Abstract
Background
Increasing evidence suggests that surgical removal of the axillary lymph nodes (axillary dissection, ALD) in early breast cancer yields no advantage in terms of either overall or disease-free survival, even in women with involvement of sentinel nodes. The optimal role of sentinel node biopsy (SNB) in neo-adjuvant therapy is currently under discussion.
Methods
This review is based on a selective search in the Medline, EMBASE, Cochrane Library, and G.I.N. (Guidelines International Network) databases for relevant articles on the role of axillary dissection in node-positive breast cancer and the role of SNB in neo-adjuvant chemotherapy.
Results
Although no single study provides adequate evidence, the available literature increasingly casts doubt on the putative therapeutic benefit of ALD as part of a multimodal treatment strategy for breast cancer. It is currently unclear what group of patients, if any, might benefit from ALD. Nor is any definitive judgment possible, from the available evidence, regarding the optimal role of SNB in neo-adjuvant therapy. The most recent evidence indicates that SNB after neo-adjuvant chemotherapy in ycN0 patients who had suspect lymph nodes before systemic treatment has a low rate of sensitivity.
Conclusion
Current evidence indicates that the radicality of lymph node surgery in the treatment of breast cancer can be reduced, even if the node status is positive.
Until the beginning of the last decade, axillary dissection was an established part of breast cancer surgery, alongside surgical removal of the primary tumor (1, 5– 10). Its main aim was to establish lymph node status as the most important parameter in prognosis, in order to select adjuvant therapy on a risk-adjusted basis. Studies showed a clear benefit for conservative sentinel node biopsy, which in recent years has become established as the new standard for axillary staging in histologically confirmed invasive breast cancer with lymph nodes that appear normal on clinical examination, palpation, and sonogram (1, 3) (Julian et al. ASCO 2013 J Clin Oncol 2013; 31: [Suppl; abstr. 1000]). In a recent meta-analysis, the rate of complications (particularly lymphedema: 19.9% versus 5.6%) during long-term follow-up was four times higher following axillary dissection than following sentinel node biopsy (4). Because the diagnostic accuracy of sentinel node biopsy in establishing lymph node status is comparable to that of axillary dissection, sentinel node biopsy is now the standard procedure for axillary staging of breast cancer. If performed according to the standard, quality-assured procedure, the accuracy of sentinel node biopsy in staging is high (more than 90%) (3, 5– 8), and morbidity is significantly reduced (9– 10).
Very recently, after sentinel node biopsy replaced axillary dissection as a procedure for diagnosing lymph node status, the therapeutic benefit of completion lymphadenectomy in those with sentinel lymph node tumor involvement has also been questioned (1, 5– 10). This article systematically examines the literature on this issue and discusses the clinical consequences.
The value of sentinel node biopsy as part of neoadjuvant therapy has not yet been unambiguously established. Reliable data is only available for sentinel node biopsy’s detection rates (feasibility, diagnostic accuracy) before systemic therapy. Sentinel node biopsy after neoadjuvant chemotherapy could reduce the axillary dissection rate, because 20 to 40% of node-positive patients are node-negative after chemotherapy and would therefore not benefit from axillary dissection. The effect of previous chemotherapy on sentinel node biopsy’s detection rates is unclear. In recent years there have been intense discussions on whether patients who are clinically node-positive may achieve complete axillary remission as a result of neoadjuvant chemotherapy, and whether this may increase the rate of axilla-conserving surgery, just as the rate of breast-conserving surgery is optimized using neoadjuvant therapy.
Methods
A selective search of the literature (from 2000 to 2013) was performed using the established databases PubMed, the Cochrane Library, G-I-N (Guideline International Network), and EMBASE, in order to assess the therapeutic effect of axillary dissection in positive sentinel lymph nodes. The search terms used were “breast cancer,” “sentinel node biopsy,” and “axillary dissection.” The search included methodically sound observational studies and prospective randomized trials conducted since 1998. Recent conference submissions were also taken into account when they concerned prospective randomized trials. A search of the literature was also performed on the subject of the value of sentinel node biopsy as part of neoadjuvant therapy, and the currently available data was summarized.
