Abstract
One of three full-length infectious molecular clones of SHIVDH12R, designated SHIVDH12R-CL-7 and obtained from productively infected rhesus monkey peripheral blood mononuclear cells, directed rapid and irreversible loss of CD4+ T cells within 3 weeks of its inoculation into Indian rhesus monkeys. Induction of complete CD4+ T-cell depletion by SHIVDH12R-CL-7 was found to be dependent on inoculum size. The acquisition of this pathogenic phenotype was accompanied by the introduction of 42 amino acid substitutions into multiple genes of parental nonpathogenic SHIVDH12. Transfer of the entire SHIVDH12R-CL-7 env gene into the genetic background of nonpathogenic SHIVDH12 failed to confer the rapid CD4+ T-lymphocyte-depleting syndrome; similarly, the substitution of gag plus pol sequences from SIVsmE543 for analogous SIVmac239 genes in SHIVDH12R-CL-7 attenuated the pathogenic phenotype. Amino acid changes affecting multiple viral genes are necessary, but insufficient by themselves, to confer the prototypically rapid and irreversible CD4+ T-cell-depleting phenotype exhibited by molecularly cloned SHIVDH12R-CL-7.
Simian immunodeficiency virus (SIV)/human immunodeficiency virus (HIV) chimeras (SHIVs) have proven to be useful reagents for studies of primate lentivirus pathogenesis and vaccine development (2, 3, 10, 21). Because they bear the HIV type 1 (HIV-1) envelope glycoprotein, SHIVs have been valuable for assessing vaccine-induced anti-HIV-1 neutralizing antibodies (2, 3, 5, 18, 24). Furthermore, highly pathogenic SHIVs induce an unusually rapid, complete, and irreversible depletion of CD4+ T lymphocytes in rhesus monkeys, thereby providing a readily demonstrable endpoint in vaccine experiments. Some highly pathogenic SHIVs were initially isolated from rhesus monkeys following multiple animal-to-animal passages of nonpathogenic viruses (14, 17). We previously reported that highly pathogenic SHIVDH12R emerged during a single in vivo passage in a rhesus monkey treated with an anti-human CD8 monoclonal antibody at the time of its primary infection with nonpathogenic SHIVDH12 (11). Because the intrinsic genetic heterogeneity of available uncloned highly pathogenic SHIV stocks could be contributing to the inconsistent disease phenotypes observed in some vaccine experiments, a molecularly cloned SHIV, capable of replicating to high levels in vitro and in vivo and consistently inducing rapid and complete loss of CD4+ T cells in inoculated Indian rhesus monkeys, was constructed.
Cloning strategy.
Lambda phage vectors have previously been used to obtain full-length molecular clones of unintegrated HIV-1 DNA from productively infected cells (1, 6, 22). The identification of a restriction enzyme that cuts the viral DNA a single time, thereby permitting the cloning of one and two long terminal repeat circular DNA molecules produced in newly infected cells is an initial step in this process. Following transfer to plasmids, cloned circularly permuted DNA molecules can be readily converted to linear, correctly oriented forms of retroviral DNA (16, 22).
In preliminary experiments to generate molecular clones of highly pathogenic SHIVDH12R (11), DNA was prepared from rhesus monkey peripheral blood mononuclear cells (PBMC), infected with the SHIVDH12R-PS1 derivative (24), and subjected to digestion by several restriction enzymes. Southern blot hybridization analyses revealed that EcoRI converted the circular forms of viral DNA into linear molecules approximately 10 kbp in size, consistent with the presence of a single EcoRI site within unintegrated SHIVDH12R DNA (data not shown). Accordingly, Hirt-fractionated (9), SHIVDH12R-PS1-infected monkey PBMC DNA was digested with EcoRI and ligated to similarly cleaved lambda phage EMBL-4 DNA (Stratagene/Biocrest; Cedar Creek, Tex.) as previously described (22). Positive recombinant phage plaques, identified by using the 8.1-kbp HaeII-XhoI fragment (encompassing the gag through the env sequences) from SHIVDH12 (20) as a probe, were expanded, and the insert was transferred to pBR322. Two long terminal repeat linear forms of SHIV DNA were reconstituted from SalI-EcoRI and EcoRI-NarI subfragments of each clone as previously described (16, 22). Three of the full-length SHIVDH12R clones (SHIVDH12R-CL-7, SHIVDH12R-CL-8, and SHIVDH12R-CL-10) obtained generated progeny virions following transfection of HeLa cells, each of which exhibited robust and indistinguishable infection kinetics in rhesus monkey PBMC (data not shown). During these spreading infections, the three cloned SHIVs were highly cytopathic, inducing large syncytia similar to those observed with parental uncloned SHIVDH12R.
