined whether any of these proteins shows association with lipid rafts at the surfaces of PRV-infected cells. Lipid raft association of different proteins on the cell surface has been studied by use of their ability to copatch with raft markers (e.g., the ganglioside GM1) but not with nonraft markers (e.g., TfR) upon cross-linking (18). Therefore, single PRV cell surface proteins were again patched on the surfaces of PRV-infected SK cells by using monospecific antibodies, followed by paraformaldehyde fixation and staining for either GM1 or TfR. As can be seen from Fig. 3A, patches and caps induced by gB-, gC-, gD-, or gE-specific antibodies were enriched in GM1. In addition, as shown in Fig. 3B, patches induced by gD- or gE-specific antibodies were excluded of TfR (since only mouse antibodies against gB and gC were available, patches induced by these antibodies could not be examined for TfR localization because of antibody incompatibility). Together, these data suggest that these four major PRV cell surface proteins associate with lipid rafts. To examine whether raft association of these four major viral glycoproteins is required for their copatching behavior, the patching and copatching experiments described above were repeated in the presence of 10 mM MCD. MCD depletes cells of plasma membrane cholesterol and thereby dissociates lipid rafts (19, 38). Staining of cellular cholesterol in SK cells by using the fluorescent fungal cholesterol-binding metabolite filipin, as described elsewhere (22), confirmed that the treatment with 10 mM MCD results in a marked decrease in plasma membrane cholesterol, without affecting intracellular cholesterol levels (Fig. 3C). Figure 3D shows that the addition of MCD before and during patching of gB reduced, but did not abolish, copatching of gD and especially gE (compare with Fig. 1) but that MCD treatment before and during patching of gC and gD did not reduce the copatching efficiency of gE. However, in PRV-infected SK cells treated with MCD, capping of the patches was decreased from 32.6% ± 1.3% to 6.3% ± 2.0%, indicating that lipid raft integrity is required for capping of antibody-induced glycoprotein patches to occur (Fig. 2E).
FIG. 3.
PRV gB, gC, gD, and gE associate with lipid rafts, which has little importance for copatching efficiency between these viral proteins but is crucial for efficient polarization of the patches to cap structures. (A and B) PRV gB, gC, gD, and gE expressed on the surfaces of infected cells are associated with lipid rafts. Aggregates of the indicated viral cell surface proteins (patches), using monospecific antibodies and FITC-labeled secondary antibodies, are enriched in the lipid raft marker GM1 (A) but excluded of the nonraft marker TfR (B). Images show sections through the middle of PRV-infected SK cells. The left column shows the patched viral cell surface protein, the middle column shows localization of GM1 (A) or TfR (B), and the right column shows a merged image. (C, D, and E) Effect of MCD, an agent known to disrupt lipid raft integrity by removing cell surface cholesterol, on cholesterol content in SK cells; on efficiency of copatching between gB, gC, gD, and gE; and on efficiency of capping of the antibody-induced viral glycoprotein patches. (C) Staining of cholesterol with the blue-fluorescent filipin on SK cells before and after treatment with 10 mM MCD shows that MCD efficiently removes cholesterol from the cell surface without affecting intracellular cholesterol levels. (D) Treatment of PRV-infected SK cells with 10 mM MCD decreases copatching efficiency of gD and especially gE with gB but does not affect copatching efficiency of gD and gE with gC and gD, respectively. Images show sections through the middle of MCD-treated PRV-infected SK cells. The left column shows the patched viral cell surface protein, the middle column shows the localization of another viral protein (copatching), and the right column shows a merged image. (E) Treatment of PRV-infected SK cells strongly reduces the efficiency of polarization of antibody-induced patches of viral cell surface glycoproteins to a cap structure. Bars, 5 μm. Error bars indicate standard deviations.
Together, these data suggest that at least four major PRV cell surface proteins associate with lipid rafts. This lipid raft association seems to be of limited importance for efficient copatching of the different glycoproteins but of crucial importance for efficient polarization of the antibody-induced glycoprotein patches towards a cap structure.
PRV glycoprotein gB, but not gC, gD, or gE, shows a strong affinity for lipid rafts.