Results
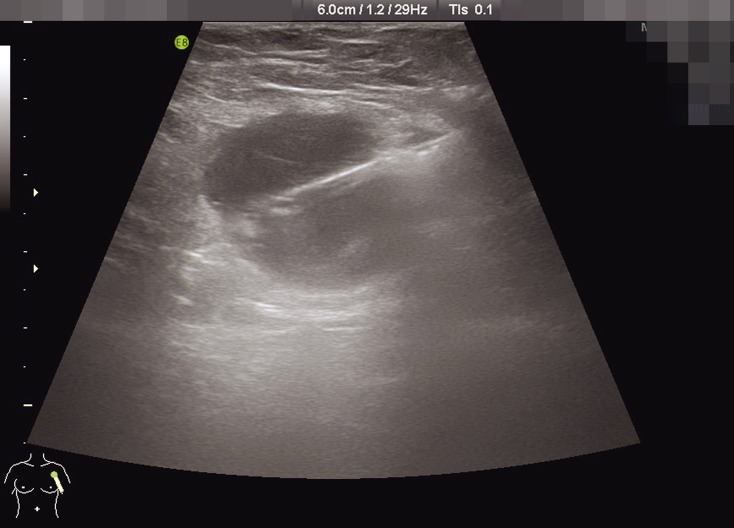
Sentinel node biopsy is accepted as standard procedure for axillary staging of breast cancer in all significant guidelines. Its superiority to axillary dissection alone has been established with the highest level of evidence (1– 3, 5– 10). Because sentinel node biopsy is a procedure used to diagnose lymph node status, it is not indicated where there is manifest clinical suspicion of advanced lymph node involvement or where a tumor has penetrated lymph nodes (11– 13). In order to establish preoperatively whether clinically and/or sonographically abnormal lymph nodes do indeed indicate lymph node metastasis, ultrasound-guided fine-needle aspiration, or high-speed punch biopsy—which has a higher predictive value—of the suspected lymph nodes is increasingly used in everyday clinical practice (Figure 1). Axillary dissection is indicated for patients with cytological or histological evidence of lymph node metastasis.
Figure 1.
Punch biopsy of an axillary lymph node with abnormal findings on sonogram
Axillary dissection in cases of positive sentinel node biopsy
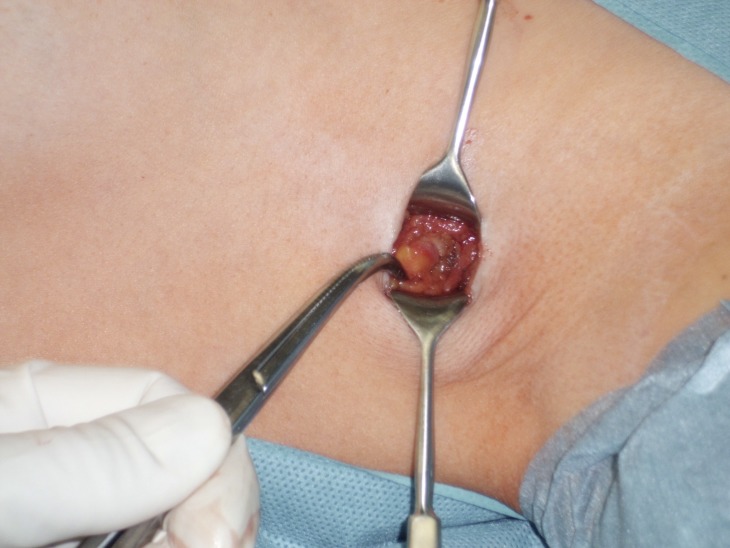
The axillary recurrence rate in invasive breast cancer is less than 1% (14– 17). Surgical removal of the remaining axillary lymph nodes has until now been considered necessary in patients with histological evidence of sentinel lymph node tumor involvement (Figure 2). In node-positive patients the number of affected lymph nodes or the ratio of positive nodes to the number of excised lymph nodes provides information which is important to the indication and selection of subsequent systemic antineoplastic therapy, as well as adjuvant radiotherapy (18– 19). Recently, however, new data has been discussed as a potential change of direction: a randomized trial (the ACOSOG Z0011 trial) (20) investigated the clinical impact, in terms of locoregional tumor control and overall survival, of not performing axillary dissection in patients with T1 and T2 tumors and one or two positive sentinel lymph nodes who had undergone breast-conserving therapy followed by percutaneous radiotherapy of the entire affected breast using tangential radiation fields. The median follow-up time in the Z0011 trial was 6.3 years. The five-year survival rates were 92.5% in the patient group undergoing sentinel node biopsy alone (95% confidence interval [CI]: 90.0% to 95.1%), and 91.8% in the treatment group that underwent complete axillary dissection (95% CI: 89.1% to 94.5%). Although 27% of the patients in the group receiving axillary dissection had lymph node involvement, the trial found no difference in the recurrence or survival rates (hazard ratio: 0.87; 95% CI: 0.62 to 1.23). Since the ACOSOG Z0011 trial found no evidence of a therapeutic benefit for axillary dissection in patients with positive sentinel lymph node status, the value of surgical lymphadenectomy for breast cancer patients has been questioned increasingly critically.
