Abstract
The aim of this work is the evaluation of the design for a nonconventional PET scanner, the voxel imaging PET (VIP), based on pixelated room-temperature CdTe detectors yielding a true 3-D impact point with a density of 450 channels cm3, for a total 6 336 000 channels in a seamless ring shaped volume. The system is simulated and evaluated following the prescriptions of the NEMA NU 2-2001 and the NEMA NU 4-2008 standards. Results show that the excellent energy resolution of the CdTe detectors (1.6% for 511 keV photons), together with the small voxel pitch (1×1×2 mm3), and the crack-free ring geometry, give the design the potential to overcome the current limitations of PET scanners and to approach the intrinsic image resolution limits set by physics. The VIP is expected to reach a competitive sensitivity and a superior signal purity with respect to values commonly quoted for state-of-the-art scintillating crystal PETs. The system can provide 14 cps/kBq with a scatter fraction of 3.95% and 21 cps/kBq with a scatter fraction of 0.73% according to NEMA NU 2-2001 and NEMA NU 4-2008, respectively. The calculated NEC curve has a peak value of 122 kcps at 5.3 kBq/mL for NEMA NU 2-2001 and 908 kcps at 1.6 MBq/mL for NEMA NU 4-2008. The proposed scanner can achieve an image resolution of ~ 1 mm full-width at half-maximum in all directions. The virtually noise-free data sample leads to direct positive impact on the quality of the reconstructed images. As a consequence, high-quality high-resolution images can be obtained with significantly lower number of events compared to conventional scanners. Overall, simulation results suggest the VIP scanner can be operated either at normal dose for fast scanning and high patient throughput, or at low dose to decrease the patient radioactivity exposure. The design evaluation presented in this work is driving the development and the optimization of a fully operative prototype to prove the feasibility of the VIP concept.
Keywords: Brain, nuclear imaging, system design
I. Introduction
THIS PAPER describes a novel design of brain positron emission tomography (PET) scanner that is currently under development in the framework of the voxel imaging PET (VIP) pathfinder project [1]. The VIP-PET uses electronically pixelated room temperature solid-state CdTe detectors to overcome the limitations of current PET systems based on scintillator detectors. The use of semiconductor detectors, such as CdTe or CdZnTe (CZT), for photon detection in PET applications is described in [2]–[5]. The VIP modular design allows us to package the detector in different shapes and for different functionalities: e.g., a whole-body (WB) PET, a small-animal PET, a positron emission mammography (PEM) scanner [6], a Compton camera [7]. Since the clinical applications related to brain pathologies are among the challenging screenings, we present and evaluate the VIP as a human brain dedicated PET. Its particular design features suppress the huge amount of scattered events that are due to high density passive material such as human skull in the field-of-view (FOV) and that would lead to lower contrast and higher noise images. Moreover, a high-resolution PET scanner can increase the specificity of a brain scan by improving metastasis localization and intratumoral inhomogeneity estimation [2], also crucially important for the efficiency of any radiation therapy.
This work proves that the new design of the PET scanner allows to:
achieve a competitive detection efficiency for 511 keV photons with a seamless ring geometry and a stopping power of 4 cm CdTe.;
eliminate the parallax error by yielding very precisely reconstructed lines of response (LOR) with a true 3-D detector and a density of ~ 450 channels per cm3;
achieve very high signal-to-noise ratio by rejecting most of the scattered events due to the excellent energy resolution of room temperature solid-state CdTe detectors.
The VIP project is currently developing a PET prototype with two small detector stacks (150 K channels in total) operating in a back-to-back coincidence mode. Though a discussion of the packaging strategy is beyond the scope of this manuscript, it must be noticed that moving from a small detector unit to a full ring-shaped stack (~ 6.6 M channels) is challenging. Other studies in the framework of the VIP project address the engineering challenges and the data processing issues dealing with the very high number or readout channels. The following sections describe in detail the design, the simulation technique, and the evaluation of the counting and imaging performance of the VIP scanner.