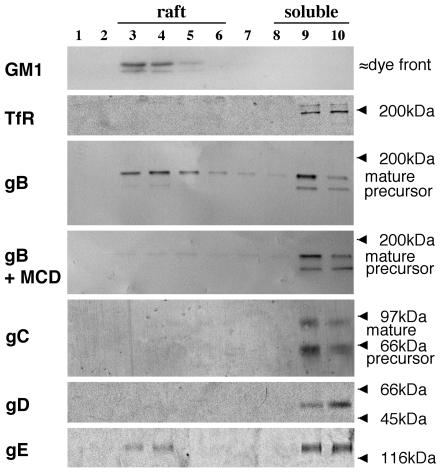
Another major tool, besides copatching experiments, to study raft association of proteins depends on the relative resistance of lipid rafts towards solubilization in Triton X-100 at 4°C. Such detergent lysis leads to the formation of a light membrane fraction that contains the remnants of lipid raft domains aggregated together, which then can be purified by density centrifugation (43). Using this method with PRV-infected monocytes, PRV glycoprotein gB and GM1 were found to fractionate to lipid rafts, whereas only a small proportion of gE was found in the raft fraction and gC, gD, TfR, and the gB precursor were not detectable in raft fractions (Fig. 4). Similar results were obtained for PRV-infected SK cells (not shown). As expected, treatment of cells with 10 mM MCD prior to cold Triton X-100 lysis abolished the migration of PRV gB to light density fractions (Fig. 4).
FIG. 4.
A significant amount of PRV gB and a small amount of PRV gE, but no detectable PRV gC or gD, float to lipid raft fractions upon cold Triton X-100 lysis of PRV-infected cells. SK cells, at 13 h p.i., were either first incubated for 45 min at 37°C with 10 mM MCD or immediately lysed with 1% ice-cold Triton X-100. Cell lysates were separated by density ultracentrifugation, and different fractions from top (light) to bottom (heavy) were collected. Samples from each fraction were subjected to SDS-PAGE and Western blotting and analyzed for the presence of GM1, TfR, gB, gC, gD, or gE. Light fractions correspond to lipid raft fractions, and heavy fractions correspond to soluble fractions.
It has been shown before that moderate affinities for lipid rafts, which are visible in copatching experiments, can be disrupted by nonionic detergents, whereas strong affinities are much more resistant to detergent lysis (20, 43). Thus, our data suggest that on the surfaces of different PRV-infected cells, the mature form of PRV gB is strongly associated with lipid rafts, gE shows an intermediate raft association, and gC and gD may be weakly raft associated.
DISCUSSION
Earlier, we and others have shown that upon addition of polyspecific, polyclonal immune serum IgG to PRV- or HSV-infected cells, specific viral proteins (gB, gD, and/or gE) may initiate cell type-dependent redistribution processes of all viral cell surface antigens that are recognized by the antibodies (8, 10, 39). Such redistribution may lead to capping, shedding, or internalization of the viral cell surface proteins, processes which have been suggested to be implicated in interference with antibody-dependent cell lysis, enhancement of cell-to-cell spread, and/or suppression of the ongoing intracellular virus replication (11, 39, 45).
Here, we show that patching of any one of four major PRV glycoproteins (gB, gC, gD, and gE) by the addition of monospecific antibodies to PRV-infected SK cells or monocytes results in copatching of the others. This patching-copatching was found not to depend on antibody bipolar bridging by the gE-gI Fc receptor and indicates that these four viral glycoproteins are linked on the surfaces of infected SK cells and monocytes, which may explain how single viral cell surface proteins may direct redistribution of many others. In addition, we found that gB and, to a lesser extent, gE show association with lipid raft microdomains, whereas gC and gD may be weakly raft associated. Disruption of lipid rafts was found to only slightly affect the observed link between the different viral glycoproteins at the cell surface but strongly decreased capping of antibody-induced viral glycoprotein patches.
We used two established methods to examine lipid raft association of PRV glycoproteins gB, gC, gD, and gE in infected SK cells and monocytes. First, we found that monospecific antibody-induced patches of any one of the four viral glycoproteins induced by monospecific antibodies were enriched in GM1, a typical lipid raft marker, but were devoid of TfR, a typical nonraft marker, which is an established method to examine raft association of proteins (18). Lipid raft association of the four major PRV glycoproteins was also examined via another widely used method, which is based on the insolubility of lipid rafts in cold Triton X-100. Cold Triton X-100 lysis followed by density gradient centrifugation allows purification of aggregates of lipid raft remnants (43). Interestingly, by using this method, only PRV gB was found in large quantities in raft fractions, compared to only a small proportion of gE and no gC and gD. This apparent discrepancy between copatching and detergent solubilization experiments supports the concern that has been put forward before that the use of detergent insolubility as the only criterion to monitor association of proteins with lipid rafts has limitations (18, 20, 41, 43). Indeed, copatching experiments using GM-1 and TfR as raft and nonraft markers, similar to those described here, have revealed that several transmembrane proteins, such as the T lymphocyte receptor, the IgA receptor, and cross-linked low-density lipoprotein receptor, all display a significant association with lipid rafts and that this association is not resistant to cold Triton X-100 solubilization (18, 20, 25). Several authors have suggested that the differences in anionic detergent solubility of different lipid raft-associated proteins are most likely due to differences in their affinity for lipid rafts. Proteins which strongly interact with rafts are Triton X-100 insoluble, whereas weakly raft-associated proteins are Triton X-100 soluble (18, 20, 43). Combined with our other results, this suggests that in PRV-infected SK cells and monocytes, PRV gB is strongly associated with rafts, whereas gE shows intermediate raft association and gC and gD may be weakly raft associated.