Figure 2.
Axillary sentinel lymph node excision
A randomized trial published subsequently investigated the therapeutic value of axillary dissection in patients with evidence of sentinel node micrometastasis. Up to 25% of these patients are known to experience further lymph node involvement. This trial also failed to find a benefit for axillary dissection in terms of event-free or overall survival (26).
The limits of the evidence
In revising the German S3 Guideline Diagnosis, Treatment, and Aftercare for Breast Cancer (Diagnose, Therapie und Nachsorge des Mammakarzinoms) (21), it was not possible to refer to any individual study regarding fundamental change in the existing standard (axillary dissection for positive sentinel lymph nodes), due to methodology regulations (22). Because of this, an external evidence report (23– 24) was commissioned, to assess other studies in addition to the methods used in the Z0011 trial. A literature search performed for this report found only three methodologically sound studies out of a total of more than 300 primary publications. On the one hand, the risk of bias in the only prospective Z0011 trial was assessed as “unclear”: a general problem affecting the study is its very low statistical predictive power. It proved impossible to recruit as many women in the Z0011 trial as originally planned between 1999 and 2004, so its statistical predictive power is too low to identify a clinically significant difference with any statistical significance. The calculations performed by Gartlehner et al. and Glechner et al. indicated that approximately 5900 recruited individuals would have been needed in order to identify a two-percentage-point difference in mortality with statistical significance (23– 24).
The other two studies are retrospective cohort studies involving registry data (25– 26). The methodologically superior of the two is based on data from more than 97 000 patients from the US National Cancer Data Base (between 1998 and 2005) (26). The second retrospective cohort study used data from more than 26 000 patients in the SEER (Surveillance, Epidemiology, and End Results) database (27). The evidence report also assessed the bias risk of both these studies as unclear. Ultimately, the available individual studies were insufficiently reliable to address this issue, although the studies were consistent in the effects they found and showed no therapeutic benefit for axillary dissection in sentinel node-positive patients as part of multimodal therapy (23– 24).
Other studies that were not included in the evidence report for methodological reasons support the idea that the therapeutic effect of axillary dissection in breast cancer is marginal at best. Several available randomized trials conducted in the “pre-sentinel era” compared axillary dissection with no lymphadenectomy. Although these studies were conducted in selected low-risk patient populations, none of them found a therapeutic effect for axillary dissection (28– 32).
However, the proposition that lymph nodes themselves have no metastasis potential of their own (34) seems to be contradicted by two recent studies. In the MA.20 trial, 1800 node-positive patients and high-risk, node-negative women who had undergone breast-conserving therapy followed by tangential-field, whole-breast radiation and adjuvant chemotherapy received either additional irradiation of the supraclavicular, infraclavicular, and mediastinal lymph nodes or no further regional therapy. The additional lymph node irradiation led to a significant reduction in distant disease-free survival (DDFS) and a borderline significant improvement in overall survival (p = 0.07) (Whelan et al. ASCO 2011 J Clin Oncol 2011; 29: [Suppl; abstr. BA 1003]). Another phase III EORTC trial was presented at ESMO 2013 (Poortmans et al. 2013 ESMO abstr. 2). In this trial, patients with medial tumors received either standard treatment (breast-conserving therapy with tangential-field radiation, mastectomy with or without chest wall radiation) or additional radiotherapy of the mediastinal and medial supraclavicular lymph nodes. The additional regional treatment led to a significant improvement in disease-free survival (69.1% to 72.1%, p = 0.044) and metastasis-free survival (75% versus 78%, p = 0.02) after a median follow-up time of 10.9 years. The effect on overall survival was borderline significant, at 80.7% versus 82.3% (p = 0.056). The results of these latest studies therefore indicate that treatment of regional lymph nodes in appropriate risk populations may be associated with a therapeutic benefit.
