The phosphoinositide 3-kinase (PI3K) pathway has a crucial role in tumor progression and drug resistance, including both conventional chemotherapeutics as well as novel agents.1 Although no mutations have been described in the PI3K/Akt genes in multiple myeloma (MM), it was shown that this pathway is constitutively activated in MM cells and has pleiotropic effects influencing proliferation, drug resistance, angiogenesis and cell adhesion.2
PI3Ks are divided into three subclasses, and of these, class I PI3Ks—p110α (also known as PIK3CA), p110β (also known as PIK3CB), p110γ (also known as PIK3CG) and p110δ (also known as PIK3CD)—are well described in terms of their role in cancer development and progression.1, 3 PIK3CA is frequently mutated in solid tumors including carcinoma of the prostate, breast colon and endometrium.4, 5 However, there have been no reports of cancer-specific mutations in MM.6
Recently, a number of potential therapeutics targeting specific PI3K groups or isoforms were developed.3, 4 Previous studies have indicated that p110α, p110β and p110δ might be potential targets for MM.7, 8, 9 Although the basic framework of PI3K signaling has been uncovered, the contribution of the different PI3K isoforms is not well understood.4 In the current study, we investigated the functional role of class I PI3K isoforms in modulating MM cell trafficking in vivo and in vitro.
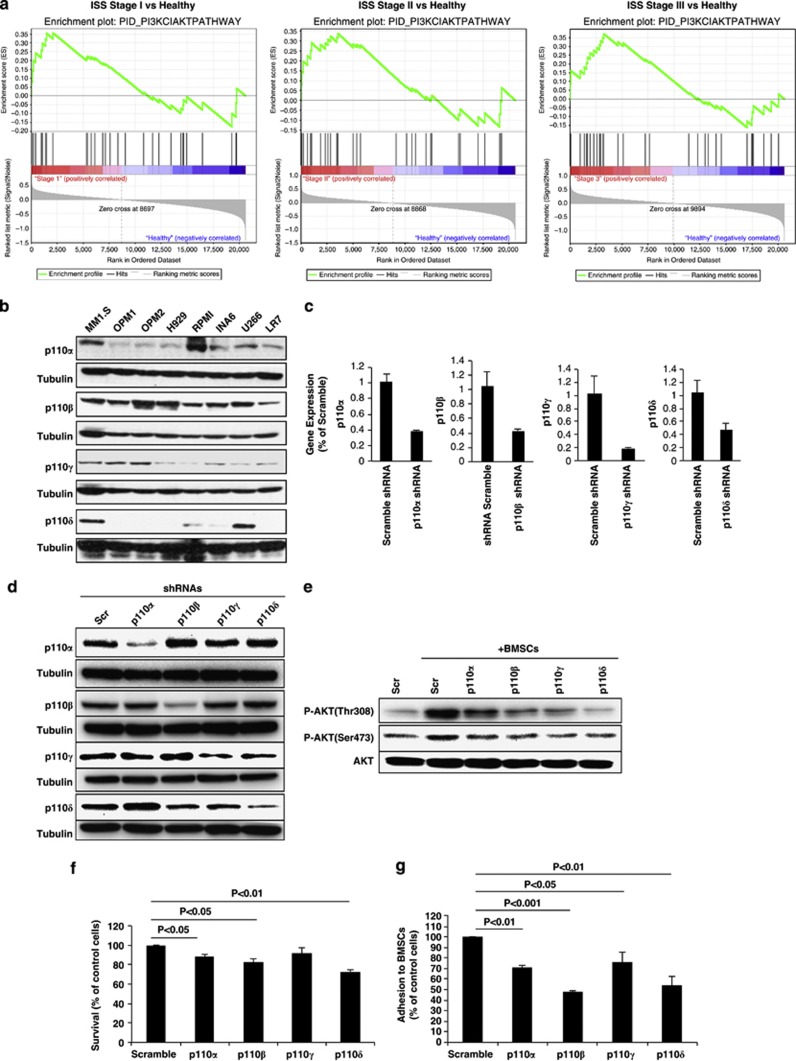
To examine activation of the PI3K/Akt pathway in MM, we first performed gene set enrichment analysis10 on the gene-expression data set (Shaughnessy et al. ref. GSE24080) of patients in different International Staging System stages of MM compared with normal donors;11 and found enrichment of genes related to class I PI3K-activated AKT signaling events. These findings were observed in stage I, II and III MM patients compared with healthy individuals (Figure 1a).
Figure 1.
