Abstract
Purpose
Delivery of radiation therapy (RT) to unresectable liver tumors is sometimes limited by proximity of radiosensitive bowel. We sought to determine if biological mesh spacers (BMS) could be used in this situation.
Methods and Materials
BMS composed of acellular human dermis were placed via a laparoscopic or open approach to displace bowel away from unresectable liver tumors in patients previously unable to receive RT due to risk of bowel toxicity.
Results
In one year, 14 patients were treated. Median age was 64. Diagnoses included intrahepatic cholangiocarcinoma (IHC) (n=6), hepatocellular carcinoma (n=3), and metastases (n=5). A solitary lesion was present in 8 patients, while 4 patients had 2 lesions and 2 patients had 3 lesions. Median largest tumor size was 6.3 cm (range 1.6-17.5 cm). Limited extrahepatic disease was present in 5 patients. The surgical approach was laparoscopic in 10 patients and open in 4 patients. Median length of stay was 2.5 days (1-8), and 3 patients developed low-grade complications. Folded, extra thick (2.3-3.3 mm) BMS, with a median area of 384 cm2 (256-640 cm2), were used to displace stomach (n=9), duodenum (7), colon (6) and/or small bowel (2). The mean displacement of these organs on post-procedure imaging was 23 mm, 23 mm, 24mm, and 20 mm, respectively. Two patients did not receive RT due to extrahepatic disease progression. The remaining patients had 3-D conformal proton RT (n=5), stereotactic body RT (4), or intensity-modulated RT (3). Median dose delivered was 54 Gy (40-58.5) in 5-15 fractions with only 1 patient with grade 3-4 toxicity. At short term followup of at least 6 months, local disease control was obtained in 11 of 12 patients.
Conclusions
Initial dual institution experience with this novel strategy demonstrates feasibility, allowing previously untreatable liver tumor patients to receive high-dose RT.
Keywords: liver tumors, biological mesh, spacers, radiation therapy
INTRODUCTION
The liver has a limited tolerance for radiation therapy (RT) and is susceptible to radiation-induced liver disease and other toxicities, making RT of primary liver tumors and liver metastases difficult.1 The past decade has seen the development of more advanced and accurate techniques for delivering RT to liver tumors.2 Despite this, the ability to deliver ablative doses of RT to liver tumors can be limited by the immediate proximity of radiation-sensitive organs including bowel. Adjacent organ toxicity becomes an even greater issue when hypofractionated doses are used. The use of spacers to displace radiation-sensitive organs may allow the use of higher doses of RT in certain circumstances. Here we describe the use of biological mesh spacers (BMS) to displace stomach, duodenum, small bowel, or colon away from liver tumors prior to the delivery of high-dose RT.
METHODS AND MATERIALS
Clinical Data
This retrospective review was approved by both institutions’ Internal Review Boards. Radiological imaging included a computed tomographic (CT) scan of the chest, abdomen and pelvis. In some instances, patients also underwent magnetic resonance imaging (MRI), positron emission tomography (PET)/CT. Cases were reviewed in a multidisciplinary fashion, and treatment plans were individualized for each patient. Liver tumors were labelled unresectable if an experienced liver surgeon deemed the tumor to be unresectable or if extrahepatic disease was present. Patients were selected for BMS placement when the treating radiation oncologist determined that the proximity of bowel would limit the dose of RT that could be delivered. Generally, tumor was in direct contact or within one cm with bowel.
Procedures were performed between May 2011 and April 2012. One patient was lost to followup, and data was last obtained on all other patients in February 2013. Median follow-up on all patients was 10 months. Local progression was defined by the RECIST definition of progressive disease, i.e. at least a 20% increase in the sum of the longest diameter of target lesions.3. Distant progression was defined as the development or growth of metastatic disease beyond the primary site.
