Abstract
Hemoglobinopathies are the most common single gene disorders worldwide with a considerable frequency in certain area particularly Mediterranean and Middle Eastern countries. Hemoglobinopathies include structural variants of hemoglobin (Hb S, Hb C, HbE,…) and thalassaemias which are inherited defects in the globin chains synthesis. The present study was conducted to determine the prevalence of hemoglobinopathies in western Iranian patients. A total of 344 patients (151 males and 193 females) with abnormal CBC and/or hemoglobin electrophoresis were enrolled in the present study. Cellulose acetate gel electrophoresis was performed for all patients and abnormal bands were identified by citrate agar gel electrophoresis and PCR based methods. Iron deficiency anemia (IDA) was present in 156 (45.3%) individuals. Thirty four (9.8%) patients had both iron deficiency anemia and α-thalassemia trait trait, 41(11.9%) patients were with both iron deficiency anemia and minor β-thalassemia. There were 31(9%) patients with α-thalassemia trait and 5 (2.2%) patients with Hb H disease. Fifty six (16.2%) patients had minor β-thalassemia. Also, there were 10 (2.9%) individuals homozygous for hemoglobin D-Punjab and one patient with hemoglobin G (0.3%). There was one sample with hemoglobin C. Further, we found 3 patients (0.9%) with sickle cell trait and more 3 patients (0.8%) with S/ β +–thalassemia. Our results indicated that the most frequent cause of hypochromic and/or microcytic anemia in our population was IDA and the minor β-thalassemia was the second cause that needs to more attention in screening programs.
Keywords: Hemoglobinopathies, α-thalassemia, β-thalassemia, Iron deficiency anemia, Anemia
INTRODUCTION
Hemoglobinopathies are the most common single gene disorders worldwide with a considerable frequency in certain areas particularly Mediterranean and Middle Eastern countries, including Iran. Hemoglobinopathies are a group of autosomal recessive disorders that classified to two main groups of thalassemia syndromes (α- and β-thalassaemia) and structural variants of hemoglobins. In a few cases both phenotypes are observed with reduced synthesis of a hemoglobin variant such as Hb E.1, 2
Structural variants of hemoglobin (Hb) typically are due to a point mutation in globin gene that produces a single amino acid substitution in the globin chain. Although most of these variants are of limited clinical significance, a few important subtypes have been identified with some frequency. Homozygous Hb S (sickle cell anemia) produces significant clinical manifestations, whereas Hb E and Hb D homozygotes may be mildly symptomatic. Although heterozygotes for these variants are typically asymptomatic, diagnosis may be important for genetic counseling.3
In contrast to structural hemoglobin variants, thalassemia syndromes are the hereditary disorders of hemoglobin synthesis in which production of one or two globin chains is either decreased or absent. The most common types of thalassemias are α -thalassemia and β-thalassemia, in which one or both globin chains are synthesized in reduced quantities. Clinical symptoms of thalassemia are from mild microcytic anemia in heterozygous thalassemia trait to life-threatening anemia in homozygous or compound heterozygous thalassemia major.4–6
The genetics of α-thalassemia is complex, and the variable levels of hemoglobin Barts in newborns has been used as a marker for the presence of α-thalassemia genes in many populations. The gene mapping in humans has shown that α-globin gene loci are duplicated (αα/αα), and the α-thalassemias are the result of deletion of one of the four structural genes responsible for α-globin synthesis. Loss of one α gene produces only minimal red cell abnormalities which are not detectable by globin chain synthesis. Deletion of two α genes, as in homozygous α-thalassemia (-α/-α) or heterozygous α-thalassemia (--/αα) produces more severe red cell changes and markedly lower α-chain production, but carriers remain asymptomatic. Hb H disease is due to deletion of three α genes (--/-α) resulted from combined heterozygosity for α-thalassemia 1 and α-thalassemia 2. Finally, when all four genes are lost (--/--), a physiologically useless condition, hydrops fetalis syndrome is produced that is the predominant hemoglobin in the fetus.7
β-thalassemia is the most common genetic disorder in Iran affecting 25,000 β-thalassemia major patients and there is two millions of carriers in the country. In β-thalassemia syndromes, there is an excess of α chain synthesis relative to β chain synthesis. The mode of inheritance of β-thalassemia is autosomal and the defects are expressed in both the heterozygous and homozygous condition. There are two main types of β-thalassemia. The heterozygous state is known as thalassemina minor or trait, whereas the homozygous condition is known as thalassemia major or Cooley's anemia. The severity of these types of thalassemia is influenced by a variety of factors, including race and interaction with other inherited erythrocytic disorders.7, 8
The Kermanshah province locates in the Western Iran and the Kurds are the prominent ethnic group living in the area.9 The aim of present study was to investigate the prevalence of iron deficiency anemia (IDA) and hemoglobinopathies among individuals with an abnormal hemoglobin band on cellulose acetate electrophoresis and/or low hematological indices who referred to the hematology clinics in Kermanshah.