The role of class I PI3K-mediated Akt signaling in MM. (a) Gene set enrichment analysis software analyzed functionally related genes in class I-mediated Akt activation with statistically significant enrichment (false-discovery rate q-values <0.25; <0.25 is considered significant), using gene-expression data set (GSE24080). Plots show enrichment results for the gene set (left, stage I MM vs normal subjects; middle, stage II MM vs normal subject; right, stage III MM vs normal subjects). (b) Baseline expression of the different PI3K isoforms (p110α, β, γ and δ) in MM cell lines was detected by immunoblotting using isoform-specific antibodies. MM tumor cells (MM.1S-GFP+/luc+) were infected with lentivirus-mediated small hairpin (sh)RNA. Reverse transcription quantitative PCR (c) and immunoblotting (d) were performed to show infection efficiency and isoform specificity, respectively. Scramble and knockdown tumor cells (p110α, β, γ and δ) were cocultured with BMSCs overnight, and MM cells were then separated from the BMSCs, lysed and whole-cell lysates were subjected to immunoblotting (e) with Akt and P-Akt (Thr308 and Ser473), which shows decreased phosphorylation of Akt in knockdown cells. The effects of inhibition of PI3K isoforms by shRNAs on cell survival were assessed by 3-(4,5-dimethylthiazol-2-yl)2-2-diphenyltetrazolium bromide (MTT) assay (f). Adhesion assay (g) was performed to show the ability of knockdown cells to adhere to BMSCs after 2 h of incubation.
To study the role of each isoform (p110α, β, γ, and δ) in regulating MM cell survival and trafficking in vivo and in vitro, the expression of PI3K isoforms was examined in a panel of eight MM cell lines showing different levels of expression of PI3K isoforms with only MM.1S expressing all isoforms (Figure 1b). Thus, MM.1S-GFP+/luc+ was infected with lentivirus-mediated small hairpin RNAs targeting the different PI3K isoforms. Stable cell lines were generated, and efficiency of knockdown for each isoform was confirmed by reverse transcription quantitative PCR (Figure 1c). Specificity of knockdown was demonstrated by immunoblotting in cell lines using specific antibodies against each isoform (Figure 1d). Then, we evaluated the effect of each isoform on PI3K–Akt signaling in MM cells in the context of primary MM bone marrow mesenchymal stromal cells (BM-MSCs) and found inhibition of BM-MSC-dependent