Pathogenicity of cloned SHIVs in rhesus monkeys.
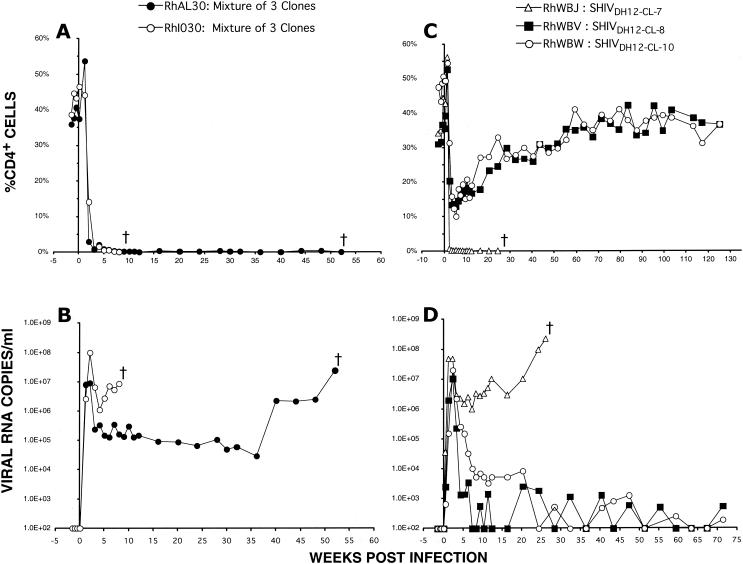
In the initial test of pathogenicity, 5 ml of HeLa cell supernatant from each of the three cloned SHIV transfections was subjected to ultracentrifugation (1.5 × 105 × g for 30 min) and the pelleted virions were resuspended in 500 μl of Dulbecco's minimal essential medium. Equal volumes (250 μl) of the suspended virus preparations were combined, and 300 μl of this mixture was inoculated intravenously into two rhesus monkeys. As shown in Fig. 1A and B, animals RhAL30 and RhI030 rapidly lost CD4+ T cells, reaching levels of less than 20 cells/μl of plasma by week 3, generated high levels of peak and postpeak plasma viremia, and were euthanized at weeks 8 and 57 because of intractable diarrhea and marked weight loss, respectively. This result indicated that one or more of the three SHIVDH12R molecular clones conferred the highly pathogenic phenotype. In a follow-up experiment, virus stocks of each clone were prepared in rhesus monkey PBMC as previously described (13) and high doses of each were inoculated individually into three different macaques. Figure 1C shows that only the recipient of SHIVDH12R-CL-7 (inoculum size, 3 × 104 50% tissue culture infective doses [TCID50]) experienced the complete depletion of CD4+ T lymphocytes (8 CD4+ T cells/μl of plasma at week 3) typically observed with parental uncloned SHIVDH12R (7, 10). The two monkeys inoculated with SHIVDH12R-CL-8 or SHIVDH12R-CL-10 (receiving 1.6 × 104 and 2.5 × 104 TCID50, respectively) had marked but transient declines (to 170 and 113 cells/μl of plasma at week 3, respectively) of their CD4+ T cells, which gradually returned to preinoculation levels (929 and 1,139 cells/μl of plasma at week 120, respectively). While all three virus-inoculated animals experienced high peak plasma virus loads (>107 RNA copies/ml) at weeks 2 to 3 postinfection, only monkey RhWBJ, which received SHIVDH12R-CL-7, continued to produce high and sustained postpeak levels of viral RNA (Fig. 1D). Animal RhWBJ was euthanized at week 26 with severe anorexia and marked weight loss.
FIG. 1.
One of three SHIVDH12R molecular clones induces rapid and complete depletion of CD4+ T cells in rhesus monkeys. Rhesus macaques RhAL30 and RhIO30 were inoculated intravenously (300 μl) with a mixture of undiluted supernatants from transfected HeLa cells, and levels of CD4+ T lymphocytes (A) and plasma viral RNA (B) were determined as previously described (7, 10, 11, 19). Animals RhWBJ, RhWBV, and RhWBW were individually inoculated with SHIVDH12R-CL-7 (3 × 104 TCID50), SHIVDH12R-CL-8 (1.6 × 104 TCID50), and SHIVDH12R-CL10 (2.5 × 104 TCID50), respectively. The resulting CD4+ T-cell levels (C) and plasma viremia (D) were determined.
