Abstract
Large polyanionic molecules, such as sulfated polysaccharides (including soluble heparin and dextran sulfate), synthetic polyanionic polymers, and negatively charged proteins, have been shown to broadly inhibit several enveloped viruses. We recently reported the antiviral activity of a peptide derived from amino acids 77 to 95 of a potential binding partner of respiratory syncytial virus F protein (RSV F), the GTPase RhoA. A subsequent study with a truncated peptide (amino acids 80 to 94) revealed that optimal antiviral activity required dimerization via intermolecular disulfide bonds. We report here that the net negative charge of this peptide is also a determining factor for its antiviral activity and that it, like other polyanions, inhibits virus attachment. In a flow cytometry-based binding assay, peptide 80-94, heparin, and dextran sulfate inhibited the attachment of virus to cells at 4°C at the same effective concentrations at which they prevent viral infectivity. Interestingly, time-of-addition experiments revealed that peptide 80-94 and soluble heparin were also able to inhibit the infectivity of a virus that had been prebound to cells at 4°C, as had previously been shown for dextran sulfate, suggesting a potential role for postattachment effects of polyanions on RSV entry. Neutralization experiments with recombinant viruses showed that the antiviral activities of peptide 80-94 and dextran sulfate were diminished in the absence of the RSV attachment glycoprotein (G). Taken together, these data indicate that the antiviral activity of RhoA-derived peptides is functionally similar to that of other polyanions, is dependent on RSV G, and does not specifically relate to a protein-protein interaction between F and RhoA.
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory illness in infants worldwide and is increasingly recognized as a significant cause of morbidity and mortality in adult populations, especially among the institutionalized elderly and the immunocompromised (8). Because there is currently no licensed vaccine for RSV, there is substantial interest in the identification and development of RSV-specific antiviral agents (41, 46, 48). Among currently described inhibitors of RSV are many that appear to target the process of viral entry (1, 12, 13, 26, 28, 42, 47).
RSV enters uninfected cells by fusion of its host-cell-derived lipid envelope with the plasma membrane of the target cell. This process is mediated by the RSV F glycoprotein (53), a type 1 viral fusion protein (9). The two additional viral surface glycoproteins, G and SH, appear to play a role in augmenting F-mediated fusion (24) and are important for viral viability in vivo, but they have been shown to be dispensable for the replication of RSV in cultured cells (6, 10, 27, 50). Although G was originally identified as the attachment protein for RSV (31), the viability of viruses lacking G indicates that F alone can mediate both attachment and fusion. The role of SH in RSV entry is unclear.
The binding of RSV to host cells is facilitated by the presence of cellular glycosaminoglycans (GAGs), particularly heparan sulfate or other iduronic-acid-containing sugar chains (21, 29, 34). Feldman et al. showed that both G and F can bind to immobilized heparin and that soluble GAGs can inhibit the binding and replication of both wild-type RSV and the cold-passaged isolate cp-52, which lacks the G and SH glycoproteins (16). A recombinant virus expressing only F on its envelope also remains GAG sensitive (51), although this effect may be modulated by the cellular substrate (52). Whether there are host cell proteins on the surfaces of virions that may also contribute to viral attachment has not been reported, although it has been shown that the presence of heparin-like molecules on the virus surface is important for viral entry (4). Given the importance of sulfated GAGs for RSV entry, it is not surprising that many natural and synthetic polyanions, which are capable of competing with the binding of RSV to these sulfated moieties, have been shown to inhibit RSV infection (25, 32).
Members of our laboratory previously described the antiviral activity of a 19-mer peptide derived from the small intracellular GTPase RhoA. This peptide was effective at reducing the replication of RSV in vitro and in vivo in a mouse model of RSV disease (44). Based on truncation studies, it was shown that a slightly shorter peptide comprising amino acids (aa) 80 to 94 of RhoA was optimal for anti-RSV activity. Interestingly, the antiviral activity of this peptide was dependent upon the oxidation of an internal cysteine residue, resulting in the formation of peptide dimers (5). This dependence on an increased molecular weight, coupled with the anionic nature of the optimal peptide sequence, suggested that the antiviral activities of RhoA-derived peptides are unrelated to a specific in vivo F-RhoA interaction, but rather are a reflection of their similarities to other antiviral polyanions.
The purpose of the present study was to determine the mechanism of action of the RhoA-derived peptide 80-94. We report that the inhibition of RSV by this peptide is largely due to a disruption of viral attachment, that this inhibition is dependent on both the net charge and multimerization of the peptide, and that to be potently inhibited by peptide 80-94, viruses must express the G glycoprotein, suggesting that RSV G is a primary target of peptide binding.
MATERIALS AND METHODS
Viruses and cells.
HEp-2 cells were propagated in minimum essential medium supplemented with glutamine, amphotericin B (Fungizone), gentamicin, and 10% fetal bovine serum (MEM 10). The A2 strain of RSV was kindly provided by Robert Channock. Recombinant green fluorescent protein-expressing viruses were a gift of Mark Peeples and have been described in detail elsewhere (50). The nomenclature used for these viruses is based on the surface glycoproteins they express: rgRSV-F expresses only the F glycoprotein, rgRSV-GF expresses G and F, and rgRSV-SGF expresses S, G, and F. All viruses were propagated in HEp-2 cells, and working stocks of virus were prepared as previously described (19).
Synthetic peptides and soluble GAGs.