induction of phospho(p)-Akt in MM cells with all PI3K isoforms silenced in the tumor clone (Figure 1e). Although p110α, β, and δ showed a modest reduction in cell survival in vitro (Figure 1f), cell cycle analysis revealed no significant difference on cell cycle distribution patterns (Supplementary Figure 1). We next performed adhesion assay of MM cells to primary MM-derived BM-MSCs; and found that by silencing each of class I PI3K isoforms, MM cells inhibited their adhesion properties, with the p110β and p110δ knockdown being the most effective (53% reduction and 47% reduction, respectively; P<0.001, P<0.01; Figure 1g).
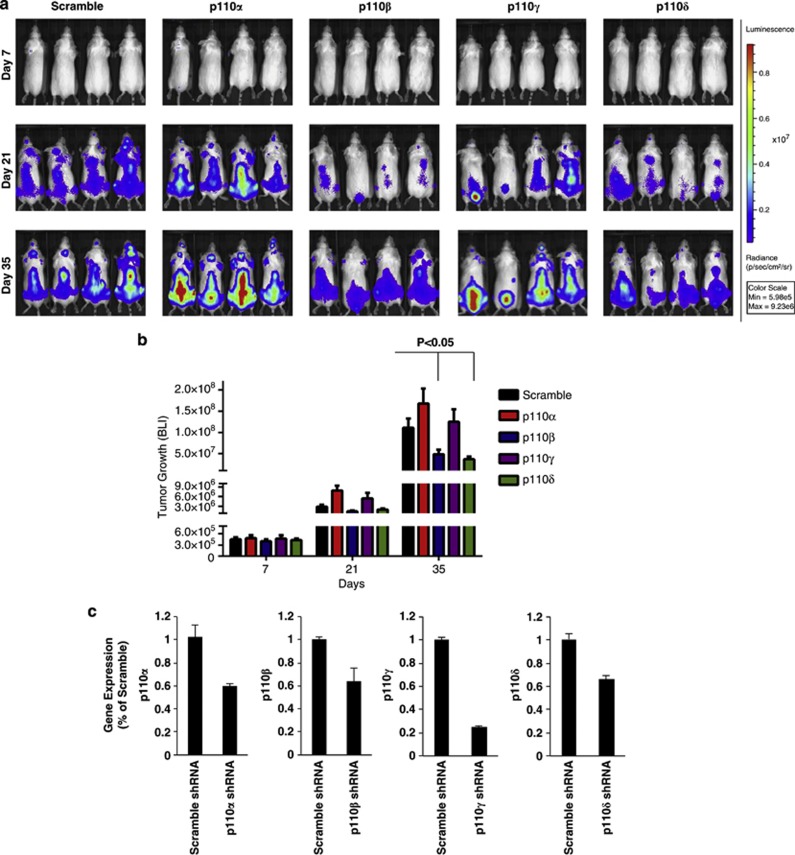
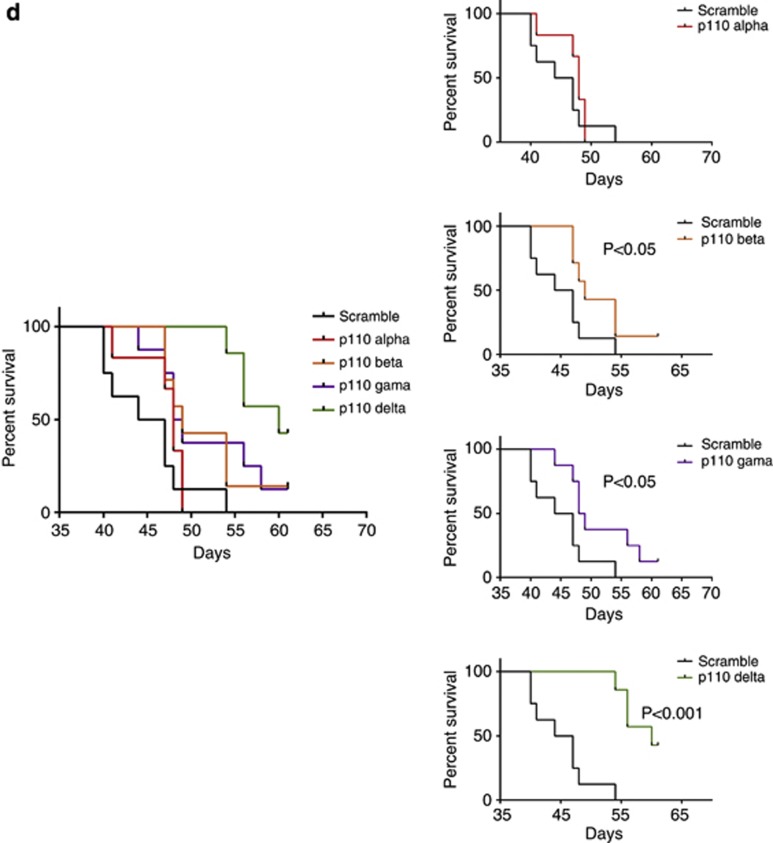
To test the effect of the different p110 isoforms on MM tumor progression in vivo, SCID-Bg mice were injected with MM cells silenced for p110α, β, γ and δ, and tumor development was monitored by bioluminescence imaging. Scramble-infected cells were used as control. In consistent with in vitro data demonstrating that the most significant changes were observed for adhesion of MM cells to BM-MSCs in p110β and p110δ knockdown cells, tumor progression was significantly lower in p110β- and p110δ-knockdown cell-injected mice compared with scramble cell-injected mice (P<0.05); whereas tumor growth observed in p110α- and p110γ-knockdown cell-injected mice was similar to control mice (Figures 2a and b). We speculate that this might be due to markedly decreased tumor cell growth triggered by MM cell adhesion to BM-MSCs, as the adhesion of MM cells to BM-MSCs activates many pathways and has a vital role in MM pathogenesis and disease progression.12 We further confirmed that tumor cells showed knockdown for each p110 isoform, as demonstrated ex vivo on tumor cells harvested from each cohort of mice (Figure 2c). Mice were followed until the development of hind limb paralysis or death, and Kaplan–Meier analysis was performed showing prolonged survival in all groups except p110α mice (p110β and p110γ, P<0.05; p110δ, P<0.001; Figure 2d). Despite similar tumor burden observed between p110γ mice and scramble control-injected mice, mice injected with p110γ knockdown cells had improved survival compared with control mice. This might be due to the different extent of tumor involvement of various organs13 between the two groups, thus explaining the differences in survival.