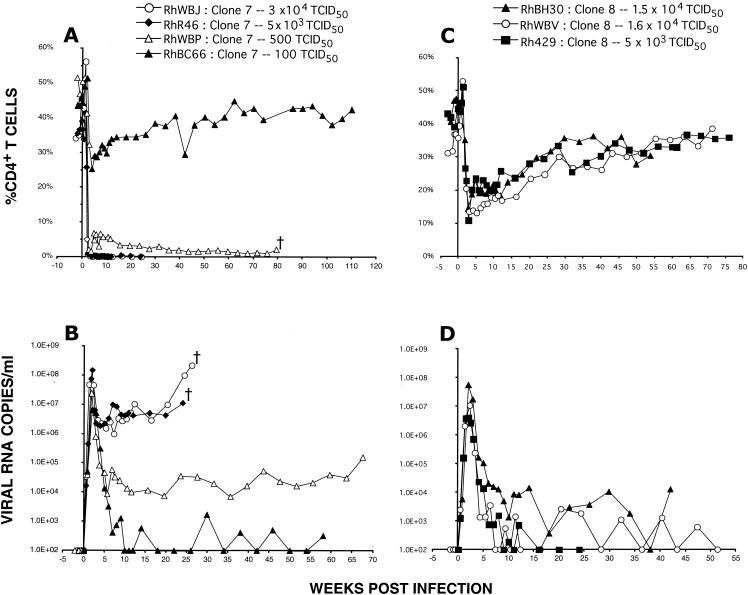
We previously reported that the rapid and irreversible loss of CD4+ T lymphocytes induced by uncloned SHIVDH12R was dose dependent (7, 12). This unusual phenotype could reflect the effect of a small subpopulation of highly pathogenic virions, present in the uncloned virus stock, which was solely responsible for the rapid and complete loss of CD4+ T cells in animals inoculated with larger amounts of virus (e.g., 625 TCID50 or greater [7]). To ascertain whether this dose-dependent phenotype was also an intrinsic property of molecularly cloned SHIVDH12R-CL-7, four monkeys were inoculated with decreasing amounts of SHIVDH12R-CL-7. As can be seen in Fig. 2A, the two macaques receiving 30,000 and 5,000 TCID50 of SHIVDH12R-CL-7 both sustained rapid declines of their CD4+ T cells and were euthanized at weeks 25 and 26 because of diarrhea and marked weight loss, a clinical outcome that was accompanied by high plasma virus loads (Fig. 2B). Three other macaques inoculated with 5,000 TCID50 also experienced complete depletion of CD4+ T lymphocytes within weeks of inoculation and were sacrificed between weeks 13 and 25 because of their deteriorating clinical condition (data not shown). The systemic depletion of CD4+ T lymphocytes by SHIVDH12R-CL-7 was similar histopathologically to that previously described for the uncloned parental SHIVDH12R virus stock (10). CD4+ T-cell loss from peripheral and internal (mesenteric and colonic) lymph nodes began by day 10 postinoculation and predominantly affected lymphocytes in paracortical regions; by day 21 postinfection, an 80 to 90% reduction of this T-cell subset was observed in multiple lymph node specimens. Virus-producing cells peaked on day 10 in the T-cell-rich regions of lymph nodes and on day 14 in the thymic medulla, coincident with the observed slightly delayed loss of CD4+ T cells from the thymus. Postmortem examination revealed diffuse lymphoid depletion, marked thymic atrophy, and evidence of cryptosporidiosis and disseminated candidiasis in several of the SHIVDH12R-CL-7-infected monkeys.
FIG. 2.
The rapid and complete loss of rhesus monkey CD4+ T cells induced by SHIVDH12R-CL-7 is inoculum size dependent. Monkeys RhWBJ, RhR46, RhWBP, and RhBC66 were inoculated intravenously with the indicated amounts of SHIVDH12R-CL-7, and the levels of CD4+ T lymphocytes (A) and plasma viral RNA (B) were determined. Monkeys RhBH30, RhWBV, and Rh429 were inoculated intravenously with the indicated amounts of SHIVDH12R-CL-8, and the levels of CD4+ T lymphocytes (C) and plasma viral RNA (D) were determined.