The AMAROS trial, which was presented at ASCO 2013 by Rutgers et al. (Rutgers et al. ASCO 2013 J Clin Oncol 2013; 31: [Suppl; abstr. 1001]), compared axillary dissection and lymph vessel irradiation in patients with T1/T2 cancer and a positive sentinel node. No significant differences were found between surgical and radiooncological regional therapy in terms of disease-free or overall survival. The limitation of the AMAROS trial’s low rates of axillary recurrences must not be overlooked: this may subsequently have caused an undetectable difference in the recurrence rates. Because of the limitations discussed here, replacement of axillary dissection with lymph vessel irradiation cannot currently be considered standard treatment in patients with a positive sentinel node. Current knowledge does not provide grounds for a general expansion of the radiation field due to axillary dissection not having been performed.
In summary, current data shows that the therapeutic effect of axillary dissection in patients with clinically normal axillae and positive sentinel lymph node status is marginal at best. It seems that it must be part of appropriate multimodal treatment, possibly also requiring regional therapy. This means that systemic therapy and locoregional therapy are both part of lymph node treatment, rather than being alternatives. The role of axillary dissection will probably continue to become less important. It remains unclear whether it is possible to define populations that do benefit from axillary dissection. Patients with a clinically detectable axillary tumor burden will probably still require axillary dissection in the future. The German INSEMA (Intergroup-Sentinel-Mamma) trial will be a prospective multicenter trial investigating whether limited axillary surgery or even no axillary staging at all is comparable to standard procedure.
Sentinel node biopsy and neoadjuvant chemotherapy
Two important trials on the role of sentinel node biopsy in the context of neoadjuvant chemotherapy were presented at the San Antonio Breast Cancer Symposium 2012: the German SENTINA trial recruited patients to four cohorts in order to investigate the value of sentinel node biopsy before and after primary systemic therapy (35). A total of 1022 women underwent sentinel node biopsy before neoadjuvant therapy. In this group, the detection rates were high: 99.1% (95% CI: 98.3 to 99.6, 1013 of 1022). In women with positive sentinel node status before chemotherapy, the trial investigated whether a second sentinel node biopsy following systemic therapy might be a suitable tool to identify the population of patients who were node-negative patients following chemotherapy. However, the detection rate was only 60.8%, and the false negative rate 51.6%. In women who presented suspicious lymph nodes before neoadjuvant therapy and normal lymph node status after chemotherapy, the detection rate was 80.1% and the false negative rate 14.2%; this was significantly less favorable than in patients receiving primary surgery. A notable feature of this trial was that the false negative rate depended to a great extent on the number of sentinel lymph nodes removed. In women in whom only one sentinel lymph node was identified the false negative rate was 24.3%. If two sentinel nodes were removed, the false negative rate was 18.5%. Only if three sentinel lymph nodes were removed could a false negative rate of less than 10% reliably be achieved. This is the rate considered the minimum standard for diagnostic accuracy in sentinel node biopsy.
The results reported for the ACOSOG 1071 trial, which was presented at the same time, were very similar (35). Here too the detection and false negative rates of sentinel lymph node biopsy were investigated in patients who converted from positive to negative node status following neoadjuvant chemotherapy. The results were comparable to those of the SENTINA trial, with a false negative rate of 14.7% in the total patient population. The false negative rate was 31.5% for patients with one sentinel node, and 21.1% for those with two. Only if three or more sentinel nodes were detected was the false negative rate below 10%. This means that the detection rates for sentinel lymph node biopsy in patients following systemic therapy were significantly less favorable than in patients receiving primary surgery if node status before treatment was positive. Axillary dissection is therefore necessary in such cases.
Current recommendations in Germany
Both the German S3 Guideline and the Breast Committee of the Gynecological Oncology Working Group (Organkommission Mamma der Arbeitsgemeinschaft für gynäkologische Onkologie) recommend not performing axillary dissection in selected patient populations when sentinel node status is positive. Micrometastases (metastases measuring less than 2 mm) in a sentinel node are no longer an indication for axillary dissection (37). As in the Z0011 trial, patients with T1 or T2 tumors and one or two positive sentinel lymph nodes can be offered the option of not undergoing axillary dissection if they receive breast-conserving therapy, provided that patients are informed of the current state of the evidence. Due to the limitations discussed here, the replacement of axillary dissection with irradiation of the lymph vessels, as in the AMAROS trial, cannot currently be considered standard therapy for patients with positive sentinel nodes. Expansion of the radiation field due to axillary dissection not having been performed is therefore not generally indicated on the basis of current knowledge. Axillary dissection must be performed in patients who undergo mastectomy or who do not receive postoperative radiotherapy of the affected breast. The S3 Guideline and the recommendations of the Gynecological Oncology Working Group are essentially the same in this regard.