II. Materials and Methods
A. VIP Design
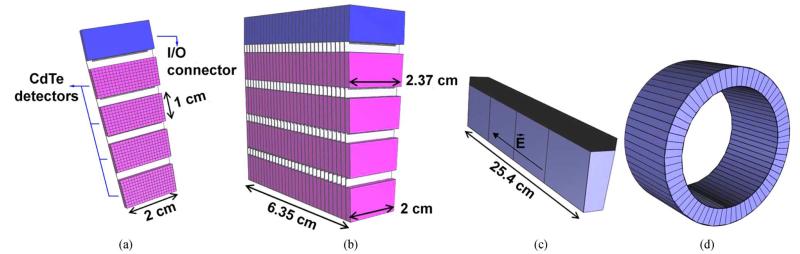
The VIP scanner has a modular design based on the detector module shown in [Fig. 1(a)]. The module hosts 4 CdTe detectors with 10 mm × 20 mm size and 2 mm thickness. The 2000 V high voltage (HV) is applied such that the electric field is perpendicular to the surface with resulting 1000 V/mm bias and an expected energy resolution of 1.6% for 511 keV photons at room temperature [8]. Each of the CdTe detectors is electronically pixelated into 200 voxels of 1 × 1 × 2 mm3 pitch, for an accurate photon impact point measurement, and bonded to a thinned read-out channel (ROC) and then mounted on a kapton printed circuit board (PCB). One of the essential ideas is reducing the passive material by thinning the ROC and the kapton PCB down to 50 μm each. The conductive glue between the ROC, the CdTe detectors, and the kapton PCB will occupy an additional thickness of 15 μm. The combined attenuation coefficient of the passive material accounts for less than 2% compared to 2 mm CdTe. A distinctive characteristic of the VIP is that the module can be given a trapezoidal shape to form a scanner ring without cracks to boost the system sensitivity. This idea has been already implemented by using continuous NaI scintillating crystals [9].
Fig. 1.
(a) Design of an individual VIP detector module. Blue piece at one end of the kapton PCB is a connector to couple the detector module to a bus of signals. (b) VIP module block. (c) VIP ring section formed from four module blocks connected to the same bus (black region). (d) General view of the VIP scanner. Due to the trapezoidal shape of the CdTe detectors the system does not have cracks in between ring sections.
The VIP module, by design, is made to point to the center of the PET cylinder with the 511 keV photons entering from the edge of the CdTe detector. Therefore, even though CdTe has lower density and thus significantly lower stopping power than scintillating crystals such as lutetium oxyorthosilicate (LSO), the depth of the VIP module can be extended in radial direction by adding CdTe detectors as much as needed to reach the necessary detection efficiency. In the proposed design, incident radiation traverses a minimum of 4 cm CdTe with 70% of singles 511 keV photons being completely absorbed.
The next unit of the VIP scanner is a module block that consists of 30 detector modules stacked together [Fig. 1(b)]. Thereafter, four such module blocks connected to the same electronic bus form a VIP section [Fig. 1(c)]. Note that the electric field inside the solid-state detector is directed as shown in Fig. 1(c) and therefore it is parallel to the magnetic field of a possible magnetic resonance (MR) scanner. It was shown in [8] that in such a configuration, with , the response of the solid-state detector is not affected by the strength of the magnetic field and the VIP design can be in principle a potential candidate for developing simultaneous MR-PET imaging systems. However, the impact of radio frequency (RF) on the VIP system, as well as the influence of the VIP scanner itself with the accompanying electronics to the signal-to-noise ratio of an MR image, have not been assessed.
Finally, when 66 sections are put together, they form a cylindrical seamless PET scanner [Fig. 1(d)] with a total of 6 336 000 detector voxels. The complete scanner has an inner diameter of 42 cm, an outer diameter of 54 cm, and an axial length of 25.4 cm to match the typical size of a brain PET.