Surgical procedures and biological mesh
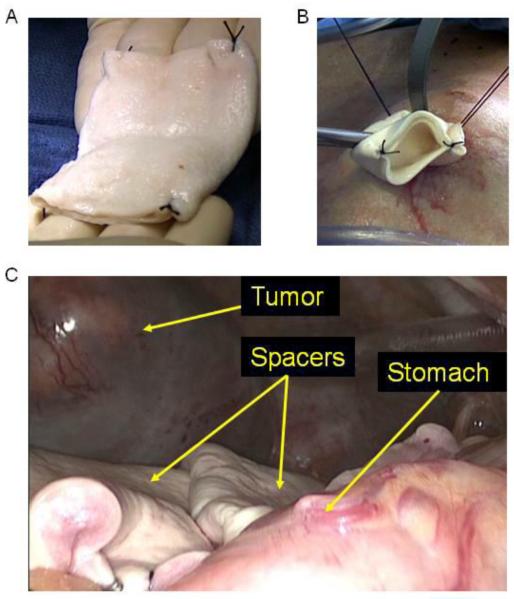
The BMS used in all cases was Alloderm (Lifecell). Alloderm is derived from donated human skin from cadavers and chemically processed to preserve the structural and biologically active dermal matrixBMS were placed laparoscopically whenever possible. A 12 mm Hasson port was placed in the periumbilical position followed by placement of two 5 mm ports and one 12 mm port. Lysis of adhesions was performed if necessary followed by mobilization of the stomach, duodenum, small bowel, or colon depending on the specific situation. Sheets of extra-thick BMS 8 × 16 cm in size were then folded into a 3 layer sandwich and sewn together at the 4 corners (Fig. 1A). The sheets were then introduced into the peritoneal cavity through the Hasson port site (Fig. 1B) and positioned between the liver tumor and the mobilized bowel (Fig. 1C). The BMS were secured to adjacent soft tissue or liver using 10 mm clips and/or intracorporeal sutures. For open or laparoscopically-assisted cases, folded 16 × 20 cm BMS were used.
Figure 1.
Photos demonstrating (A) creation of 3 layer BMS spacer from an 8 × 16 cm sheet of Alloderm with sutures at the 4 corners, (B) insertion of the BMS into the abdominal cavity via the Hasson port site, and (C) placement of two BMS between a large left lateral segment liver tumor and stomach.
External beam radiotherapy
Following recovery from surgery, patients were treated using either proton beam RT (PBRT) or photons. Techniques for radiation delivery included 3-D conformal PBRT, stereotactic body RT (SBRT), and intensity-modulated RT (IMRT).
PBRT was delivered at Institution A using 240 MeV protons generated from a cyclotron. PBRT was delivered using 3D passively scattered protons. RT was planned using a CT scan obtained with the patient immobilized in the treatment position. All Institution A patients underwent placement of fiducials near the tumor prior to RT planning. Two 4-D CT scans were obtained on separate days to assess interfractional variation. Additionally, the scans each day were done with and without abdominal compression depending on whether abdominal compression was found to decrease tumor motion. A helical scan with IV contrast was obtained on the second day.
All patients at Institution B were treated with photons using IMRT with a breath-hold technique. Internal fiducial markers were not placed. A 4-D CT scan was obtained after a moderate inspiration during simulation using the Varian Real-time Position Management (RPM) system to monitor the respiratory cycle. The same technique was used for treatment. All patients were treated using a CT-on-Rails system for target localization. A daily CT scan was obtained immediately prior to daily treatment using the same inspiration breath hold technique. 3-D soft tissue alignment was accomplished and verified prior to each fraction.
In all cases, the locations of the BMS were identified on the planning CT scan and the postoperative clinical target volumes were contoured in conjunction with the surgical oncologist.
Measurement of organ displacement by spacers
Abdomen CT scans before and after placement of BMS were reviewed by two radiologists. The closest distance between the tumor to stomach, duodenum, small bowel, and colon was determined before and after BMS placement, and the change in distance was calculated. Bowel that was more than 25 mm away from the tumor before and after BMS placement was excluded from this analysis based on the authors’ clinical judgment
RESULTS
Patients and treatment
Table 1 summarizes the patient and tumor characteristics of the 14 patients in this study. The most common histology was cholangiocarcinoma. Three patients had hepatocellular carcinoma in the setting of Child-Pugh A or B cirrhosis. Most patients had just a solitary liver tumor, with the median size of the largest tumor being 6.25 cm (range 1.6-17.5 cm). Five patients had extrahepatic disease in the form of nodal metastases (n=3), bone metastases (n=1), or primary tumor still in situ (n=1). Prior to BMS placement, half the patients had received some form of therapy, most commonly chemotherapy, for the targeted liver tumor or tumors. The primary reasons for not performing surgical resection of the liver tumor(s) were the following: presence of extrahepatic disease (n=5), tumor deemed unresectable by surgeon (n=5), underlying cirrhosis precluding surgical resection (n=3), and unfavorable histology (i.e. pancreatic cancer, n=1). RT was chosen over RFA in 10 cases due to size of tumor being greater than 4 cm. In the four cases where maximal tumor size was less than 4 cm, RFA was not chosen due to proximity of the tumor to major vessels (i.e. heat sink).