Peptides 80-94 and 83A were synthesized by 9-fluorenylmethoxy carbonyl solid-phase chemistry (Chiron Technologies, San Diego, Calif.) by the FDA Facility for Biotechnology Resources, Bethesda, Md. Peptide 80-94-N was purchased from Biosynthesis Incorporated (Loveland, Tex.). Peptide 80-94 is a linear peptide corresponding to aa 80 to 94 of RhoA, with the sequence ILMCFSIDSPDSLEN. Peptide 83A contains the same sequence with a single substitution of an alanine residue for cysteine 83: ILMAFSIDSPDSLEN. Peptide 80-94-N, ILMCFSINSPNSLQN, contains a substitution of the corresponding amide for each acidic amino acid, resulting in a peptide with no net charge. All peptides were purified by high-performance liquid chromatography to ≥80% purity and were analyzed by mass spectrometry to verify the correct molecular mass. Peptides 80-94 and 83A were dissolved in sterile-filtered ammonium bicarbonate buffer (1% [wt/vol]), pH 8.0, containing 20% dimethyl sulfoxide (DMSO) to promote the oxidation of the cysteine residue of 80-94. Peptide 80-94-N was generally dissolved in 100% DMSO. Heparin from porcine intestinal mucosa (Sigma, St. Louis, Mo.) and dextran sulfate, of approximately 8,000 kDa (Sigma), were dissolved to 10 mg/ml in sterile phosphate-buffered saline (PBS) and stored in aliquots at −20°C.
Oxidation of peptides.
Lyophilized peptides were reconstituted in 1% ammonium bicarbonate buffer containing 20% DMSO and allowed to incubate at room temperature overnight. Peptide samples were tested for free SH content before and after room temperature incubation by the use of Ellman's reagent (Pierce Biotechnology, Rockford, Ill.) according to the manufacturer's protocol, adapted to a microwell format. Briefly, cysteine standards and peptide stocks were diluted 1:50 into 200 μl of freshly prepared reaction buffer (0.1 M sodium phosphate [pH 8.0], 1 mM EDTA) containing freshly dissolved Ellman's reagent (1 mM) in 96-well microtiter plates. Samples were mixed for ∼30 s and then incubated for 15 min at room temperature. The absorbance at 405 nm was then measured with an MRX microplate reader (Dynex Technologies, Chantilly, Va.), and free SH values were calculated based on a standard curve in the range of 0.078 to 10 mM. Both peptides 80-94 and 80-94-N were oxidized to undetectable levels (≤5% of preincubation values) after an overnight incubation.
Assessment of antiviral activity by antigen reduction assay.
Peptides, heparin, or dextran sulfate was diluted by serial fourfold dilutions in MEM 10 to give a range of final test concentrations from 0.1 to 100 μg/ml. An equal volume of MEM 10 containing RSV was then added to the diluted peptides. Unless otherwise indicated, viruses were diluted to give a final concentration of approximately 500 PFU/well. Ninety microliters of virus-peptide mixture was added in triplicate to HEp-2 cells in 96-well plates that had been seeded the previous day at 1.5 × 104 to 2.0 × 104 cells per well. At 2 to 3 days postinfection, infected and mock-infected cells were fixed in methanol and the extent of viral replication was determined by enzyme-linked immunosorbent assay (ELISA) as described below.
ELISA.
Virus replication was measured by detecting the amount of F protein expressed in RSV-infected cells. Briefly, fixed cells were washed in blocking buffer (PBS containing 3% nonfat dry milk) and then incubated for approximately 1 h at room temperature with a mix of anti-F monoclonal antibodies 1200, 1269, and 1243 (2, 40) diluted 1:1,000 in blocking buffer. Cells were then washed three times with PBS-Tween 20 (0.5%), followed by incubation with an anti-mouse horseradish peroxidase-conjugated secondary antibody (Sigma) similarly diluted 1:1,000 in blocking buffer. After three additional washes in PBS-Tween 20, 100 μl of ABTS substrate solution (KPL, Gaithersburg, Md.) was added and allowed to develop until the optical density values of infected control wells with no inhibitor reached approximately 1.0 absorbance unit above that of mock-infected blanks. The absorbance of the wells at 405 nm was read with an MRX microplate reader (Dynex Technologies). To allow for the comparison of multiple experiments, we normalized all data points in each experiment to the level of the internal controls (virus with no inhibitor), and they are represented in figures as fractions of the control value.
Immunofluorescence attachment assay.
HEp-2 cells in 96-well tissue culture dishes (Costar) were chilled for approximately 1 h at 4°C. The medium was then removed and serial dilutions of RSV in ice-cold MEM 10 were added and allowed to bind for 30 min at 4°C. After this incubation, the cells were washed three times in cold PBS to remove unbound virus and were fixed in methanol. Bound virus was then detected by indirect immunofluorescence. After a brief incubation in blocking buffer (PBS with 3% nonfat dry milk), cells were incubated with a 1:500 dilution of primary antibody (the pool of anti-F monoclonal antibodies described above or anti-G monoclonal antibody MAB 858-2 [Chemicon, Temecula, Calif.]) or an isotype control monoclonal antibody for 1 h at room temperature. Cells were then washed three times in PBS-Tween and incubated with a 1:500 dilution of an Alexa Fluor 488-labeled anti-mouse secondary antibody (Molecular Probes, Eugene, Oreg.). After a final series of washes in PBS-Tween, 100 to 200 μl of PBS was added to each well and images of each well were captured with a Zeiss Axiovert 200 M fluorescence microscope (Carl Zeiss Microimaging, Thornwood, N.Y.) equipped with an ORCA-ER digital camera (Hamamatsu, Bridgewater, N.J.). After acquisition, all images were uniformly adjusted for viewing with Zeiss Axiovision software and Adobe Photoshop.
Inhibition of attachment assessed by immunofluorescence.
RSV at 4 × 106 PFU/ml was premixed with 25 μg of heparin, dextran sulfate, or peptide/ml in ice-cold MEM 10, added to HEp-2 cells at 4°C, bound, and detected as described above.
Flow-based attachment assay.