Rapid and severe loss of CD4+ T cells (51 cells/μl of plasma at week 3) also occurred in the animal (RhWBP) inoculated with 500 TCID50 of SHIVDH12R-CL-7 (Fig. 2A), but in this case, the depletion did not reach baseline levels (<1% of CD4+ T lymphocytes, <20 cells/mm3) until week 63 postinfection. Monkey WBP had to be euthanized at week 79 because of anorexia and intractable diarrhea. Finally, the inoculation of macaque RhBC66 with 100 TCID50 of SHIVDH12R-CL-7 resulted in only a modest and transient CD4+ T-cell decline (557 cells/μl of plasma at week 5), with a return to preinfection levels by week 40 (1,070 cells/μl of plasma) (Fig. 2A). This moderate CD4+ T-lymphocyte depletion was nonetheless associated with a high peak plasma virus load (6.3 × 106 viral RNA copies/ml) but low to undetectable postpeak viremia (Fig. 2B). Thus, as was the case for uncloned SHIVDH12R, the clinical outcome of SHIVDH12R-CL-7 infection was also dependent on inoculum size, indicating that this was an intrinsic property of both the uncloned and cloned viruses.
To be certain that the partial and transient depletion of CD4+ T lymphocytes induced by SHIVDH12R-CL-8 in monkey RHWBV (Fig. 1C) was an intrinsic property of this clone and did not represent an idiosyncratic animal-specific response to virus, two additional animals were inoculated with high doses of SHIVDH12R-CL-8. In both cases, marked but incomplete loss of CD4+ T cells occurred during the first weeks of infection (to 270 cells/μl [RhBH30] and 170 cells/μl [Rh429] at week 3 [Fig. 2C]); this depletion was associated with prompt control of plasma viremia (Fig. 2D). The patterns of CD4+ T-lymphocyte depletion and restoration in the three SHIVDH12R-CL-8-infected monkeys were similar to one another and clearly distinguishable from that observed with the animals inoculated with SHIVDH12R-CL-7. It is also worth noting that SHIVDH12R-CL-7 and SHIVDH12R-CL-8 exhibited indistinguishable infection kinetics and cytopathicity following infection of cultured PBMC (data not shown).
Determinants of SHIV pathogenicity.
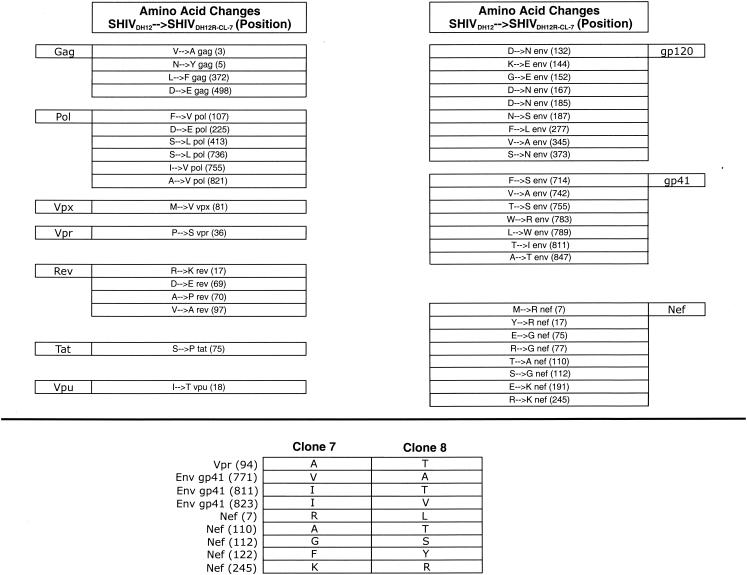
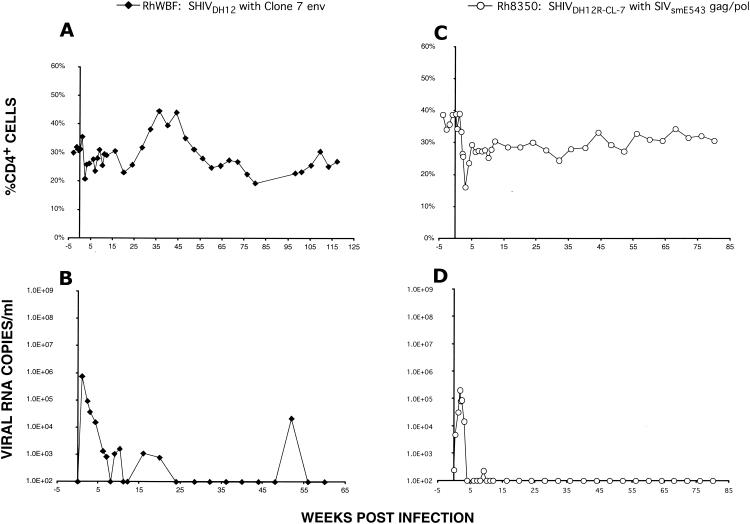
Complete nucleotide sequencing of SHIVDH12R-CL-7 revealed that 42 amino acid changes, relative to the starting sequence of nonpathogenic SHIVDH12 (previously designated SHIVMD14YE [20]), had accompanied the acquisition of the highly pathogenic phenotype (Fig. 3, top). Although these changes were distributed throughout the viral genome, they primarily affected the env and nef genes. On the basis of increased chemokine receptor binding affinity, membrane fusion capacity, and/or neutralization resistance, earlier analyses of the molecularly cloned SHIVKB9 derivative of SHIV89.6P (15) and the molecularly