Table 1.
Patient and Tumor Characteristics
| Patients (n=14) | Median or n | Range or % |
|---|---|---|
| Age | 64 | 46-83 |
| Male | 8 | 57% |
| Tumor type | ||
| Cholangiocarcinoma | 6 | 43% |
| Hepatocellular carcinoma | 3 | 21% |
| Mestastatic colorectal cancer | 2 | 14% |
| Metastatic pancreatic cancer | 2 | 14% |
| Mestastatic esophageal cancer | 1 | 7% |
| Number of liver tumors | ||
| One | 8 | 57% |
| Two | 4 | 29% |
| Three | 2 | 14% |
| Size of largest tumor (cm) | 6.25 | 1.1 - 17.5 |
| Location of liver tumors | ||
| Right | 5 | 36% |
| Left | 6 | 43% |
| Bilateral | 3 | 21% |
| Extrahepatic disease | ||
| Yes | 5 | 36% |
| No | 9 | 64% |
| Prior therapy for liver tumors | ||
| None | 7 | 50% |
| Chemotherapy | 5 | 36% |
| Chemotherapy plus radiation | 1 | 7% |
| Chemotherapy plus TACE | 1 | 7% |
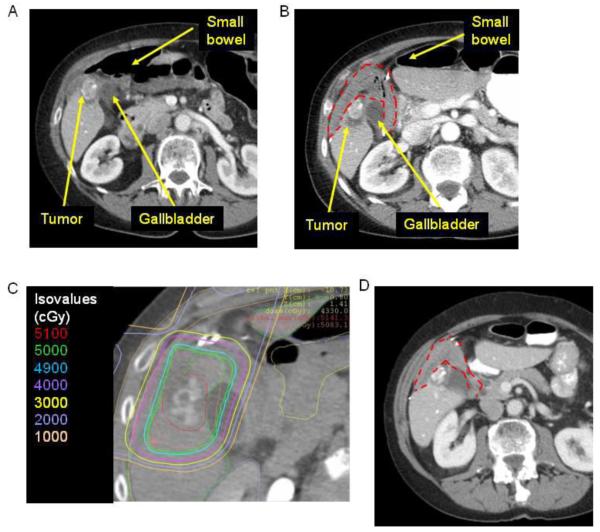
The first patient treated with this technique was a 72 year old woman with a 5.4 cm cholangiocarcinoma in segment V of her right liver. PET/CT at the time of diagnosis showed FDG avidity in the primary tumor along with metastatic periportal and periaortic lymph nodes. She received chemotherapy on a clinical protocol. PET/CT three years after initial diagnosis showed calcification of her primary tumor, resolution of her lymphadenopathy, and no FDG activity. One year later, PET/CT showed two foci of increasing FDG activity in her primary tumor. The patient was offered surgical resection or RT following laparoscopic BMS placement, and she chose the latter option. BMS placement was required because small bowel was immediately adjacent to the tumor (Fig. 2A). She underwent laparoscopic mobilization of stomach, duodenum, and small bowel followed by placement of BMS. Post-procedure abdomen CT scan demonstrated displacement of the small bowel to >3.6 cm (Fig. 2B). She then received proton beam SBRT at a dose of 50 Gy in 5 fractions ending in June of 2011 (Fig. 2C). Abdomen CT scan one year after RT demonstrated regression of the tumor (Fig. 2D).
Figure 2.
A) Axial CT image of an intrahepatic cholangiocarcinoma adjacent to the gallbladder and small bowel prior to placement of biological mesh spacer. Tumor is partially calcified. B) Axial CT image following placement of BMS (red dotted line). C) Axial CT image with radiation planning after placement of BMS (red dotted line). D) Axial CT image 18 months after BMS placement and radiation.