HEp-2 cells were dissociated from tissue culture flasks by use of a nonenzymatic cell dissociation solution (Cellstripper; Mediatech Inc., Herndon, Va.) and were resuspended in cold MEM 10. After 10 to 15 min on ice, cells were pelleted by centrifugation for 5 min at 800 × g and then resuspended to 106 cells/ml in MEM 10 containing RSV A2 at 1.6 × 105 to 4 × 107 PFU/ml. After being incubated on ice for 30 min to allow binding, cells were pelleted and washed in cold PBS to remove unbound virus. After being washed, cells were resuspended in cold PBS with a 1:1,000 dilution of anti-F, anti-G, or control monoclonal antibodies plus 2 μg of propidium iodide (PI)/ml and were incubated for 15 min on ice, followed by a single wash in PBS and a second 15-min incubation with a 1:1,000 dilution of anti-mouse Alexa Fluor 488 secondary antibody. Cells were then washed and fixed in 1% paraformaldehyde in PBS and evaluated by flow cytometry with a FACSCalibur instrument (Becton Dickinson, San Jose, Calif.). Dead cells were excluded from analysis based on PI staining. Data were analyzed with FlowJo, version 4.2 (Tree Star, San Carlos, Calif.).
Inhibition of attachment assessed by flow cytometry.
HEp-2 cells were dissociated and resuspended in cold MEM 10 as described above. Peptides, heparin, or dextran sulfate was serially diluted in ice-cold MEM 10 to give a range of concentrations and was premixed with RSV A2 at 107 PFU/ml. Cells were pelleted, resuspended in premixed virus plus inhibitor, and incubated for 30 min on ice. After the incubation, cells were washed three times in PBS and bound virus was detected by flow cytometry using a pool of anti-F monoclonal antibodies as described above.
Calculation of EC50 values.
Data from the antigen reduction assay and the flow-based binding assay were used to calculate 50% effective concentration (EC50) values by use of nonlinear curve fit analysis software (SigmaPlot 2001; SPSS Inc., Chicago, Ill.); EC50 values represent the amount of inhibitor calculated to give a 50% reduction in viral infectivity or binding. Briefly, EC50 values were individually calculated for each of three or more individual experiments by nonlinear regression analysis based on the best possible curve fit for each data set (curve fits were compared based on r2 values and were generally in the range of 0.950 to 0.999). The geometric mean of the individual EC50 values was then calculated.
Assessment of postattachment inhibition of viral infectivity.
HEp-2 cells in 96-well plates were chilled to 4°C. RSV (103 PFU/well) was then added and allowed to bind at 4°C for 30 min. After binding, cells were washed three times in ice-cold PBS to remove any unbound inoculum. Fresh medium was then added at 4°C and plates were transferred to a 37°C incubator for warming. A peptide or soluble heparin, at a final concentration of 25 μg/ml in MEM 10, was added or removed at various time points as follows. For inhibitor pretreatment of cells, cells were treated for 30 min at 4°C with the inhibitor, which was then washed out before the virus was added. For binding inhibition only, the inhibitor and virus were added to the cells concurrently and washed out after a 30-min binding period. For postattachment inhibition, inhibitors were added after the inoculum was removed, but prior to the transfer to 37°C. For inhibition throughout, the inhibitor and virus were added concurrently and neither was subsequently removed. For postwarming inhibition, the inhibitor was added 2 h after the transfer of cell monolayers to 37°C. Each sample group was compared in quadruplicate to similarly treated control wells containing no inhibitor.
RESULTS
Antiviral activity of RhoA-derived peptides is dependent upon polyanionic charge.
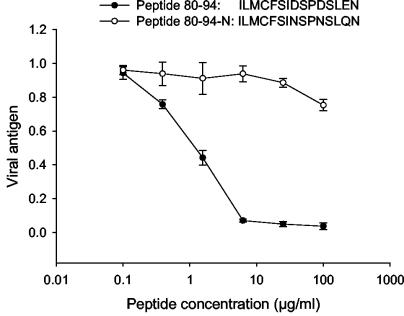
We observed previously that the antiviral activity of RhoA-derived peptide 80-94 was dependent on the formation of dimers and/or aggregates. This dependence on molecular weight, coupled with the net negative charge of the molecule, suggested that the peptide might function similarly to other polyanions with antiviral activities. Peptide 80-94 has a net charge of −3, caused by three internal acidic amino acids, namely Asp87, Asp90, and Glu93. To assess the importance of the negative charges on the peptide function, we replaced each of the acidic amino acids with its corresponding amide, as shown in Fig. 1. The resulting peptide, termed 80-94-N, was >100-fold less active at reducing RSV replication in cell culture than peptide 80-94 when it was similarly oxidized. These data indicate that the net negative charge of the RhoA-derived peptide is an important determinant of its antiviral potency. In agreement with this, we have also observed that the addition of positively charged lysine residues to RhoA-derived peptides from the aa 77 to 95 region abolishes their antiviral activity. This observation was made when lysine residues were added to several previously tested peptide 77-95 derivatives (5) in an effort to simplify their elution from a reverse-phase high-performance liquid chromatography column. In each instance, the addition of three C-terminal lysines resulted in a loss of antiviral activity compared to the corresponding peptide without the C-terminal lysines (data not shown).
FIG. 1.
Antiviral activity of peptide 80-94 is dependent upon net negative charge. Peptides 80-94 and 80-94-N were oxidized by incubation in 20% DMSO and then were tested for antiviral activity against RSV. The virus was premixed with an inhibitor and added to HEp-2 cells. At 2 days postinfection, cells were fixed and the extent of viral replication was assessed by ELISA. The curves shown are from separate experiments and are representative of three or more experiments with each peptide. Data points are the means of triplicate samples and error bars represent 1 standard deviation.
Peptide 80-94 inhibits RSV attachment.