cloned SHIVHXBc2P-3.2 derivative of SHIVKU-1 (4) concluded that changes in the HIV-1 envelope glycoprotein component of highly pathogenic SHIVs were the primary determinant for inducing the rapid loss of CD4+ T lymphocytes in vivo. To ascertain whether the 17 amino acid substitutions in SHIVDH12R-CL-7 Env, by themselves, could confer the rapid and irreversible CD4+ T-cell-depleting phenotype, the 2.7-kbp HindIII fragment (positions 6333 to 9079) encoding the entire env gene of SHIVDH12R-CL-7 was transferred into the background of original nonpathogenic strain SHIVDH12. A stock of the resultant chimera (SHIVDH12[gp160-CL-7]) was prepared in rhesus monkey PBMC and used to inoculate macaque RhWBF with 75,000 TCID50. As shown in Fig. 4A, SHIVDH12[gp160-CL-7] did not induce the signature rapid loss of CD4+ T lymphocytes, replicating to only modest levels (peak plasma viremia of 7.5 × 105 RNA copies/ml) during the initial weeks of infection (Fig. 4B). The inability of the SHIVDH12R-CL-7 env gene to confer the unique pathogenic phenotype was also consistent with nucleotide sequence analyses of the pathogenic SHIVDH12R-CL-7 and nonpathogenic SHIVDH12R-CL-8 genomes, which indicated that both viruses have identical gp120s at both the nucleotide and amino acid levels (Fig. 3, bottom). More importantly, the comparison of the two viruses revealed that the distinctive CD4+ T-lymphocyte-depleting properties of SHIVDH12R-CL-7 versus SHIVDH12R-CL-8 are due to sequences encoding Nef, gp41, and Vpr; no changes affecting cis-acting elements such as the Rev responsive element, transcriptional regulatory sequences, or packaging signals were noted.
FIG. 3.
Amino acid changes associated with the acquisition of a highly pathogenic phenotype. The 42 amino acid differences between nonpathogenic parental SHIVDH12 and highly pathogenic SHIVDH12R-CL-7 (top) and between nonpathogenic SHIVDH12R-CL-8 and highly pathogenic SHIVDH12R-CL-7 (bottom) are shown. Note that some of the differences between SHIVDH12R-CL-8 and SHIVDH12R-CL-7 are not listed in the top panel because they represent amino acid substitutions present in SHIVDH12R-CL-8 and not in SHIVDH12R-CL-7.
FIG. 4.
Altered gag, pol, and env sequences in SHIVDH12R-CL-7 are necessary but not sufficient to confer the highly pathogenic phenotype. Macaque RhWBF was inoculated intravenously with SHIVDH12[gp160-CL-7] (75,000 TCID50), in which only the env gene from SHIVDH12R-CL-7 was inserted into nonpathogenic SHIVDH12 (A and B). Animal Rh8350 was inoculated with SHIVDH12R-E543(GPV) (16,000 TCID50), in which the 5-kbp NarI-BstBI fragment bearing gag, pol, and vif sequences from SIVsmE543 was introduced into SHIVDH12R-CL-7 (C and D). Plasma viremia and CD4+ T-cell levels were determined as indicated.