MATERIALS AND METHODS
The samples of present study were collected from September 2010 to January 2012. The samples consisted of 344 (151 males and 193 females) individuals with an abnormal hemoglobin band on cellulose acetate electrophoresis and/or MCV < 80 fl and / or MCH < 27 pg who referred to the hematology clinics of Kermanshah. Before sample collection informed written consent was obtained from each individual. Using a questionnaire the demographic information was obtained. Five milliliter whole blood was aspirated into two tubes. One of the tubes was used for evaluation of iron status. Reduced transferrin saturation percent (<15%) was considered as an indicator of iron deficiency. Another EDTA treated whole blood tube was used for hematological tests. First, cellulose acetate electrophoresis at pH 8.4 was performed on blood samples. In the presence of an abnormal band the EDTA treated samples were further processed by citrate agar gel electrophoresis and an automated ion-exchange high performance liquid chromatography system using the β-thalassaemia short program on the Bio–Rad variant instrument (Bio-Rad Laboratories, Belgium). The presence of β-thalassaemia minor was confirmed with hypochromia and/or microcytosis along with HbA2 > 3.5%. Increased Hb F level (5-20%) with normal Hb A2 was considered as δβ -thalassaemia trait. A presumptive diagnosis of α-thalassaemia was made if β-thalassaemia minor, δβ-thalassaemia trait and iron deficiency were excluded. We used Mentzer index to confirm the differential diagnosis of α-thalassemia from IDA. Also, a standardized manual hemoglobin H inclusion body screen using commercially prepared brilliant cresyl blue (BDH Chemicals, Middlesex, England) was applied if hemoglobin H band was observed in electrophoresis.10–12 For differential diagnosis of structural variants of hemoglobin the PCR and PCR based techniques (ARMS and RFLP) were used.14 All statistical analysis was performed by using SPSS software version 16.0.
RESULTS
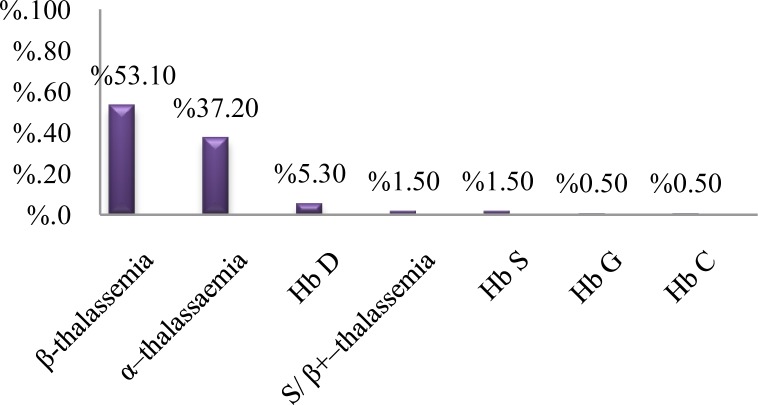
The age of studied subjects was from 1 to 63 years, with a median of 23 years for males and 20 years for females. Our findings indicated that the most frequent cause of hypochromic and/or microcytic anemia was only iron deficiency anemia (45.3%). The remaining cases had some types of hemoglobinopathies (Table 1). Different types of hemoglobinopathies in our study are demonstrated in Figure 1. β -thalassemia minor was the most frequent (53.1%) abnormal hemoglobin among cases with hemoghlobinopathies (Figure 1). There was samples with concomitant presence of a thalassemia syndrome and IDA including 41 (11.9%) with β-thalassemia minor and IDA and 34 (9.8%) with both α-thalassemia trait and IDA (Table 1). There was one sample with hemoglobin C (0.3%). Also, there were 10 homozygous hemoglobin D-Punjab samples (2.9%) and one case with hemoglobin G (0.3%). According to abnormal red blood cell indices and the percent of hemoglobin G (45%) the case was characterized as a double heterozygous (-αG/-α) α-gene.11 Further, 3 patients were detected as HbS/ β+–thalassemia. In the present study, we did not observe sickle cell anemia (Hb SS). Overall, we detected 188 out of 344 (54.7%) samples with abnormal hematological indices that had one type of hemoglobinopathies.
Table 1.
The frequency, type of anemia and hemoglobin variants in our studied samples
| Parameter | N | % |
|---|---|---|
| Iron deficiency anemia | 156 | 45.3 |
| β-thalassemiaminor | 56 | 16.3 |
| α-thalassemia trait | 31 | 9 |
| β-thalassemia minor+IDA | 41 | 11.9 |
| α -thalassemia minor+IDA | 34 | 9.9 |
| Hb H disease | 5 | 1.4 |
| Hb D-Punjab | 10 | 2.9 |
| S/ β+–thalassemia | 3 | 0.9 |
| dβ-thalassaemia trait | 3 | 0.9 |
| Sickle cell trait (AS) | 3 | 0.9 |
| Hb G | 1 | 0.3 |
| Hb C | 1 | 0.3 |
| Total | 344 | 100 |
Figure 1.