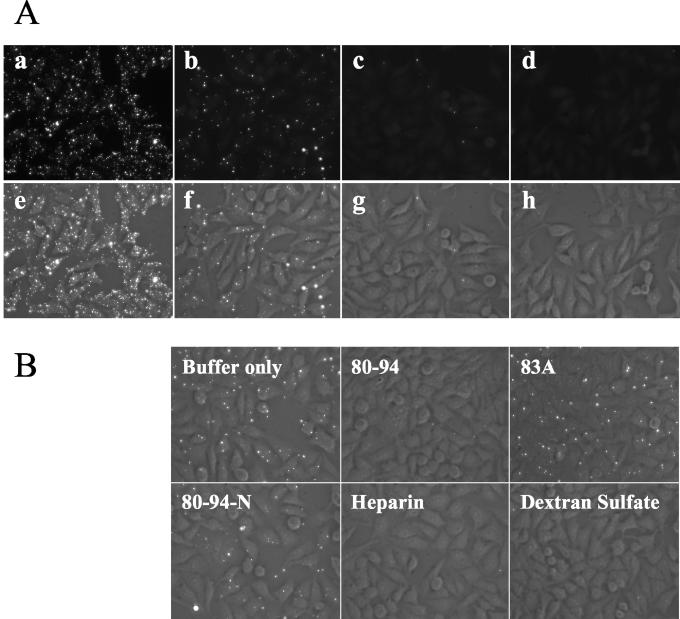
Since other polyanionic compounds have been shown to block the binding of RSV to host cells (25), we next examined whether the 80-94 peptide was able to interfere with the binding of virus to HEp-2 cells. To do this, we developed an immunofluorescence assay for virus attachment. The binding of RSV to cells occurs readily at 4°C but does not progress to fusion at temperatures below 18°C (49). To determine whether we could detect virus bound to the surfaces of host cells at 4°C, we incubated various amounts of RSV with HEp-2 cells at 4°C. After washing away of the unbound inoculum, the cells were fixed and stained for surface-associated viral glycoproteins. As shown in Fig. 2A, staining for bound F resulted in a punctate pattern of fluorescence that directly correlated with the amount of input virus. The same pattern was observed when cells were stained for surface-bound G, while no detectable staining was observed with a control antibody (data not shown), indicating that the staining was specific for RSV.
FIG. 2.
Peptide 80-94 inhibits viral attachment. (A) Validation of immunofluorescence attachment assay. One hundred microliters of RSV in cold MEM 10 was added at 4 × 107 (a and e), 4 × 106 (b and f), or 4 × 105 (c and g) PFU/ml to HEp-2 cells in 96-well plates at 4°C. After an incubation for 30 min at 4°C to allow binding, virus-treated cells and mock-treated controls (d and h) were washed three times in cold PBS and then fixed in methanol. Bound virus was detected by indirect immunofluorescence with an anti-F monoclonal antibody, followed by an Alexa Fluor 488-labeled secondary antibody. Fluorescence and phase-contrast images of each sample were taken. The fluorescence images alone are shown in the top row, and the merged phase-contrast and fluorescence images are shown in the bottom row. (B) HEp2 cell monolayers in 96-well plates were incubated with 4 × 106 PFU of RSV/ml at 4°C in the presence of 25 μg of peptide, heparin, or dextran sulfate/ml, as indicated. After 30 min at 4°C, the cells were washed and bound virus was detected by immunofluorescence as described above.
We then examined the effects of peptide 80-94 on viral attachment. Peptide 80-94 efficiently blocked viral binding under these conditions, as did heparin and dextran sulfate, while peptide 80-94-N did not. Peptide 83A, which lacks the critical cysteine residue responsible for 80-94 dimerization (5), was also unable to inhibit viral attachment (Fig. 2B).
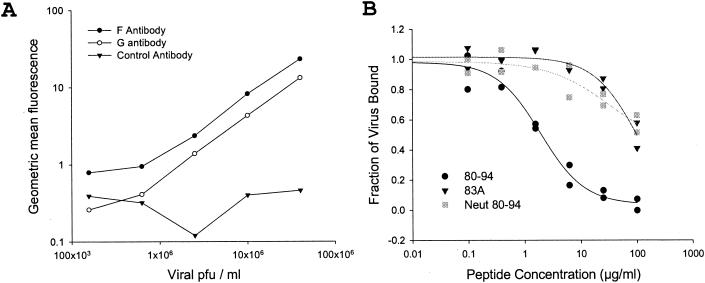
In order to more carefully quantitate the ability of the various inhibitors to block viral attachment, we adapted the immunofluorescence assay for flow cytometry. In agreement with a previous study (34), we observed that the binding of virus can be detected by flow cytometry and that the magnitude of binding is linear when cells are incubated with 2.5 × 105 to 4 × 107 PFU/ml (Fig. 3A). We tested the ability of peptides and sulfated polysaccharides to interfere with viral attachment by mixing 107 PFU of RSV/ml with a range of test concentrations of each inhibitor before allowing attachment at 4°C. This analysis revealed that peptide 80-94, soluble heparin, and dextran sulfate were able to efficiently interfere with the binding of virus to HEp-2 cells at 4°C, while peptides 83A and 80-94-N were not (Fig. 3B and Table 1). The amount of each compound necessary for a 50% inhibition of viral infectivity (EC50) correlated extremely well with the effective doses that inhibited the binding of virus in the flow cytometry assay (Table 1). Because the viral infectivity assay and the attachment assay are both dependent on the detection of viral antigen, we went on to verify that the polyanions used did not interfere with the binding of antibody to viral antigen. At the maximum concentration used for our assays, none of the compounds tested had a significant effect on the binding of the detecting antibody to RSV-infected cells (data not shown). Thus, the decrease in antigen detected in the presence of inhibitors was an accurate reflection of the amount of viral binding or replication and was not an artifact of the detection process. The strong correlation between the EC50 values for attachment and infectivity inhibition suggests that the ability of peptide 80-94 to inhibit viral attachment may be sufficient to account for its antiviral activity.
FIG. 3.