Different research groups have recently reported that at least some alphaherpesvirus proteins show raft association. By using detergent solubilization experiments, HSV gB (but not gC, gD, or gH) has been shown to localize to raft fractions during virus entry (2). These findings seem to be consistent with our present findings which suggest that PRV gB shows a strong, detergent-resistant raft association whereas gC and gD show a weak, detergent-sensitive raft association in infected cells. Interestingly, those researchers suggest that the ectodomain of gB is responsible for the gB association with rafts, suggesting that gB may interact, via its ectodomain, with a raft-residing protein (2). The HSV type 1 vhs tegument protein and, to a lesser extent, gH glycoprotein have also been shown to be associated with rafts in infected cells (26). Further, the HSV type 2 UL56 protein, a protein with significant similarities to the PRV Us9 type II-anchored membrane protein (3), has also been shown to be raft associated (24). Recently, it has been shown that the PRV UL11 tegument protein is membrane associated and is a diacylated protein, a hallmark of many raft-associated proteins, and may therefore be raft associated (23). Together with our present data, this indicates that many alphaherpesvirus membrane (glyco)proteins and tegument proteins may interact with lipid rafts, in different ways and with different affinities.
Although the exact determinants that direct specific proteins to lipid rafts are very diverse and still largely unresolved, it seems unlikely that all of these alphaherpesvirus proteins contain specific signals that link them directly to lipid rafts. Although it is speculative, an attractive alternative explanation may be that the numerous protein-protein interactions which are believed to exist between tegument proteins and the cytoplasmic domains of viral membrane (glyco)proteins (31) may allow a specific subset of strongly raft-associated membrane (glyco)proteins and tegument proteins to serve as nucleation sites to concentrate many of the others indirectly to rafts. Indeed, it is known that proteins, such as Fc gamma receptors, can be indirectly raft associated through their affinity for other raft-associated proteins (16). Importantly, since lipid raft formation and protein association with lipid rafts occur in the Golgi complex (14), such a putative glycoprotein and tegument protein concentrating function of lipid rafts may also be significant for efficient alphaherpesvirus budding and particle formation, as has been hypothesized recently (17, 23, 26). In this context, it is interesting that lipid rafts have recently been shown to serve as budding platforms for several enveloped viruses, such as human immunodeficiency virus, Ebola virus, influenza virus, and measles virus (1, 28, 35, 36, 40), and that such lipid raft-mediated budding has been hypothesized to explain pseudotyping of different viruses, including HSV (37). Further, the viral glycoprotein distribution in the HSV virion envelope has been shown to be nonrandom, which has been hypothesized to be caused by association of viral glycoproteins with lipid rafts (17). In addition, we have found that, like for human immunodeficiency virus (15, 27), cholesterol in the PRV envelope is required for infectivity, since depletion of cholesterol from PRV preparations by using 10 mM MCD completely abolished PRV's ability to successfully infect susceptible cells (data not shown). Therefore, it will be interesting to dissect the exact role of lipid rafts during alphaherpesvirus particle formation.
We found that disruption of lipid raft integrity had only a minor effect on the observed copatching between different viral cell surface glycoproteins. This indicates that lipid rafts may be redundant for maintaining viral cell surface protein links, but it does not exclude the possibility that lipid rafts may be of importance in establishing these links. Indeed, as mentioned above, lipid rafts may serve as nucleation sites to collect and aggregate many glycoproteins and perhaps other viral proteins during the process of the establishment of a link between the different viral cell surface proteins. Further research will be necessary to evaluate this. It is likely that the complex network of interactions between alphaherpesvirus tegument and membrane glycoproteins (31), as mentioned above, is involved in both establishing and maintaining links between the different viral cell surface proteins. However, this complex network is not yet fully understood, which makes it at this time extremely difficult to carefully examine the exact roles of these interactions in the copatching between different PRV cell surface proteins that we observe here. We did find that a PRV mutant that does not express the UL49 protein, which has been shown to interact with the cytosolic domain of gE (13), still showed efficient copatching of gB, gC, and gD with gE, but because of the many redundancies that are thought to exist in the tegument-glycoprotein interaction network (31), this does not necessarily exclude a possible crucial role of these interactions for the observed copatching behaviors of PRV cell surface glycoproteins.