The amino acid comparison of SHIVDH12R-CL-7 with its nonpathogenic parent, SHIVDH12, shown in Fig. 3 (top) indicates that the acquisition of a more aggressive pathogenic phenotype was accompanied by 10 amino acid substitutions mapping to the SIVmac239 gag and pol genes present in SHIVDH12R-CL-7. The contribution of these SIV changes was evaluated by substituting the 5,041-bp NarI-BstBI fragment, carrying gag, pol, and vif sequences, from pathogenic SIVsmE543 (8) for analogous SIVmac239 sequences in SHIVDH12R-CL-7. The molecular clone, designated SHIVDH12R-E543(GPV), was transfected into HeLa cells, and the culture supernatant was used to prepare a virus stock in rhesus monkey PBMC. Despite receiving 16,000 TCID50 of SHIVDH12R-E543(GPV) intravenously, monkey Rh8350 experienced only a modest loss of CD4+ T cells (248 cells/μl of plasma at week 2) and a relatively low peak plasma viremia (5 × 105 viral RNA copies/ml) (Fig. 4C and D). This attenuated in vivo response very likely reflects differences between the gag and pol genes of SIVsmE543 and SIVmac239, as well as differences between SIVsmE543 and the 10 amino acid changes introduced into the gag and pol sequences attending the evolution of SHIVDH12R-CL-7 from its nonpathogenic SHIVDH12 parent. Taken together, these results indicate that changes affecting gag-pol and env are necessary, but insufficient by themselves, to confer the prototypically rapid and irreversible disease phenotype exhibited by SHIVDH12R-CL-7.
SHIVDH12R-CL-7 uses CXCR4 for entry into rhesus monkey PBMC.
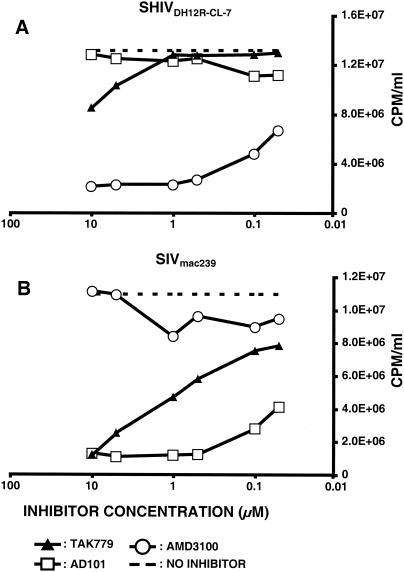
The env gene of nonpathogenic SHIVDH12 was derived from HIV-1DH12 (19), previously reported to use CXCR4 and CCR5 for entry into the human glioblastoma cell line U87MG expressing CD4 (27). To assess the chemokine receptor used by SHIVDH12R-CL-7 for replication in rhesus monkey PBMC, cells were infected in the presence of small-molecule coreceptor-targeted inhibitors specific for CCR5 or CXCR4. The production of progeny virus was measured as reverse transcriptase activity released into the medium on day 5 postinfection. As shown in Fig. 5A, infection of monkey PBMC by SHIVDH12R-CL-7 was blocked by the CXCR4 inhibitor AMD3100 and not by two CCR5 inhibitors (TAK-779 and AD-101). The opposite result was obtained with SIVmac239, which was blocked by the two CCR5 inhibitors and not by AMD3100 (Fig. 5B). The latter result is in agreement with a previous report showing that CCR5 is the coreceptor used by SIVmac239 for infection of macaque PBMC (26). SHIVDH12R-CL-7 also appears to be exclusively T cell tropic since it was unable to establish spreading infections in alveolar macrophage (13).
FIG. 5.
Coreceptor usage of SHIVDH12R-CL-7 and SIVmac239 for entry into macaque PBMC. SHIVDH12R-CL-7 and SIVmac239 were spinoculated onto rhesus PBMC in the presence of the indicated small-molecule coreceptor inhibitors. Rhesus monkey PBMC (5 × 104 cells) were dispensed into 96-well round-bottom plates. The inhibitor concentrations used were 0.05, 0.1, 0.5, 1.0, 5.0, and 10 μM. Reverse transcriptase activity released into the medium on day 5 postinfection was determined in the absence (dashed line) or presence of inhibitor.
Conclusions.