Quantitation of attachment inhibition by flow cytometry. (A) HEp-2 cells were incubated on ice with serial fourfold dilutions of RSV to allow viral attachment. After 30 min, cells were washed three times in cold PBS and bound virus was detected with anti-F (closed circles), anti-G (open circles), or control (closed triangles) monoclonal antibodies. (B) HEp-2 cells at 106/ml were incubated with 107 PFU of RSV/ml for 30 min on ice in the presence or absence of peptide 80-94 (circles), 83A (triangles), or 80-94-N (squares), as shown. Unbound virus was removed by washing in PBS, and bound virus was detected by flow cytometry with anti-F monoclonal antibodies. Geometric mean fluorescence values from two separate experiments with each peptide are shown with nonlinear curve fits.
TABLE 1.
Inhibition of RSV infectivity and binding by tested inhibitors
| Inhibitor | EC50 value (μg/ml) for inhibition ofa:
|
|
|---|---|---|
| Infectivity | Viral binding | |
| Peptide 80-94 | 1.23 (0.851-1.76) | 1.60 (1.05-2.13) |
| Peptide 80-94N | >100 | >100 |
| Peptide 83A | >100 | >100 |
| Dextran sulfate | 1.02 (0.620-1.76) | 0.642 (0.480-0.794) |
| Soluble heparin | 0.314 (0.179-0.517) | 0.430 (0.379-0.462) |
EC50 values are presented as the geometric means from three or more separate experiments followed by the ranges for those experiments in parentheses.
Postattachment inhibition by peptide 80-94 and heparin.
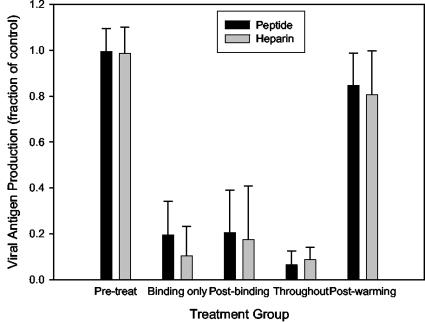
To confirm that the antiviral activity of the RhoA-derived peptide was due to an inhibition of viral binding, we tested its ability to decrease virus infectivity when present only at the time of viral attachment. The process of RSV entry can be separated into two distinct steps: (i) initial attachment, which occurs readily at 4°C, and (ii) a subsequent fusion event, which does not occur at temperatures below 18°C (49). In order to separate the effects of the peptide on the attachment of RSV from subsequent entry events, we tested the ability of the peptide to inhibit viral infection when present at different times during the entry process. As shown in Fig. 4, neither peptide nor heparin had any effect on viral infection when it was preincubated with cells in the absence of virus or added after the completion of viral entry (after warming to 37°C). As expected, both peptide 80-94 and soluble heparin were generally as effective at reducing viral infectivity when present only during the attachment of virus to cells at 4°C as when they were present throughout the assay. Interestingly, each inhibitor was also able to inhibit infection when it was added to cells after the attachment of virus at 4°C, but before warming to 37°C. This inhibition represents the neutralization of prebound virus, not of the residual inoculum, since the unbound virus had been removed by multiple washes in cold PBS prior to the addition of the inhibitor. These data indicate that heparin and peptide 80-94 are not only able to prevent the binding of unbound virions to host cells, but also to neutralize virions that have already attached. Previous studies have shown the same to be true for other polyanions, including dextran sulfate (25). Taken together, these data show that peptide 80-94 and other polyanions can interfere with viral entry when they are present both during and after the initial attachment of RSV.
FIG. 4.
Peptide inhibition of RSV occurs at the time of entry with similar functional properties to soluble heparin. RSV was added to prechilled HEp-2 cells and allowed to bind at 4°C for 30 min. Cells were then washed three times in ice-cold PBS to remove the unbound inoculum, replenished with MEM 10 at 4°C, and transferred to 37°C to allow viral entry. Peptide 80-94 or soluble heparin was added and/or removed at various time points. x axis labels indicate when peptide or heparin was present during the assay. Pretreat, cells were treated for 30 min at 4° with inhibitor and then washed before virus was added; binding only, the inhibitor and virus were added to the cells concurrently and inhibitors were washed out with the virus after a 30-min binding period; postbinding, inhibitors were added after the inoculum was removed but prior to the transfer to 37°C; throughout, the inhibitor and virus were added concurrently and neither was subsequently removed; postwarming, the inhibitor was added 2 h after transfer of the plate to 37°C.
G is required for efficient antiviral activity by peptide 80-94.