Figure 2.
Knockdown of PI3K isoforms regulates tumor progression and survival in vivo. MM.1S-GFP+/luc+ tumor cell lines (Scr, p110α, β, γ and δ) were injected intravenously into SCID-Bg mice and tumor growth was assessed by in vivo bioluminescence imaging (BLI). (a) Representative BLI of each group in different time points is shown. (b) Quantification of BLI signals demonstrated that p110β and δ mice showed significant reduction in tumor growth (P<0.05) compared with scramble mice. (c) Reverse transcription quantitative PCR was performed on tumor cells that were harvested from hind leg bones of animals by bone marrow flushing. (d) Survival of mice was evaluated until complete hind limb paralysis or death using Kaplan–Meier curves. Compared with scramble mice, all groups except p110α showed prolonged survival (p110β and p110γ, P<0.05; p110δ, P<0.001).
Interestingly, our data indicate that p110α is not critical for the survival of MM cells in vivo. Unlike most solid tumor malignancies, where PI3KCA (p110α) mutation is the leading cause of activation of this pathway and is the target of many therapeutic agents in development,3 there have been no reports of this specific mutations in MM.6 Moreover, it was shown that unlike wild-type p110α, overexpression of the wild-type p110β, p110γ and p110δ is sufficient to induce an oncogenic transformation of fibroblasts in cell culture.14
In this study, p110β was highly expressed in all MM cell lines, whereas only a minor subset expressed p110δ at the protein level (Figure 1b), which is consistent with a recent report9 showing expression of p110β in 38 MM cell lines in comparison to the detectable expression of p110δ in only 4 cell lines. In addition, another study8 reported similar findings in cell lines showing lack of p110δ expression in most MM cell lines. Of note, we found discrepancies in p110δ expression in cell lines between our study and prior published studies but our data was confirmed in the Cancer Cell Line Encyclopedia data at the mRNA level (data not shown).15 Importantly, Ikeda et al.8 evaluated p110δ levels in patient samples and detected its expression in all 24 MM patients. This may provide a clinical rationale for targeting p110δ despite the lack of expression of p110δ in MM cell lines.
Overall, our data suggest that, in contrast with solid tumors, MM may be more dependent on PI3K p110β and p110δ and less dependent on PI3Kα, and these may be the focus of drug development in this hematological malignancy.
Acknowledgments
This work was supported by R01CA154648.
IMG is on the advisory board for Onyx, BMS and Celgene, and receives research lab support from Genzyme and BMS. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Author contributions
IS: designed and performed the research, analyzed the data and wrote the manuscript; MM, YM, SVG, BT, FA, YZ, PM, AS, AKA and AMR: performed the research and analyzed the data; IMG: supervised the study and wrote the manuscript.
Supplementary Material
References
- Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Lonial S. PI3 kinase/AKT pathway as a therapeutic target in multiple myeloma. Future Oncol. 2007;3:639–647. doi: 10.2217/14796694.3.6.639. [DOI] [PubMed] [Google Scholar]
- Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Zhao L, Vogt PK, Class I. PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail SI, Mahmoud IS, Msallam MM, Sughayer MA. Hotspot mutations of PIK3CA and AKT1 genes are absent in multiple myeloma. Leuk Res. 2010;34:824–826. doi: 10.1016/j.leukres.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Glauer J, Pletz N, Schon M, Schneider P, Liu N, Ziegelbauer K, et al. A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo. Blood Cancer J. 2013;3:e141. doi: 10.1038/bcj.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116:1460–1468. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munugalavadla V, Mariathasan S, Slaga D, Du C, Berry L, Del Rosario G, et al. The PI3K inhibitor GDC-0941 combines with existing clinical regimens for superior activity in multiple myeloma. Oncogene. 2013;33:316–325. doi: 10.1038/onc.2012.594. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009;37 (Database issue:D674–D679. doi: 10.1093/nar/gkn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahindra A, Hideshima T, Anderson KC. Multiple myeloma: biology of the disease. Blood Rev. 2010;24 (Suppl 1:S5–S11. doi: 10.1016/S0268-960X(10)70003-5. [DOI] [PubMed] [Google Scholar]
- Huang YW, Richardson JA, Tong AW, Zhang BQ, Stone MJ, Vitetta ES. Disseminated growth of a human multiple myeloma cell line in mice with severe combined immunodeficiency disease. Cancer Res. 1993;53:1392–1396. [PubMed] [Google Scholar]
- Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.