The prevalence of various types of hemoglobinopathies in the present study
DISCUSSION
Hypochromic and/or microcytic anemia are the most common hematologic abnormality encountered by physicians. The findings of present study indicate that iron deficiency anemia is the most common cause of hypochromic and/or microcytic anemia in our population. We observed 54.7% of samples with abnormal hematological indices had one type of hemoglobinopathies.
In the present study, there were 100 cases (53.1%) with β-thalassemia syndromes as the most common abnormal hemoglobin that caused hypochromic and/or microcytic anemia. Among β-thalassemia syndromes the β-thalassemia minor was the most frequent with 97 patients (51.5%). Studies demonstrate that Iran has the highest prevalence of β-thalassemia syndromes in Middle Eastern countries15 and ethnic differences play a strong role in presenting various prevalence of hemoglobinopathies.10 Among Saudi Arabian patients around 36% of hemoglobinopaties cases were β-thalassemia minor.16 Further, in a southern city of Iran, Movahed et al., in a population with hemoglobinopathies reported the presence of 70.2% cases with β-thalassemia minor that is higher than that in our studied population.17 According to Abolghasemi et al., report the thalassemia is more prevalent in the northern Caspian Sea coast, southern Persian Gulf and Oman Sea coasts areas of the country18 that is in agreement with the results of Movahed et al.17 In other hand, similar to our findings Jalal et al., in Sulaimani, a city of Iraq, bordering the Kermanshah province with Kurdish ethnic background reported that 50.8% patients with hemoglobinopathies had β-thalassemia minor and 1.6% of patients had δβ-thalassaemia trait.12 It is noteworthy, according to latest report of Kermanshah University of Medical Sciences, there are 280 patients with major β-thalassemia that are under follow up in a special clinic and we excluded these patients in the present study.8
We found 70 patients (37.2% of hemoglobinopathies) with α–thalassaemia syndromes as the second cause of hemoglobinopathies in our region that need to pay more attention in genetic counseling programs to prevent children born with severe form of α–thalassaemia. According to previous studies, the most common mutations in Iran that causes α–thalassaemia are α3.7, --MED and αpolyA2.19 Jalal et al., among Sulaimani population reported that 40.8 and 0.8% of hemoglobinopathies were α–thalassaemia minor and Hb H disease, respectively.12
Hb D-Punjab is one of the most commonly encountered Hb variants worldwide. According to our previously report,14 the study of abnormal Hb variants in Kurdish people from Western Iran revealed that similar to Asian and Middle East countries Hb D-Punjab is the most prevalent β-globin chain structural variant in this area that the present study confirmed it.
Also, Hb S was found to be the second prevalent Hb variantin our population. We found 6 individuals (3% of hemoglobinopathies) who carried Hb S. Three of these patients were compound heterozygous as S/ β+–thalassemia. Ashtiani et al., reported that 0.2% of hemoglobinopathies in Tehran, the capital, was Hb S.21 In the Fars province the sickle gene has the highest frequency among structural variants of hemoglobin.20 However, Jalal et al., reported that HbS consisted the 3.3% of hemoglobinopathies in Sulaimani.12 This issue shows the effect of different ethnicity in the prevalence of hemoglobinopathies.
In summary the results of present study demonstrates the high prevalence of IDA and thalassemias syndrome as the most causes of hypochromic microcytic anemia. The results of present study are useful for screening programs and clinical management of hemoglobinopathies in our area.
ACKNOWLEDGEMENT
This work was financially supported by a grant from Vice Chancellor for Research of Kermanshah University of Medical Sciences, Kermanshah, Iran.