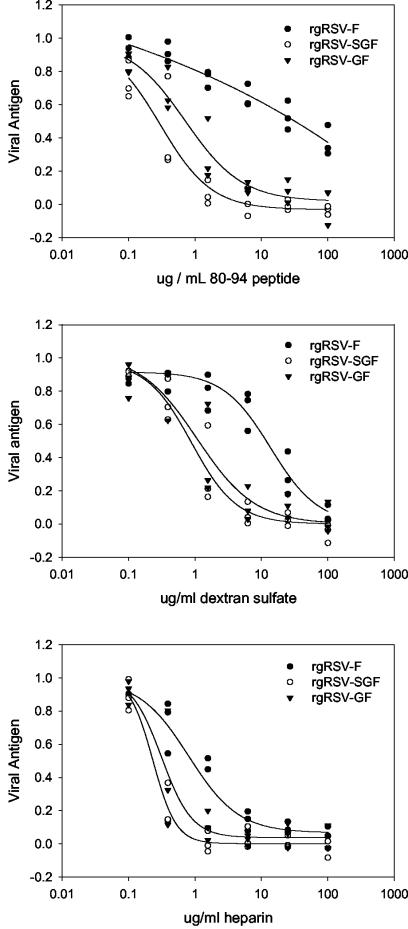
To determine the relative importance of F, G, and SH as targets of peptide 80-94 binding, we tested the ability of peptide 80-94 to inhibit the infectivity of recombinant viruses expressing F alone (rgRSV-F) or in combination with G (rgRSV-GF) or expressing G and SH (rgRSV-SGF). We compared the peptide inhibition to the inhibition seen with both heparin and dextran sulfate. For all three inhibitors, the presence of SH did not seem to be a significant factor in determining viral susceptibility, since the inhibition curve for each inhibitor against the rgRSV-SGF virus was similar to that against the rgRSV-GF virus (Fig. 5). G, on the other hand, was an important factor in the susceptibility of the virus to inhibition by peptide 80-94 and dextran sulfate, as the recombinant virus lacking G and SH (rgRSV-F) was much less susceptible to either of these inhibitors than the virus lacking only SH. The presence of G was not as critical for the inhibition by heparin, although rgRSV-F was somewhat less susceptible to the inhibitory effects of heparin than rgRSV-SF or rgRSV-SGF. This may relate to the fact that both RSV F and RSV G can mediate viral attachment to cell surface proteoglycans and that soluble heparin may more closely resemble a natural F ligand than dextran sulfate or peptide 80-94. Although peptide 80-94 did have some effect on rgRSV-F at high concentrations, it is clear that the efficient inhibition of RSV by peptide 80-94 requires the presence of the RSV G protein. This dependence on RSV G is consistent with the effect of the peptide on viral attachment, but it is somewhat unexpected in the context of the postattachment neutralization and the antifusion activity previously described for the RhoA-derived peptide 77-95 (44; also discussed below).
FIG. 5.
Inhibitory effects of peptide 80-94, dextran sulfate, and heparin on recombinant RSV. Recombinant RSV strains were tested for their susceptibility to inhibition by the 80-94 peptide, soluble heparin, or dextran sulfate. Briefly, the virus and inhibitors were premixed and added to HEp-2 cells. After 48 to 72 h at 37°C, the extent of viral replication was assessed based on the amount of viral antigen (F protein) produced. Virus rgRSV-SGF expresses all three surface glycoproteins, SH, G, and F. rgRSV-GF does not express SH, and rgRSV-F expresses neither SH nor G. Data from three independent experiments with each virus, normalized to internal controls, are shown.
DISCUSSION
Large (≥5 kDa) polyanionic molecules have been shown to be excellent in vitro inhibitors of attachment and entry for several enveloped viruses, including herpesviruses, retroviruses, orthomyxoviruses, and paramyxoviruses (11, 32). For many of these viruses, this has been shown to be due to the blocking of important charge-charge interactions involved in the binding of the virus to its cellular receptor(s). Such an inhibition is generally dependent upon the molecular weight and extent of the negative charge of the polyanions used and is somewhat nonspecific, in the sense that the interactions are thought to be largely charge mediated and of low affinity and may involve several sites on a particular viral or cellular protein (32). The inhibition of human immunodeficiency virus (HIV) by dextran sulfate, for example, has been shown to involve the association of dextran sulfate with several positively charged regions of the surface glycoprotein gp120 (7, 14, 22, 37, 39) and to interfere with the binding of gp160 to its receptor (30, 35), coreceptor (39), and cell surface heparan sulfate (38, 45). Although not as thoroughly studied, RSV is also inhibited by large polyanions, and for RSV this inhibition was shown to occur at the levels of both binding and fusion (25).
When the antiviral activity of a RhoA-derived peptide was originally described, it was in the context of a recently described interaction between RSV F and RhoA (43), and the possibility of a direct interference upon F-RhoA interaction was considered as a mechanism of action (44). While our present data do not directly address this hypothesis, they do indicate that the antiviral activity of the RhoA-derived peptide 80-94 is likely to be more general in nature. Previous work indicated that the antiviral potency of RhoA peptides is not correlated with their structural similarity to RhoA (5), nor does their mechanism of activity seem to be specific for RSV F, given the relative insensitivity of the rgRSV-F virus to inhibition by peptide 80-94. In this light, the ability of RhoA-derived peptides to inhibit RSV entry should be viewed as a phenomenon separate from and perhaps unrelated to the previously described interaction between F and RhoA.
A more plausible explanation for the antiviral effects of the 80-94 peptide is suggested by the polyanionic nature of the peptide itself and its dependence on multimerization for its antiviral activity, both of which are hallmarks of previously described polyanionic inhibitors of enveloped viruses. The RhoA-derived peptide 80-94, like other polyanions, blocks the binding of RSV to host cells with approximately the same effective concentration at which it inhibits viral replication. This inhibition is dependent on the G protein of RSV, in agreement with its role as the primary attachment protein. Interestingly, the peptide was also effective at inhibiting the infectivity of a virus that had been prebound at 4°C. This agrees with the previous observation that polyanions can block both binding and postbinding entry events of RSV (25) and with the observation of a fusion-inhibiting activity of the 77-95 peptide in previous studies (44). The basis for the inhibition of RSV entry at a postbinding step by heparin, oxidized peptide 80-94, and other polyanions (25) is not clear and is the object of ongoing research. It has previously been shown that the N-terminal fusion peptide of gp41, the analogous viral fusion protein of HIV, can interact with polyanions (17), suggesting that polyanions may (at least for HIV) have a direct effect on viral fusion. However, the relative ineffectiveness of peptide 80-94 in inhibiting replication of the F-only virus indicates that direct effects of peptide 80-94 on the F protein are minimal. Given the dependence of peptide 80-94 on G for its antiviral effect, it may be that the peptide binds to complexes of F and G at the virion surface or to a conformation of F that is dependent upon the coexpression of G. Alternatively, the binding of peptide or other large polyanions to G may sterically block important sites on F that are necessary for the process of fusion. A requirement for steric hindrance or multisite binding would be consistent with the observation that the peptide activity requires multimerization. The identification of the specific binding targets of the 80-94 peptide is an ongoing area of research.