A molecular clone, designated SHIVDH12R-CL-7, has been obtained that consistently causes rapid, complete, and irreversible loss of CD4+ T lymphocytes. This disease phenotype has been previously reported for three independently derived uncloned SHIVs (11, 14, 17) and is applicable to only one of the three full-length infectious SHIVDH12R clones obtained in this study. The acquisition of these unusual pathogenic characteristics appears to be multigenic: 42 amino acid substitutions, relative to the starting nonpathogenic SHIVDH12 clone (20), distributed among several viral genes, were present in SHIVDH12R-CL-7. In contrast to studies of the envelope glycoproteins associated with molecularly cloned SHIVKB9 and SHIVHXBc2P-3.2, which were reported to be the principal determinants inducing CD4+ T-lymphocyte depletion (4, 15), the entire env gene of SHIVDH12R-CL-7, by itself, failed to confer the rapid and irreversible CD4+ T-cell-depleting properties following its insertion into the genome of nonpathogenic parental strain SHIVDH12. Similarly, substitution of gag-pol sequences from pathogenic SIVE543 could not replace analogous SIVmac239 genes in SHIVDH12R-CL-7. This result implies that the 10 amino acid substitutions introduced into the SIVmac239 gag and pol genes (Fig. 3) also contributed to the rapid CD4+ T-lymphocyte-depleting phenotype. In both cases, peak levels of plasma viremia were reduced compared to those caused by SHIVDH12R-CL-7 and the capacity of each virus to induce CD4+ T-cell depletion was minimal (Fig. 4). In the studies described here, our working definition of SHIV pathogenicity was rapid, irreversible, and complete elimination of CD4+ T lymphocytes coupled with high and sustained levels of viral RNA in plasma. SHIVDH12R-CL-7 was the only cloned SHIV that fulfilled these criteria.
The mechanism(s) underlying the rapid elimination of CD4+ T lymphocytes and induction of immunodeficiency by pathogenic SHIVs remains unknown, although the increased fusogenicity of the envelope glycoprotein and the infection of a substantial fraction of CD4+ T cells in lymphoid tissue during the first weeks postinoculation have been proposed to explain the unusual disease phenotype (4, 10, 15). Because the fraction of CD4+ T cells in blood and lymphoid tissues expressing CXCR4 is very large (>80%) (23, 25) (Y. Nishimura and M. Martin, unpublished data), complete and systemic elimination of this T-lymphocyte subset by X4-tropic SHIVDH12R-CL-7 could simply reflect the targeting and unrelenting depletion of these cells. It is also possible that a threshold level of systemic virus production must be reached during the first week of infection to cause the overwhelming and systemic killing of virtually all CD4+ T-lymphocyte subsets in lymphoid tissues. A delay of only a few days in exceeding such a threshold, such as that due to infections initiated with low-dose virus inocula, may permit the development of immune responses capable of controlling virus replication. However, monkeys inoculated with large amounts of SHIVDH12R-CL-8 generated peak levels of plasma viremia that were similar to those seen in animals infected with SHIVDH12R-CL-7 (Fig. 2B and D), yet the CD4+ T-cell depletion in SHIVDH12R-CL-8-inoculated macaques was incomplete and transient (compare Fig. 2A and C). Assuming that the concentration of virus particles circulating in the blood is a reflection of SHIV replication systemically, the level of progeny virion production, per se, during the acute infection cannot be the principal determinant causing the unrelenting and complete elimination of CD4+ T lymphocytes. Since SHIVDH12R-CL-7 and SHIVDH12R-CL-8 differ by only nine amino acids, it is likely that one or more of these residues contribute to the signature SHIV disease. Chimeric SHIVs containing individual amino acid substitutions or combinations of these amino acid substitutions are currently being constructed for inoculation into rhesus monkeys to ascertain which viral gene(s) confers the rapid CD4+ T-cell-depleting phenotype.