Disruption of lipid rafts by cholesterol depletion resulted in a strong reduction in polarization of the patches to a cap structure, a gE-mediated process that has been observed in specific PRV- and HSV-infected cells (10, 39). Interestingly, lipid rafts have been shown to collect or recruit specific signal transduction proteins at their cytoplasmic leaflet that are of critical importance in allowing capping of specific cellular surface proteins, such as the B-cell receptor (BCR) and T-cell receptor (TCR) (32, 42, 43). Indeed, antigen-stimulated lipid raft association of the BCR and TCR has been shown to allow these protein complexes to interact with raft-associated Src kinases, which subsequently phosphorylate specific tyrosine-based amino acid motifs (immunoreceptor tyrosine-based activation motifs) in the BCR and TCR as a first step in the signal transduction cascade leading to capping of the BCR and TCR and subsequent lymphocyte activation (6). The present data my therefore further support the hypothesis that has been put forward before (9) that the mechanism underlying capping of cross-linked viral glycoproteins, mediated by PRV gE and HSV gE, may be related to capping of cross-linked BCR and TCR in lymphocytes. Indeed, like BCR and TCR capping, efficient PRV gE-mediated capping has been shown to depend on immunoreceptor tyrosine-based activation-like motifs in the gE tail and on tyrosine kinase activity (9), and, as shown here, it seems to depend on lipid raft integrity.
It has been shown before that during the first 4 h of infection, PRV gB and gE, but not gC, undergo spontaneous endocytosis in PRV-infected PK 15 cells (44). This endocytosis is inhibited at later times in infection (44). If, as we show here, gB, gC, and gE all are linked, partly through raft association, then how can gB and gE spontaneously internalize while gC remains on the cell surface? One possible hypothesis may be that the complex interaction between lipid rafts, viral membrane (glyco)proteins, and tegument proteins that we suggest to exist may depend on sufficient levels of protein. Most structural proteins (envelope proteins and tegument proteins) are most abundantly expressed at later stages in infection (from 5 to 6 h p.i. onwards). It may be that at earlier stages in infection, not all factors necessary to allow a link between the different glycoproteins are present in sufficient amounts, thereby allowing endocytosis of some, but not all, major viral cell surface proteins. In support of this hypothesis, we found that when patching gC on the surfaces of PRV-infected SK cells, gD copatching is less pronounced at 5 h p.i. than at 13 h p.i. (data not shown).
In conclusion, we show that at least four major PRV glycoproteins (gB, gC, gD, and gE) on the plasma membranes of infected cells coaggregate when incubated with monospecific antibodies, indicating that they are linked. Further, we have indications that these four viral glycoproteins all may be associated with lipid rafts, although raft association of gC and gD is likely to be very weak. Lipid raft association was found to have only minor importance in allowing the observed copatching events but to be required for efficient capping of the antibody-induced patches of viral glycoproteins, indicating a possible involvement of lipid raft-associated transmembrane signaling events during this process.
Acknowledgments
We thank Carine Boonen for excellent assistance in many experiments; Fernand De Backer, Chantal Vanmaercke, Geoffrey Labarque, Peter Meerts, and Steven Van Gucht for help in isolating porcine blood monocytes; and Geert Van Minnebruggen, Peter Delputte, Kristin Geenen, and the other lab members for fruitful discussions. We also thank S. Brockmeier for swine anti-gD antibodies, K. Bienkowska-Szewczyk for rabbit anti-gE antibodies, and A. Brun for monoclonal anti-gC antibodies.
This research was supported by a Concerted Research Action Fund of the Research Council of Ghent University.