In patients whose lymph node status is positive before neoadjuvant chemotherapy, axillary dissection is necessary after systemic therapy. For patients whose clinical node status before neoadjuvant chemotherapy is negative, sentinel node biopsy before chemotherapy is recommended in Germany.
Conclusion
For patients with early breast cancer, a T1/T2 tumor, and one or two positive sentinel nodes, not performing axillary dissection is currently an option of which patients should be informed in the context of breast-conserving therapy. Discussion with the patient must include careful consideration of the potential benefit versus the risk, ultimately leading to a joint decision on axillary staging. Recent studies and aggregate evidence show a general trend, in assessment of endpoints, that suggests that in the future axillary dissection in primary breast cancer therapy may become less important in view of risk–benefit ratio. However, the exact population of patients who do not benefit from axillary dissection must be clearly established in future studies.
Key Messages.
Sentinel node biopsy is the gold standard for axillary staging in patients with early breast cancer and clinically normal axillae.
The current data indicates that sentinel node biopsy should usually be performed before neoadjuvant chemotherapy in patients with cN0 status. The current data is not sufficient for a general recommendation of repeat sentinel node biopsy after chemotherapy or sentinel node biopsy alone after neoadjuvant chemotherapy in patients with ycN0 status following pretreatment cN1 status.
It is likely that in the future risk–benefit analysis will favor sentinel node biopsy alone more often and general systematic axillary lymphadenectomy less often for patients with a positive sentinel node. However, the exact patient population that does not benefit from axillary lymphadenectomy must be established in future studies.
Current data is already sufficient to allow sentinel node biopsy alone to be discussed and indicated as an option for a selected population of patients with early breast cancer (T1/T2) when breast-conserving surgery is performed, if they have a positive sentinel node.
On the basis of current data (one prospective randomized trial), the option of lymph vessel irradiation alone for patients with positive sentinel nodes cannot be considered standard procedure.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
The authors would like to thank Prof. A. Scharl for his careful revision of the manuscript of this article.
Footnotes
Conflict of interest statement
Prof. Janni, Prof. Fehm, Prof. Wöckel, Dr. Schwentner, and Prof. Kuhn declare that no conflict of interest exists.
Prof. Kreienberg has received consultancy fees from Astra Zeneca.
References
- 1.Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18:2553–2559. doi: 10.1200/JCO.2000.18.13.2553. [DOI] [PubMed] [Google Scholar]
- 2.Helms G, Kühn T, Moser L, Remmel E, Kreienberg R. Schulter-Arm-Morbidität nach Sentinel-Node-Biopsie und kompletter Axilladissektion - Daten einer prospektiv randomisierten Studie. Senologie. 2008:A5–A65. [Google Scholar]
- 3.Kuehn T, Vogl FD, Helms GV, et al. Sentinel Node Biopsy is a reliable method for axillary staging in breast cancer: results from a large prospective German multiinstitutional trial. Eur J Surg Oncol. 2004;30:252–259. doi: 10.1016/j.ejso.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 4.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncology. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 5.Bergkvist L, Frisell J, Liljegren G, Celebioglu F, Damm S, Thorn M. Multicentre study of detection and falsenegative rates in sentinel node biopsy for breast cancer. Br J Surg. 2001;88:1644–1648. doi: 10.1046/j.0007-1323.2001.01948.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early stage breast carcinoma: a metaanalysis. Cancer. 2006;106 doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 7.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 8.Tafra L, Lannin DR, Swanson MS, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233 doi: 10.1097/00000658-200101000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleissig A, Fallowfield LJ, Langridge CI, et al. Postoperative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 11.Kuehn T, Bembenek A, Decker T, et al. A concept for the clinical implementation of sentinel lymph node biopsy in patients with breast carcinoma with special regard to quality assurance. Cancer. 2005;103:451–461. doi: 10.1002/cncr.20786. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Gampenrieder SP, Rinnerthaler G, Greil R. Neoadjuvant chemotherapy and targeted therapy in breast cancer: past, present, and future. J Oncol. 2013 doi: 10.1155/2013/732047. dx.doi.org/10.1155/2013/732047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palesty JA, Foster JM, Hurd TC, Watroba N, Rezaishiraz H, Edge SB. Axillary recurrence in women with a negative sentinel lymph node and no axillary dissection in breast cancer. J Surg Oncol. 2006;93:129–132. doi: 10.1002/jso.20408. [DOI] [PubMed] [Google Scholar]