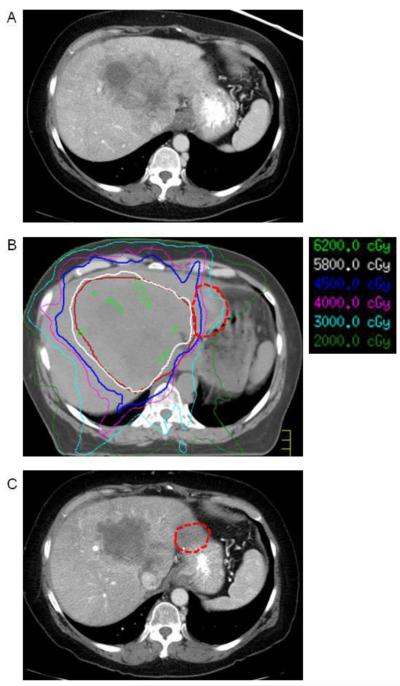
The last patient treated was a 63 year old woman with a 17.5 cm intrahepatic cholangiocarcinoma occupying much of the left lobe of the liver extending into the caudate. The tumor was deemed to be unresectable due to inferior vena cava involvement. She underwent two rounds of transarterial chemoembolization with minimal response. She subsequently underwent 12 cycles of gemcitabine and cisplatin chemotherapy with some decrease in size of tumor, but with persistent abutment of the tumor to the stomach (Fig. 3A). She then underwent laparoscopic-assisted placement of a BMS between the stomach and liver followed by IMRT to a total dose of 58.1 Gy over 15 fractions (Fig. 3B). Three months post-treatment abdomen CT demonstrated no extrahepatic progression, near complete necrosis of the tumor(Fig. 3C).
Figure 3.
(A) Axial CT image demonstrating large central intrahepatic cholangiocarcinoma invading inferior vena cava. B) Axial CT image from radiation planning CT demonstrating displacement of stomach away from tumor by BMS (red dotted line). C) Axial CT image 3 months after biological mesh placement and radiation.
Surgical placement of BMS
The surgical approach was laparoscopic in 9 patients, laparoscopically-assisted in 1 patient, and open in 4 patients. One patient had an initial attempt at laparoscopic placement but was converted to an open operation due to significant adhesions. Organs mobilized for spacer placement according to the operative report included stomach (n=11), duodenum (n=7), colon (n=6), and small bowel (n=1). Extra thick (2.3-3.3 mm) BMS were used, with a median total surface area of 384 cm2 (256-640 cm2). Six patients underwent additional procedures beyond lysis of adhesions during BMS spacer placement including laparoscopic incisional hernia repair with mesh (n=2), laparoscopic cholecystectomy, laparoscopic placement of gold fiduciary seeds into the liver, laparoscopic wedge resection of a liver lesion, and open wedge resection of a liver lesion along with portal lymphadenectomy. Median operating time was 118 min (range 57-232 min). Median length of stay was 2.5 days (range 1-8). Three patients developed complications including abdominal wall hematoma at a port site that required no intervention, wound cellulitis managed with antibiotics, and ileus managed with nasogastric tube decompression.
Distance between tumor/tumor bed and adjacent structures
Table 2 shows the mean distance between specific organs and the liver tumors before and after BMS placement. In 9 patients, the stomach was less than 25 mm away from the tumor with a mean distance of 6.5 mm. After BMS placement, the mean distance from stomach to tumor was 29.8 mm. For the duodenum, 7 patients had tumor(s) less than 25 mm away with a mean distance before BMS placement of 9.6 mm, and the mean distance after BMS placement was 32.9 mm.
Table 2.
Displacement of Organs
| Number of patients |
Mean distance before spacer in mm (range) |
Mean distance after spacer in mm (range) |
Mean change in mm (range) |
|
|---|---|---|---|---|
| Stomach | 9 | 6.5 (0-22) | 29.8 (9-50) | 22.8 (6-50) |
| Duodenum | 7 | 9.6 (0-24) | 32.9 (10-50) | 23.3 (5-50) |
| Small Bowel | 2 | 11.5 (0-23) | 31.5 (27-36) | 20.0 (4-36) |
| Colon | 6 | 4.6 (0-12) | 28.2 (14-48) | 23.5 (9-46) |
Radiation therapy and toxicity
Two patients did not receive RT after BMS placement due to rapid extrahepatic disease progression. For the remaining 12 patients, Table 3 shows the number of organs displaced for each patient, the mean distance before and after BMS placement, and the RT delivered. Patients 1-9 were treated at one institution where the preferred RT modality was PBRT. PBRT was delivered by proton 3-D conformal RT (3D-CRT) in 5 patients and SBRT in 4 patients.. Patients 10-12 were treated another institution by photon IMRT in 3 patients and photon SBRT in 1 patient. The median total dose was 54 Gy with a range of 40-58.5 Gy. RT was generally well tolerated with some grade 1 nausea (n=4) and fatigue (n=3) and grade 1-2 abdominal pain (n=3) (Table 4). There was only one grade 3 abdominal pain and no other grade 3-4 toxicities including no GI bleeding or ulceration, which have been previously associated with radiotherapy in this location.