Due to the large number of individual channels, the design of the electronics for the signal processing and readout is a crucial and unique feature of the VIP project. For each channel independently, the VIP readout will provide a digitized value of the energy, the time stamp of every photon detection, and the position of the channel where the detection happened. The signal processing takes place “in situ” with the electric layers located in between adjacent detector modules. To obtain the energy and time information, each channel is bonded to a microchip hosting a fully integrated front-end electronics for a total surface of 1 × 1 mm2 per channel. Each channel has its own preamplifier, a calibration setup, a pulse shaper, a tunable threshold level, a number of digital-to-analog converters (DACs) and analog-to-digital converters (ADCs) as needed, a discriminator, a digital controller, a pulse feeding, a detector leakage compensator, and a peak and hold circuit. The channel back-end contains an analog section and a digital section. The analog section includes a band-gap and current reference circuit, a temperature sensor, and a 6-bit global threshold DAC. The digital part contains the time to digital converter (TDC), the digital controller, a configuration register, and the identification register. This solution is called “smart-pixel.” Its design, the development status, and the results of the successful characterization of the preliminary prototypes are described in detail in [10]. The “smart-pixel” has the advantage of compactness and of the robustness of having the analog front-end and the analog to digital conversion optimized for minimum parasitic loading and/or coupling. To this day, no other design with the specifics and functionality of the VIP smart-pixel has been developed. In this approach, the ASIC design transforms each channel into a completely independent self-triggered detector operating stand-alone.
B. Simulation
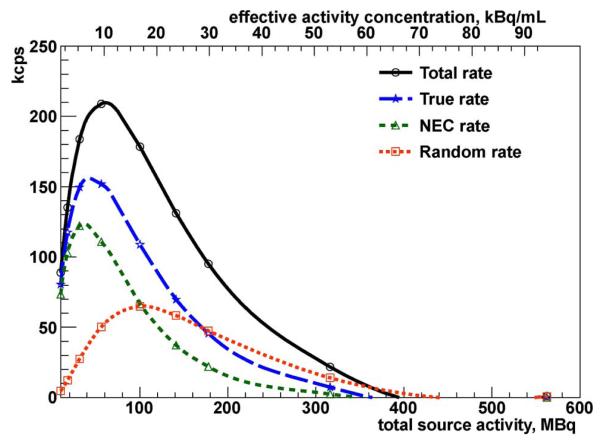
In order to evaluate the VIP system performance and assess its image quality, the whole VIP geometry is simulated using the Geant4-based Architecture for Medicine-Oriented Simulations (GAMOS) ver. 3.0.0 based on Geant4 4.9.4 [11]. The Geant4 kernel provides the accurate simulation of particle interaction with the materials within the defined geometry. For each event (e.g., a positron emission from a 18F source), the energies that primary (e.g., 511 keV photons) and secondary particles deposit in the detector’s pixels get summed to get one resulting hit per pixel per event. The smart-pixel slow shaper integrates the deposited energy with 20 μs peak time. After digitizing the peak value, the time needed to reset the pixel electronic before the next event is 130 μs. The 150 μs total signal processing time allows each channel to handle easily 6 kHz with negligible pile up [10]. To simulate the effect of the electronic measuring time and dead time, all energy depositions within the same voxel and within the first 20 μs window time contribute to the total energy of a single hit; energies deposited right after and within the 130 μs dead time, are lost. A Gaussian smearing is applied to the total hit energy to mimic the pedestal noise and the energy resolution of the CdTe. A channel triggers if the total collected energy exceeds the 20 keV trigger threshold defined as the 10 σ noise level above the base line of the amplifier. The trigger defines the time stamp of the hit as measured by the TDC with 0.1 ns resolution. Additional smearing is considered due to the asymmetry between electron and hole mobilities [12] and to the jitter of the electronic discriminator as measured in [10]. A direct measurement of the coincidence time resolution [13] shows that for energy close to the 20 keV trigger threshold, a time coincidence window as wide as 20 ns is needed to detect at least 70% of the photon pairs. The counting performance evaluation reported in the following section indicates that with such a coincidence time window no significant pile-up effect is expected up to 107 Bq activity in the FOV and the system can handle up to 108 without saturation (Figs. 4 and 5).
Fig. 4.
NEMA NU 2-2001 VIP scanner counting rates as a function of effective activity concentration and total source activity.
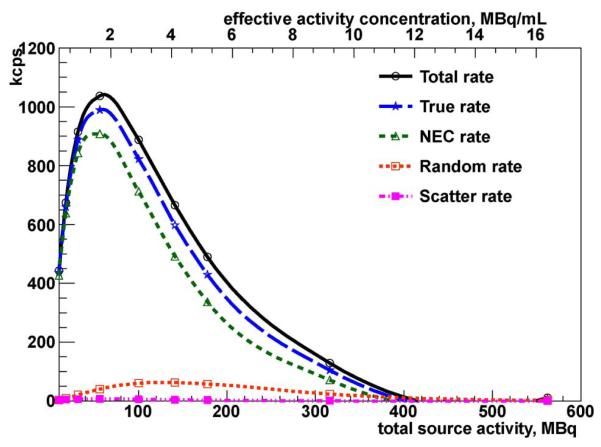
Fig. 5.