- 15.Smidt ML, Janssen CM, Kuster DM, Bruggink ED, Strobbe LJ. Axillary recurrence after a negative sentinel node biopsy for breast cancer: incidence and clinical significance. Ann Surg Oncol. 2005;12 doi: 10.1007/s10434-004-1166-0. [DOI] [PubMed] [Google Scholar]
- 16.Veronesi U, Orecchia R, Zurrida S, et al. Avoiding axillary dissection in breast cancer surgery: a randomized trial to assess the role of axillary radiotherapy. Ann Oncol. 2005;16:383–388. doi: 10.1093/annonc/mdi089. [DOI] [PubMed] [Google Scholar]
- 17.Zavagno G, Carcoforo P, Franchini Z, et al. Axillary recurrence after negative sentinel lymph node biopsy without axillary dissection: a study on 479 breast cancer patients. Eur J Surg Oncol. 2005;31:715–720. doi: 10.1016/j.ejso.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Moebus V, Jackisch C, Lueck HJ, et al. Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. J Clin Oncol. 2010;28:2874–2880. doi: 10.1200/JCO.2009.24.7643. [DOI] [PubMed] [Google Scholar]
- 19.AGO-Leitlinien. Arbeitsgemeinschaft Gynäkologische Onkologie [Google Scholar]
- 20.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreienberg R, Albert SU, Follmann M, et al. Interdisziplinäre S3-Leitlinie „Diagnostik, Therapie und Nachsorge des Mammakarzinoms“ des Onkologischen Leitlinienprogrammes der DKG, Deutschen Krebshilfe und AWMF Zuckerschwerdtverlag 2012. http://wwwawmf.org/uploads/tx_szleitlinien/032045OL_k_S3__Brustkrebs_Mammakarzinom_Diagnostik_Therapie_Nachsorge_2012-07.pdf [Google Scholar]
- 22.AWMF Regelwerk. www.awmf.org/leitlinien/awmf-regelwerk/awmf-regelwerk-offline.html. (last accessed on 31 January 2014)
- 23.Gartlehner G, Chapman A, Strobelberger M, et al. Vergleichende Wirksamkeit und Sicherheit von alleiniger Sentinel-Lymphknoten-Biopsie oder kompletter Axilladissektion bei Sentinel-positivem Mammakarzinom: Systematische Übersichtsarbeit 2011. www.krebsgesellschaft.de/download/s3_bk_update_2012_ol_evidenzbericht_prof._gartlehner.pdf. (last accessed on 31. January 2149) [Google Scholar]
- 24.Glechner A, Wöckel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U, Kreienberg R. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer. 2013;49:812–825. doi: 10.1016/j.ejca.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from SEER database. Ann Surg Oncol. 2010;17:343–351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 27.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twentyfiveyear follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 29.Martelli G, Boracchi P, De Palo M, et al. A randomized trial comparing axillary dissection to no axillary dissection in older patients with T1N0 breast cancer: results after 5 years of follow up. Ann Surg. 2005;242 doi: 10.1097/01.sla.0000167759.15670.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudenstam CM, Zahrieh D, Forbes JF, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 1093. J Clin Oncol. 2006;24:337–344. doi: 10.1200/JCO.2005.01.5784. [DOI] [PubMed] [Google Scholar]
- 31.Veronesi U, Marubini E, Mariani L, Valagussa P, Zucali R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30year results of a randomised trial. Eur J Cancer. 1999;35:1320–1325. doi: 10.1016/s0959-8049(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 32.Veronesi U, Galimberti V, Mariani L, et al. Sentinel node biopsy in breast cancer: early results in 953 patients with negative sentinel node biopsy and no axillary dissection. Eur J Cancer. 2005;41:231–237. doi: 10.1016/j.ejca.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Fisher B, Wolmark N, Redmond C, Deutsch M, Fisher ER. Findings from NSABP protocol No. B-04: Comparison of radical mastectomy with alternative treatments. The clinical and biologic significance of medial-central breast cancers. Cancer. 1981;48:1863–1872. doi: 10.1002/1097-0142(19811015)48:8<1863::aid-cncr2820480825>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Engel J, Emeny RT, Hölzel D. Positive lymph nodes do not metastasize. Cancer Metastasis Rev. 2012;31:235–246. doi: 10.1007/s10555-011-9343-7. [DOI] [PubMed] [Google Scholar]
- 35.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective multi-center cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 36.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]