Table 3.
Radiation Therapy
| Patient | Number of organs displaced |
Mean distance before spacer in mm (range) |
Mean distance after spacer in mm (range) |
Radiation therapy modality |
Number of fractions |
Dose per fraction |
Total dose |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 3 | 15.3 (0-24) | 33.0 (31-36) | SBRT | 5 | 10 | 50 |
| 2 | 2 | 6.5 (1-12) | 26.0 (23-29) | 3-D conformal | 15 | 3.9 | 58.5 |
| 3 | 1 | 24 | 33 | 3-D conformal | 15 | 3.9 | 58.5 |
| 4 | 2 | 11.0 (4-18) | 33.0 (31-35) | 3-D conformal | 15 | 3.9 | 58.5 |
| 5 | 3 | 3.3 (2-5) | 22.3 (10-30) | 3-D conformal | 15 | 2.7 | 40.5 |
| 6 | 2 | 13.5 (4-23) | 20.5 (14-27) | SBRT | 5 | 8 | 40 |
| 7 | 2 | 5.0 (3-7) | 12.5 (9-16) | SBRT | 5 | 8 | 40 |
| 8 | 1 | 13 | 29 | 3-D conformal | 5 | 10 | 50 |
| 9 | 2 | 0 (0-0) | 50.0 (48-52) | SBRT | 4 | 10 | 40 |
| 10 | 2 | 5.5 (0-11) | 27.5 (20-35) | IMRT | 13 | 4.5 | 58.5 |
| 11 | 1 | 5 | 50 | IMRT | 15 | 3.9 | 58.5 |
| 12 | 1 | 10 | 30 | IMRT | 15 | 3.7 | 58.1 |
SBRT, stereotactic body radiation therapy; IMRT, intensity-modulated radiation therapy
Table 4.
Radiation Therapy Toxicity
| Grade 1 or 2 | Grade 3-5 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Nausea | 4 | 33 | 0 | 0 |
| Abdominal pain | 2 | 17 | 1 | 8 |
| Fatigue | 3 | 25 | 0 | 0 |
| Gastrointestinal ulcer/bleeding | 0 | 0 | 0 | 0 |
Disease control
With short-term follow-up (median 10 months), there was no local progression in 11 of 12 patients, but 6 patients developed new or progressive metastatic disease. There were 6 patients with unresectable intrahepatic cholangiocarcinomas in this study including the one patient with local progression in this study. One other patient with intrahepatic cholangiocarcinoma developed a new liver lesion away from the primary liver lesion 9 months after the completion of RT. The remaining 4 patients with intrahepatic cholangiocarcinoma were without local or distant progression at last follow-up.
DISCUSSION
This manuscript describes the use of BMS in combination with RT for unresectable liver tumors. BMS were used to displace bowel abutting the target volume to allow safe delivery of external beam RT. Therapy was generally well-tolerated, and there was only one grade 3 or 4 toxicity. Two patients had local progression shortly after BMS placement and did not receive RT. With short-term followup of at least 6 months, local disease was controlled in 11 of 12 patients. There were 6 patients with unresectable intrahepatic cholangiocarcinomas, and this subgroup of patients may have benefited most from this strategy with 4 of 6 patients free of local and distant progression at last followup. Thus this is a feasible strategy that warrants further investigation in a larger number of patients.