NEMA NU 4-2008 VIP scanner counting rates as a function of effective activity concentration and total source activity of a line source for the mousesize phantom.
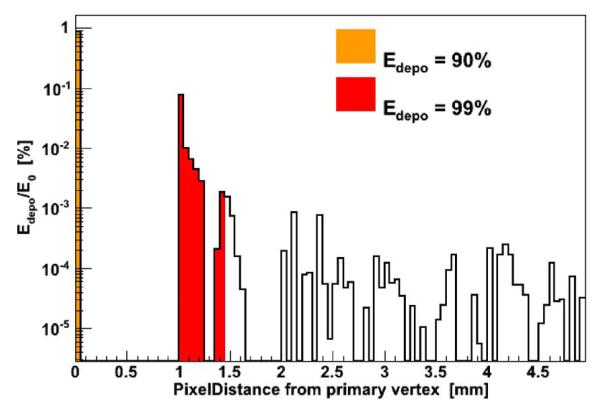
Due to the large number of channels in the full ring, each channel is treated as an independent detector with data collected in list mode (LM) and processed offline. Hit entries of the data list are characterized by the (x, y, z) position of the center of the correspondent voxel, the collected energy, and the time stamp. The coincidence searching algorithm processes the LM data to group consecutive hits lying inside a coincidence time window of 20 ns. Within a group of coincident hits, energies of hits whose reciprocal distance is below 1.45 mm are added together and the new position assigned to the E-weighted centroid. The merging radius is chosen in order to recover up to 99% of the deposited energy that secondary particles can deposit away from the original impact point (Fig. 2). After merging, only coincidences with two hits are considered, with both the corresponding energies equal to 511 keV ± 8 keV.
Fig. 2.
Deposited energy profile after photoelectric interaction of 500 keV photons in CdTe material. The gaps in the distribution are due to the pixelization of the CdTe detector where the energy collected in one pixel is always assigned to the center of the pixel.
Approximately one third of 511 keV photons in CdTe undergo one or more Compton scatterings before the final photoelectric interaction and the total photon energy must be reconstructed out of a multiple hit signature with fraction of the energy deposited in distant voxels. The high granularity of the VIP detector offers the possibility of defining elaborated algorithms to reconstruct the Compton sequence and recover the otherwise ambiguous events. The complexity of such algorithms is independent of the flexibility of the ASIC since they are applied offline to the LM data set. Currently, five different algorithms have been studied to identify the first impact point of a multi-hit sequence.
Choose the pixel with the highest energy deposition.
Choose the pixel with the second highest energy deposition.
Choose the pixel with minimum energy deposition.
Choose the pixel with the smallest radius (pixel closer to the source).
Base the choice on the reconstruction of the Compton angle.
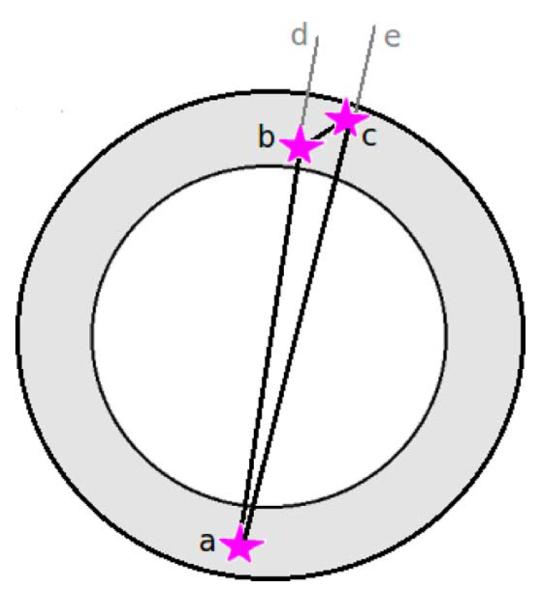
When applied to the VIP detector, the first algorithm gives 58.37% of true LORs while the second reaches 66.6%. With the third method we get 60.4% of correct LORs. The algorithm number 4 is based on the fact that with 511 keV photons the probability of a forward Compton is bigger than the probability of a back scattering. This algorithm finds 70% of right first interaction points. Finally, algorithm number 5, in its current version, is applied only to events with three total hits from the two back to back photons where one of the two photons is assumed to undergo directly a photoelectric interaction (Fig. 3, hit a) while the other one suffers a single Compton scattering before being completely absorbed (Fig. 3 hits b and c). In order to define which hit comes first between b and c, two possible Compton angles dbc and ecb are calculated from the Compton equation
| (1) |
where Eγ is initial photon energy, E’γ is energy of the photon after the Compton scattering, m0c2 is the photon rest energy and θc is a Compton angle. The same Compton angles can be calculated from the geometrical factors (spatial coordinates of the hits b and c). The configuration that best agrees with the Compton equation is chosen. This method yields 81.5% of correct LORs and it is very effective mostly because of the excellent energy and spatial resolution of the pixelated CdTe detectors.