The identification of an in vitro interaction between RhoA and RSV F (43) and a subsequent report of antiviral activity by a RhoA-derived peptide (44) have led to several investigations into the role of RhoA in RSV infections. The results presented here indicate that the inhibition by RhoA-derived peptides is a function of the intrinsic biophysical properties of the peptides themselves and has no bearing on an interaction between RSV glycoproteins and RhoA in an infected cell. While RhoA and RSV F do interact in vitro, there is no evidence that they directly interact during RSV infection, and the inhibition of RSV by RhoA-derived peptides should not be seen as substantiating this hypothesis.
Even though RhoA and RSV F may not directly interact in vivo, RhoA signaling pathways are involved in several important aspects of RSV biology. RhoA is activated during RSV infections of cultured cells (18), and this activation is necessary for the production of viral filaments, although it is not essential for viral replication (36). RhoA activation appears to be important for the budding of RSV from lipid rafts (36), and cytoskeletal rearrangements mediated by RhoA contribute to, but are not essential for, the growth of RSV in some cell lines (3). RhoA signaling may also contribute to RSV pathogenesis, since interference with RhoA signaling pathways reduces the airway hyperreactivity induced by RSV infection in sensitized mice (23). Thus, RhoA appears to play an important role in RSV infection and disease, although this is likely due to the intrinsic importance of RhoA to cellular physiology (15, 20, 33), rather than as a specific binding partner for RSV F, as was previously hypothesized (43).
Although the antiviral activity of RhoA-derived peptides is likely unrelated to the role of RhoA in RSV biology, studies of these peptides have provided insights into the ways in which RSV entry can be inhibited. We have shown here that the RhoA-derived peptide 80-94 is able to block both the attachment of RSV to host cells and a postattachment step in the RSV entry process and that this activity is dependent on both molecular weight and the net negative charge. This activity is largely dependent upon the presence of the G protein of RSV and appears functionally similar to RSV inhibition by soluble heparin or dextran sulfate. A further investigation of the postattachment neutralization of RSV by these molecules should lead to a better understanding of the process of RSV entry and the respective roles of RSV F and G in this process.
Acknowledgments
We thank Jan Lukszo and Nga Ngyuen and her staff for the synthesis and analysis of the synthetic peptides used in these studies. We also thank Lynne Crim, Susette Audet, and Manoj Pastey for helpful discussions and advice.
REFERENCES
- 1.Barnard, D. L., C. L. Hill, T. Gage, J. E. Matheson, J. H. Huffman, R. W. Sidwell, M. I. Otto, and R. F. Schinazi. 1997. Potent inhibition of respiratory syncytial virus by polyoxometalates of several structural classes. Antivir. Res. 34:27-37. [DOI] [PubMed] [Google Scholar]
- 2.Beeler, J. A., and K. van Wyke Coelingh. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J. Virol. 63:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitko, V., A. Oldenburg, N. E. Garmon, and S. Barik. 2003. Profilin is required for viral morphogenesis, syncytium formation, and cell-specific stress fiber induction by respiratory syncytial virus. BMC Microbiol. 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgeois, C., J. B. Bour, K. Lidholt, C. Gauthray, and P. Pothier. 1998. Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro. J. Virol. 72:7221-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budge, P. J., J. Lebowitz, and B. S. Graham. 2003. Antiviral activity of RhoA-derived peptides against respiratory syncytial virus is dependent on formation of peptide dimers. Antimicrob. Agents Chemother. 47:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukreyev, A., S. S. Whitehead, B. R. Murphy, and P. L. Collins. 1997. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J. Virol. 71:8973-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan, L. N., M. Phelan, M. Mallinson, and M. A. Norcross. 1991. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J. Virol. 65:1543-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 9.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 10.Crowe, J. E., Jr., P. T. Bui, C. Y. Firestone, M. Connors, W. R. Elkins, R. M. Chanock, and B. R. Murphy. 1996. Live subgroup B respiratory syncytial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and nonhuman primates. J. Infect. Dis. 173:829-839. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq, E. 2001. 2001 ASPET Otto Krayer Award Lecture. Molecular targets for antiviral agents. J. Pharmacol. Exp. Ther. 297:1-10. (Erratum, 297:1227.) [PubMed] [Google Scholar]
- 12.Douglas, J. L., M. L. Panis, E. Ho, K. Y. Lin, S. H. Krawczyk, D. M. Grant, R. Cai, S. Swaminathan, and T. Cihlar. 2003. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J. Virol. 77:5054-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubovi, E. J., J. D. Geratz, and R. R. Tidwell. 1980. Inhibition of respiratory syncytial virus by bis(5-amidino-2-benzimidazolyl)methane. Virology 103:502-509. [DOI] [PubMed] [Google Scholar]
- 14.Este, J. A., D. Schols, K. De Vreese, K. Van Laethem, A. M. Vandamme, J. Desmyter, and E. De Clercq. 1997. Development of resistance of human immunodeficiency virus type 1 to dextran sulfate associated with the emergence of specific mutations in the envelope gp120 glycoprotein. Mol. Pharmacol. 52:98-104. [DOI] [PubMed] [Google Scholar]
- 15.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 16.Feldman, S. A., S. Audet, and J. A. Beeler. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 74:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon, L. M., A. J. Waring, C. C. Curtain, A. Kirkpatrick, C. Leung, K. Faull, and P. W. Mobley. 1995. Antivirals that target the amino-terminal domain of HIV type 1 glycoprotein 41. AIDS Res. Hum. Retrovir. 11:677-686. [DOI] [PubMed] [Google Scholar]