Acknowledgments
We thank Frances Banks, Russ Byrum, Sekou Savane, and Wes Thornton for devoted animal care, Carol Clarke and Dusty Rhodes for help in veterinary procedures, and Randy Elkins for assistance in procurement and maintenance of animals.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 4.Cayabyab, M., G. B. Karlsson, B. A. Etemad-Moghadam, W. Hofmann, T. Steenbeke, M. Halloran, J. W. Fanton, M. K. Axthelm, N. L. Letvin, and J. G. Sodroski. 1999. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 73:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, M. W., Y. B. Kim, M. K. Lee, K. C. Gupta, W. Ross, R. Plishka, A. Buckler-White, T. Igarashi, T. Theodore, R. Byrum, C. Kemp, D. C. Montefiori, and M. A. Martin. 2001. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J. Virol. 75:2224-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66:7517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo, Y., T. Igarashi, Y. Nishimura, C. Buckler, A. Buckler-White, R. Plishka, D. S. Dimitrov, and M. A. Martin. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 74:6935-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch, V., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71:1608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi, T., C. R. Brown, R. A. Byrum, Y. Nishimura, Y. Endo, R. J. Plishka, C. Buckler, A. Buckler-White, G. Miller, V. M. Hirsch, and M. A. Martin. 2002. Rapid and irreversible CD4+ T-cell depletion induced by the highly pathogenic simian/human immunodeficiency virus SHIVDH12R is systemic and synchronous. J. Virol. 76:379-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi, T., Y. Endo, G. Englund, R. Sadjadpour, T. Matano, C. Buckler, A. Buckler-White, R. Plishka, T. Theodore, R. Shibata, and M. Martin. 1999. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl. Acad. Sci. USA 96:14049-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi, T., Y. Endo, Y. Nishimura, C. Buckler, R. Sadjadpour, O. K. Donau, M.-J. Dumaurier, R. J. Plishka, A. Buckler-White, and M. A. Martin. 2003. Early control of highly pathogenic simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys usually results in long-lasting asymptomatic clinical outcomes. J. Virol. 77:10829-10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imamichi, H., T. Igarashi, T. Imamichi, O. K. Donau, Y. Endo, Y. Nishimura, R. L. Willey, A. F. Suffredini, H. C. Lane, and M. A. Martin. 2002. Amino acid deletions are introduced into the V2 region of gp120 during independent pathogenic simian immunodeficiency virus/HIV chimeric virus (SHIV) infections of rhesus monkeys generating variants that are macrophage tropic. Proc. Natl. Acad. Sci. USA 99:13813-13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joag, S. V., Z. Li, L. Foresman, E. B. Stephens, L.-J. Zhao, I. Adany, D. M. Pinson, H. M. McClure, and O. Narayan. 1996. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J. Virol. 70:3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson, G. B., M. Halloran, D. Schenten, J. Lee, P. Racz, K. Tenner-Racz, J. Manola, R. Gelman, B. Etemad-Moghadam, E. Desjardins, R. Wyatt, N. P. Gerard, L. Marcon, D. Margolin, J. Fanton, M. K. Axthelm, N. L. Letvin, and J. Sodroski. 1998. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J. Exp. Med. 188:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peden, K., M. Emerman, and L. Montagnier. 1991. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology 185:661-672. [DOI] [PubMed] [Google Scholar]
- 17.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 19.Shibata, R., M. D. Hoggan, C. Broscius, G. Englund, T. S. Theodore, A. Buckler-White, L. O. Arthur, Z. Israel, A. Schultz, H. C. Lane, and M. A. Martin. 1995. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J. Virol. 69:4453-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 21.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M.-E. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 22.Theodore, T. S., G. Englund, A. Buckler-White, C. E. Buckler, M. A. Martin, and K. W. C. Peden. 1996. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res. Hum. Retrovir. 12:191-194. [DOI] [PubMed] [Google Scholar]
- 23.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willey, R. L., R. Byrum, M. Piatak, Y. B. Kim, M. W. Cho, J. L. Rossio, Jr., J. Bess, Jr., T. Igarashi, Y. Endo, L. O. Arthur, J. D. Lifson, and M. A. Martin. 2003. Control of viremia and prevention of simian-human immunodeficiency virus-induced disease in rhesus macaques immunized with recombinant vaccinia viruses plus inactivated simian immunodeficiency virus and human immunodeficiency virus type 1 particles. J. Virol. 77:1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaitseva, M. B., S. Lee, R. L. Rabin, H. L. Tiffany, J. M. Farber, K. W. C. Peden, P. M. Murphy, and H. Golding. 1998. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J. Immunol. 161:3103-3113. [PubMed] [Google Scholar]
- 26.Zhang, Y.-J., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, Y.-J., T. Dragic, Y. Cao, L. Kostrikis, D. S. Kwon, D. R. Littman, V. N. KewalRamani, and J. P. Moore. 1998. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J. Virol. 72:9337-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]