To our knowledge, this is the first study to describe the use of implanted BMS spacers to facilitate the delivery of RT for liver tumors. Komatsu et al. reported a single case in which a Gore-Tex spacer was used to displace bowel away from a 12 cm hepatocellular carcinoma prior to PBRT of 84 Gy over 20 fractions.4 We have previously described the use of BMS and RT for retroperitoneal and pelvic tumors.5
SBRT refers to the deliver of large doses of highly conformal radiation over a limited number of dose fractions to extracranial tumor sites. When applied to liver metastases, SBRT must minimize normal liver injury from RT, which is a function of both the volume of liver irradiated and the dose of radiation administered. Thus image-guided radiotherapy (IGRT) techniques are often used. Breathing-related liver motion may need to be accounted for by controlling breathing, abdominal compression, respiratory gating, or tumor tracking using implanted fiducial markers. Hoyer et al. recently reviewed 7 phase I and II prospective studies and 5 retrospective studies on SBRT for liver tumors.2 There was significant variability among the studies in terms of types of tumors treated, tumor volumes, radiation dose and fractionation, and dosimetric planning criteria. Most studies used doses of 30-60 Gy in 1-6 fractions. Two-year local control ranges from 60-90% and 2-year survival ranges from 30-83%. A dose response for local control seemed to exist, although the optimal threshold dose was uncertain. Grade 1 or 2 toxicities including liver function test elevations, nausea, and fatigue were common, but grade 3 or greater toxicity such as liver failure or severe GI toxicity were uncommon. In this study, there 3 patients with minor surgical complications and only 1 patient with grade 3-4 toxicity from radiation. Of note, the followup on this study was short and thus late complications were not assessed.
The proximity of tumors to normal bowel is becoming a more important issue as the use of hypofractionated treatment schedules increases. In the Radiation Therapy Oncology Group 1112 randomized phase III study of sorafenib versus SBRT followed by sorafenib for hepatocellular carcinoma, “…reducing the maximal dose to all luminal gastrointestinal normal tissues [is] a planning priority to reduce the risk of gastrointestinal toxicity” and maximal dose to stomach, duodenum, small bowel, and colon is set at 30 Gy.6 An alternative strategy is to intentionally underdose the planning tumor volume (PTV) in order to protect organs at risk. It remains unknown if underdosing the periphery of the PTV will impact local control as the clonogenic density at the periphery of the CTV may be lower.
There may be several disadvantages to the strategy using BMS to displace organs prior to RT. First, this invasive procedure can be associated with complications, and there were 3 low-grade complications in our 14 patients. Second, it takes some experience on the part of the surgeon to optimally place these spacers to get adequate separation between tumor and bowel, and thus there is a learning curve to this technique. Third, it can be difficult for radiologists to discern the biological mesh from recurrent tumor, thus making surveillance for tumor recurrence more difficult. Third, biological meshes are generally quite expensive, and one large sheet of extra thick Alloderm costs several thousands of dollars. Finally, patient selection is extremely important. Two patients had disease progression after BMS placement and before RT and thus in retrospect were poor choices for this procedure.
In summary, we describe the use of BMS to displace bowel away from primary and metastatic liver tumors to facilitate the delivery of external RT in patients who previously were not candidates. The idea of using spacers or implants to displace radiation-sensitive organs prior to RT has been discussed for at least 20 years. The primary innovations of this study are the use of this concept for liver tumors, the use of minimally invasive approaches to place the spacers, and the use of BMS rather than prosthetic implants or gels. The use of BMS allowed for the delivery of high doses of RT to the tumor, therapy was generally well-tolerated, and short-term local control was excellent. Thus this strategy is feasible and warrants further investigation.
Acknowledgments
The project was supported by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Society for Surgical Oncology Annual Meeting, Washington, D.C. on March 6, 2013.
Conflict of Interests Notification: The authors have no conflict of interests.
REFERENCES
- (1).Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- (2).Hoyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- (3).Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- (4).Komatsu S, Hori Y, Fukumoto T, Murakami M, Hishikawa Y, Ku Y. Surgical spacer placement and proton radiotherapy for unresectable hepatocellular carcinoma. World J Gastroenterol. 2010;16:1800–1803. doi: 10.3748/wjg.v16.i14.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Yoon SS, Chen YL, Kambadakone A, Schmidt B, DeLaney T. Surgical placement of biological mesh spacers prior to external beam radiation for retroperitoneal and pelvic tumors. Practical Radiat Oncol. doi: 10.1016/j.prro.2012.06.008. (in press) [DOI] [PubMed] [Google Scholar]
- (6).Radiation Therapy Oncology Group website: www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1112.