REFERENCES
- 1.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brideau, A. D., B. W. Banfield, and L. W. Enquist. 1998. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 72:4560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 6.Dykstra, M., A. Cherukuri, H. W. Sohn, S. J. Tzeng, and S. K. Pierce. 2003. Location is everything: lipid rafts and immune cell signaling. Annu. Rev. Immunol. 21:457-481. [DOI] [PubMed] [Google Scholar]
- 7.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favoreel, H. W., H. J. Nauwynck, H. M. Halewyck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1999. Antibody-induced endocytosis of viral glycoproteins and major histocompatibility complex class I on pseudorabies virus-infected monocytes. J. Gen. Virol. 80:1283-1291. [DOI] [PubMed] [Google Scholar]
- 9.Favoreel, H. W., H. J. Nauwynck, and M. B. Pensaert. 1999. Role of the cytoplasmic tail of gE in antibody-induced redistribution of viral glycoproteins expressed on pseudorabies-virus-infected cells. Virology 259:141-147. [DOI] [PubMed] [Google Scholar]
- 10.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J. Virol. 71:8254-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favoreel, H. W., G. R. Van de Walle, H. J. Nauwynck, T. C. Mettenleiter, and M. B. Pensaert. 2003. Pseudorabies virus (PRV)-specific antibodies suppress intracellular protein levels in PRV-infected monocytes. J. Gen. Virol. 84:2969-2973. [DOI] [PubMed] [Google Scholar]
- 12.Frank, I., and H. M. Friedman. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gkantiragas, I., B. Brugger, E. Stuven, D. Kaloyanova, X. Y. Li, K. Lohr, F. Lottspeich, F. T. Wieland, and J. B. Helms. 2001. Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell 12:1819-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, D. R., E. Chertova, J. M. Hilburn, L. O. Arthur, and J. E. Hildreth. 2003. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 77:8237-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, J. M., A. D. Schreiber, and E. J. Brown. 1997. Role for a glycan phosphoinositol anchor in Fc γ receptor synergy. J. Cell Biol. 139:1209-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grünewald, K., P. Desai, D. C. Winkler, J. B. Heymann, D. M. Belnap, W. Baumeister, and A. C. Steven. 2003. Three-dimensional structure of herpes simplex virus from cryo-electron microscopy. Science 302:1396-1398. [DOI] [PubMed] [Google Scholar]
- 18.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan, A. S., and A. E. Vatter. 1959. A comparison of herpes simplex virus and pseudorabies virus. Virology 7:394-407. [DOI] [PubMed] [Google Scholar]
- 22.Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopp, M., H. Granzow, W. Fuchs, B. G. Klupp, E. Mundt, A. Karger, and T. C. Mettenleiter. 2003. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 77:5339-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshizuka, T., F. Goshima, H. Takakuwa, N. Nozawa, T. Daikoku, O. Koiwai, and Y. Nishiyama. 2002. Identification and characterization of the UL56 gene product of herpes simplex virus type 2. J. Virol. 76:6718-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang, M. L., L. Shen, and W. F. Wade. 1999. γ-Chain dependent recruitment of tyrosine kinases to membrane rafts by the human IgA receptor FcαR. J. Immunol. 163:5391-5398. [PubMed] [Google Scholar]
- 26.Lee, G. E., G. A. Church, and D. W. Wilson. 2003. A subpopulation of tegument protein Vhs localizes to detergent-insoluble lipid rafts in herpes simplex virus-infected cells. J. Virol. 77:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mettenleiter, T. C., A. Saalmuller, and F. Weiland. 1993. Pseudorabies virus protein homologous to herpes simplex virus type 1 ICP18.5 is necessary for capsid maturation. J. Virol. 67:1236-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mettenleiter, T. C., C. Schreurs, F. Zuckermann, and T. Ben-Porat. 1987. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J. Virol. 61:2764-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran, M., and M. C. Micelli. 1998. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity 9:787-796. [DOI] [PubMed] [Google Scholar]
- 33.Nauwynck, H. J., and M. B. Pensaert. 1992. Abortion induced by cell-associated pseudorabies virus in vaccinated sows. Am. J. Vet. Res. 53:489-493. [PubMed] [Google Scholar]
- 34.Nauwynck, H. J., and M. B. Pensaert. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch. Virol. 140:1137-1146. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickl, W., F. X. Pimentel-Muinos, and B. Seed. 2001. Lipid rafts and pseudotyping. J. Virol. 75:7175-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizvi, S. M., and M. Raghavan. 2003. Responses of herpes simplex virus type 1-infected cells to the presence of extracellular antibodies: gE-dependent glycoprotein capping and enhancement in cell-to-cell spread. J. Virol. 77:701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 41.Sedwick, C. E., and A. Altman. 2002. Ordered just so: lipid rafts and lymphocyte function. Sci. STKE 122:RE2. [DOI] [PubMed] [Google Scholar]
- 42.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 43.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 44.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, and M. B. Pensaert. 2003. Antibody-induced internalization of viral glycoproteins and gE-gI Fc receptor activity protect pseudorabies virus-infected monocytes from efficient complement-mediated lysis. J. Gen. Virol. 84:939-948. [DOI] [PubMed] [Google Scholar]