Fig. 3.
Example of a coincidence event where one of the two photons is directly absorbed with a photoelectric interaction without scattering inside the detector (hit a) and the other photon suffers only a single Compton scattering (hits b and c). As one can see, in this case there are two possible LORs: ab and ac.
The choice of the right algorithm to use in the present analysis aims to maximize both the purity of the Compton reconstructed sample, and the selection efficiency. The best trade-off is obtained with algorithm number 4 because the fifth algorithm, though providing the best signal purity, is penalized in terms of efficiency because in its current version it works only with three-hit events. More elaborated algorithms are currently under study.
Since the geometry of the VIP is optimized for brain scan, the system is evaluated following the prescriptions of the National Electrical Manufacturers Association (NEMA) NU 2-2001 [14] standard for WB and head PET scanners. Nevertheless, despite the relatively big FOV, the VIP performance is closer to a high-resolution high-sensitivity small-FOV PET than to a WB PET. For this reason, the system is put through further testing following the NEMA NU 4-2008 [15] protocol for small animal PET.
III. Results
A. Scatter Fraction
For brain scanners, the NEMA NU 2-2001 protocol recommends to use a 19-cm-long cylindrical phantom filled with nonradioactive water and with a diameter of 20 cm. Inside the phantom, a 185-mm-long line source with a diameter of 2 mm and filled with 18F is placed at three different radial positions: 0, 45, and 90 mm. The activity of the source is low enough to have negligible random event rate. The protocol prescribes to estimate the average scatter fraction (SF) as
| (2) |
where Cs is the number of scatter counts and Ctot is the sum of true plus scattered counts. The subscripts 1, 2, and 3 correspond to three radial positions of the line source: 0, 45, and 90 mm, respectively. With real detectors the number of scattered events should be estimated from a sinogram profile. However, since the test is based on simulated data, the exact number of true, scattered, and random events are known. The average SF of the VIP is 3.95% while state-of-the-art brain PET scanners struggle to fall below 30%.
For comparison with small animal PET systems, we performed a test following the NEMA NU 4-2008 protocol. The VIP SF is measured using the mouse-size phantom, a line source filled with 18F, placed at a radial distance of 10 mm from the axis of a high density polyethylene, 25-mm-diameter × 70-mm-long cylinder. The data are acquired at low counting rate. The SF is required to be calculated as
| (3) |
In this case the VIP SF is 0.73%, while current state-of-the-art brain PET scanners and small animal PET systems hardly achieve SF < 10% for the same phantom. The unprecedented signal purity of VIP is due to the excellent energy resolution of the CdTe detectors and the resulting narrow energy acceptance window, something not easy to achieve with scintillating crystals.