- 18.Gower, T. L., M. E. Peeples, P. L. Collins, and B. S. Graham. 2001. RhoA is activated during respiratory syncytial virus infection. Virology 283:188-196. [DOI] [PubMed] [Google Scholar]
- 19.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 20.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 21.Hallak, L. K., D. Spillmann, P. L. Collins, and M. E. Peeples. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrop, H. A., D. R. Coombe, and C. C. Rider. 1994. Heparin specifically inhibits binding of V3 loop antibodies to HIV-1 gp120, an effect potentiated by CD4 binding. AIDS 8:183-192. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto, K., R. S. Peebles, Jr., J. R. Sheller, K. Jarzecka, J. Furlong, D. B. Mitchell, T. V. Hartert, and B. S. Graham. 2002. Suppression of airway hyperresponsiveness induced by ovalbumin sensitisation and RSV infection with Y-27632, a Rho kinase inhibitor. Thorax 57:524-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heminway, B. R., Y. Yu, Y. Tanaka, K. G. Perrine, E. Gustafson, J. M. Bernstein, and M. S. Galinski. 1994. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology 200:801-805. [DOI] [PubMed] [Google Scholar]
- 25.Hosoya, M., J. Balzarini, S. Shigeta, and E. De Clercq. 1991. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob. Agents Chemother. 35:2515-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda, S., J. Neyts, S. Verma, A. Wickramasinghe, P. Mohan, and E. De Clercq. 1994. In vitro and in vivo inhibition of ortho- and paramyxovirus infections by a new class of sulfonic acid polymers interacting with virus-cell binding and/or fusion. Antimicrob. Agents Chemother. 38:256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karron, R. A., D. A. Buonagurio, A. F. Georgiu, S. S. Whitehead, J. E. Adamus, M. L. Clements-Mann, D. O. Harris, V. B. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura, K., S. Mori, K. Tomita, K. Ohno, K. Takahashi, S. Shigeta, and M. Terada. 2000. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antivir. Res. 47:41-51. [DOI] [PubMed] [Google Scholar]
- 29.Krusat, T., and H. J. Streckert. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 142:1247-1254. [DOI] [PubMed] [Google Scholar]
- 30.Lederman, S., R. Gulick, and L. Chess. 1989. Dextran sulfate and heparin interact with CD4 molecules to inhibit the binding of coat protein (gp120) of HIV. J. Immunol. 143:1149-1154. [PubMed] [Google Scholar]
- 31.Levine, S., R. Klaiber-Franco, and P. R. Paradiso. 1987. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68:2521-2524. [DOI] [PubMed] [Google Scholar]
- 32.Luscher-Mattli, M. 2000. Polyanions—a lost chance in the fight against HIV and other virus diseases? Antivir. Chem. Chemother. 11:249-259. [DOI] [PubMed] [Google Scholar]
- 33.Mackay, D. J., and A. Hall. 1998. Rho GTPases. J. Biol. Chem. 273:20685-20688. [DOI] [PubMed] [Google Scholar]
- 34.Martinez, I., and J. A. Melero. 2000. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J. Gen. Virol. 81:2715-2722. [DOI] [PubMed] [Google Scholar]
- 35.Mbemba, E., V. Chams, J. C. Gluckman, D. Klatzmann, and L. Gattegno. 1992. Molecular interaction between HIV-1 major envelope glycoprotein and dextran sulfate. Biochim. Biophys. Acta 1138:62-67. [DOI] [PubMed] [Google Scholar]
- 36.McCurdy, L. H., and B. S. Graham. 2003. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J. Virol. 77:1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meylan, P. R., R. S. Kornbluth, I. Zbinden, and D. D. Richman. 1994. Influence of host cell type and V3 loop of the surface glycoprotein on susceptibility of human immunodeficiency virus type 1 to polyanion compounds. Antimicrob. Agents Chemother. 38:2910-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy, B. R., A. V. Sotnikov, L. A. Lawrence, S. M. Banks, and G. A. Prince. 1990. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine 8:497-502. [DOI] [PubMed] [Google Scholar]
- 41.Nikitenko, A. A., Y. E. Raifeld, and T. Z. Wang. 2001. The discovery of RFI-641 as a potent and selective inhibitor of the respiratory syncytial virus. Bioorg. Med. Chem. Lett. 11:1041-1044. [DOI] [PubMed] [Google Scholar]
- 42.Ohki, S. 2003. The compound DATEM inhibits respiratory syncytial virus fusion activity with epithelial cells. Antivir. Res. 58:115-124. [DOI] [PubMed] [Google Scholar]
- 43.Pastey, M. K., J. E. Crowe, Jr., and B. S. Graham. 1999. RhoA interacts with the fusion glycoprotein of respiratory syncytial virus and facilitates virus-induced syncytium formation. J. Virol. 73:7262-7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastey, M. K., T. L. Gower, P. W. Spearman, J. E. Crowe, Jr., and B. S. Graham. 2000. A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nat. Med. 6:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 46.Prince, G. A. 2001. An update on respiratory syncytial virus antiviral agents. Exp. Opin. Investig. Drugs 10:297-308. [DOI] [PubMed] [Google Scholar]
- 47.Razinkov, V., A. Gazumyan, A. Nikitenko, G. Ellestad, and G. Krishnamurthy. 2001. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 8:645-659. [DOI] [PubMed] [Google Scholar]
- 48.Shigeta, S. 2000. Recent progress in antiviral chemotherapy for respiratory syncytial virus infections. Exp. Opin. Investig. Drugs 9:221-235. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasakumar, N., P. L. Ogra, and T. D. Flanagan. 1991. Characteristics of fusion of respiratory syncytial virus with HEp-2 cells as measured by R18 fluorescence dequenching assay. J. Virol. 65:4063-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Techaarpornkul, S., P. L. Collins, and M. E. Peeples. 2002. Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus. Virology 294:296-304. [DOI] [PubMed] [Google Scholar]
- 52.Teng, M. N., S. S. Whitehead, and P. L. Collins. 2001. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289:283-296. [DOI] [PubMed] [Google Scholar]
- 53.Walsh, E. E., and J. Hruska. 1983. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J. Virol. 47:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]