REFERENCES
- 1.Weatherall DJ. Hemoglobin and inherited Disorders of globin synthesis. In: Hoffbrand AV, Catovsky D, Tuddenham EGD, editors. Postgraduate haematology. 5th ed. Oxford: Blackwell Scientific Publication; 2005. pp. 85–90. [Google Scholar]
- 2.Rahimi Z. Genetic epidemiology, hematological and clinical features of hemoglobinopathies in Iran. BioMed Res Int. 2013;2013:1–10. doi: 10.1155/2013/803487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke GM, Higgins TN. Laboratory investigation of hemoglobinopathies and thalassemias: review and update. ClinChem. 2000;46:1284–90. [PubMed] [Google Scholar]
- 4.Dumars KW, Boehm C, Eckman JR, et al. Practical guide to the diagnosis of thalassemia. Am J Med Gen. 1996;62:29–37. doi: 10.1002/(SICI)1096-8628(19960301)62:1<29::AID-AJMG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Rund D, Rachmilewitz E. Thalassemia. N Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 6.Lafferty J, Waye JS, Chui DH, et al. Good practice guidelines for laboratory investigation of hemoglobinopathies. Lab Hematol. 2003;9:237–45. [PubMed] [Google Scholar]
- 7.Al-Awamy BH. Thalassemia syndromes in Saudi Arabia: Meta-analysis of local studies. Saudi Med J. 2000;21:8–17. [PubMed] [Google Scholar]
- 8.Rahimi Z, Muniz A, Parsian A. Detection of responsible mutations for beta thalassemia in the Kermanshah Province of Iran using PCR-based techniques. MolBiol Rep. 2010;37:149–54. doi: 10.1007/s11033-009-9560-0. [DOI] [PubMed] [Google Scholar]
- 9.Soltani MA. Tehran: Soha Press; 1999. Historical geography and comprehensive history of Kermanshah. [Google Scholar]
- 10.Lafferty JD, Barth DS, Sheridan BL, McFarlane AG, Halchuk LM, Crowther MA. Prevalence of thalassemia in patients with microcytosis referred for hemoglobinopathy investigation in Ontario: a prospective cohort study. Am J Clin Pathol. 2007;127:192–6. doi: 10.1309/P6HM33F4D05T30YM. [DOI] [PubMed] [Google Scholar]
- 11.MacPherson RA, Pincus MR. 22nd ed. Elsevier publication; 2011. Henry's clinical diagnostic and management by laboratory methods; pp. 575–89. [Google Scholar]
- 12.Jalal S, AL-Allawi N, Faraj A, Ahmed N. Prevalence of haemoglobinopathies in Sulaimani – Iraq. Dohuk Medical Journal. 2008;2:71–9. [Google Scholar]
- 13.Lafferty JD, Barth DS, Sheridan BL, McFarlane AG, Halchuk LM, Crowther MA. Prevalence of thalassemia in patients with microcytosis referred for hemoglobinopathy investigation in Ontario: a prospective cohort study. Am J Clin Pathol. 2007;127:192–6. doi: 10.1309/P6HM33F4D05T30YM. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi Z, Muniz A, Mozafari H. Abnormal hemoglobins among Kurdish population of Western Iran: hematological and molecular features. MolBiol Rep. 2010;37:51–7. doi: 10.1007/s11033-009-9516-4. [DOI] [PubMed] [Google Scholar]
- 15.Abolghasemi H, Amid A, Zeinali S, Radfar MH, Eshghi P, Rahiminejad MS, Ehsani MA, Najmabadi H, Akbari MT, Afrasiabi A, Akhavan-Niaki H, Hoorfar H. Thalassemia in Iran: epidemiology, prevention, and management. J PediatrHematolOncol. 2007;29:233–8. doi: 10.1097/MPH.0b013e3180437e02. [DOI] [PubMed] [Google Scholar]
- 16.Alsaeed AH. Prevalence of Hemoglobinopathy Disorders in Adult Patients Sent for Diagnosis of Anemia in Saudi Arabia. Genet Test and Mole Biomarkers. 2012;16:25–9. doi: 10.1089/gtmb.2011.0087. [DOI] [PubMed] [Google Scholar]
- 17.Movahed A, Obeidi A, Khamisipour GR. Prevalence of hemoglobinopathies and their associations with different types of hemoglobin and mean cell volume in the pre university students of Bushehr, 2007. South Med J. 2008;12:54–9. [Google Scholar]
- 18.Abolghasemi H, Amid A, Zeinali S, Radfar MH, Eshghi P, Rahiminejad MS, Ehsani MA, Najmabadi H, Akbari MT, Afrasiabi A, Akhavan-Niaki H, Hoorfar H. Thalassemia in Iran: epidemiology, prevention, and management. J Pediatr Hematol Oncol. 2007;29:233–8. doi: 10.1097/MPH.0b013e3180437e02. [DOI] [PubMed] [Google Scholar]
- 19.Hadavi V, Taromchi AH, Malekpour M, Gholami B, Law HY, Almadani N, Afroozan F, Sahebjam F, Pajouh P, Kariminejad R, Kariminejad MH, Azarkeivan A, Jafroodi M, Tamaddoni A, Puehringer H, Oberkanins C, Najmabadi H. Elucidating the spectrum of alpha-thalassemia mutations in Iran. Haematologica. 2007;92:992–3. doi: 10.3324/haematol.10658. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi Z, Merat A, Gerard N, Krishnamoorthy R, Nagel RL. Implications of the genetic epidemiology of globin haplotypes linked to the sickle gene in Southern Iran. Hum Biol. 2006;78:719–731. doi: 10.1353/hub.2007.0016. [DOI] [PubMed] [Google Scholar]