B. Counting Rate
The purpose of the counting rate performance test is to measure the effect of the system dead time and the amount of random coincidence events at several levels of source activity. The test is based on the same cylindrical phantom as for the NEMA NU 2-2001 SF test, but this time the phantom is entirely filled with an 18F solution. The initial activity is 562 MBq and gets reduced down to 10 MBq, so that the total, true, random, and noise equivalent count (NEC) rates are measured at different levels of activity and plotted in Fig. 4 as a function of both total and specific activity, the latter computed as the total activity divided by the phantom volume (5966 mL). The NEC rate (RNEC) is defined as the count rate which would have resulted in the same signal-to-noise ratio in the absence of scatter and random events
| (4) |
where Rt, Rs, Rr are the true, scatter, and random count rate, respectively. The peak true counting rate (Rt,peak) and peak NEC rate (RNEC,peak) with the corresponding specific activity values at,peak and aNEC,peak are reported in Table I.
TABLE I.
Counting Rates Measurements of the VIP
| Parameter | NEMA NLI 2-2001 | NEMA NU 4-2008 |
|---|---|---|
| RNEC,peak(kcps) | 122 | 908 |
| aNEC,peak(kBq/mL) | 5.3 | 1600 |
| Rt,peak(kcps) | 152 | 989.3 |
| at,Peak(kBq/mL) | 9.43 | 1600 |
Additionally, the counting performance test is repeated following the NEMA NU 4-2008 guidelines. This test is based on the mouse-size phantom described in Section III-A. The initial activity is the same as in the previous test (562 MBq). The total active volume of the phantom is 34.34 mL. The results are shown in Fig. 5 and Table I. The excellent counting performance of the VIP system in both tests is mostly due to the very effective rejection of scattered events.
C. Sensitivity
Two tests are performed to measure the VIP sensitivity. The first test follows the prescriptions of the NEMA NU 2-2001 protocol. The attenuation free measurement is directly performed by simulating a 70-cm-long ideal 511 keV back-to-back gamma line source with no surrounding material. The source is placed in the center of the FOV along the axial direction and has an activity of 5 MBq. As required by NEMA, the activity is low enough for counting losses to be less than 1%, and the random event rate is less than 5% of the rate of true coincidences. The resulting sensitivity is 14.37 cps/kBq.
The second sensitivity test employs a point-like source as described in the NEMA NU 4-2008 standard. In this procedure, a [saru] 1 mm diameter 22Na sphere with an activity of 1 MBq is placed in the center of a 10 × 10 × 10 mm3 acrylic cube. The first measurement is done with the cube positioned in the center of the FOV. The required 104 true coincidences are acquired at this location and as the source is stepped axially with 4 mm increments, covering the axial FOV of the whole scanner. A total of 65 measurements are analyzed and the total sensitivity of the system is calculated as:
| (5) |
| (6) |
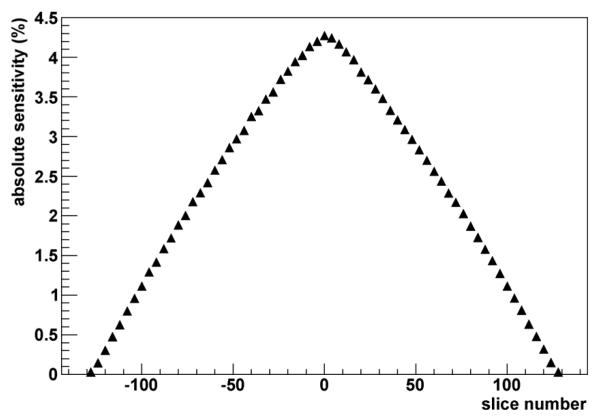
where N is the number of measurements. The VIP total sensitivity is Stot = 21 cps/kBq. The axial sensitivity profile of the VIP scanner obtained by plotting the absolute sensitivity SA for each slice number is shown in Fig. 6. According to the previous tests, the VIP scanner sensitivity is compatible with the average performance of commercial PETs.
Fig. 6.
Axial absolute sensitivity profile along z-axis of the VIP scanner for the energy window of 503–519 keV.
D. Spatial Resolution
The spatial resolution is measured using an 18F point source that is placed into a glass capillary. The point source is a 0.1-mm-diameter water sphere. All dimensions of the capillary are smaller than 1 mm. The source is placed in several different positions across the FOV at 10 mm and 100 mm radial distance from the center of the FOV and for different values of the axial coordinate, as the NEMA NU 2-2001 requires in order to estimate the radial, tangential, and axial resolution separately. At least 105 coincidences are collected per measurement. Images are reconstructed using the single-slice rebinning technique (SSRB) [16] and filtered backprojection (FBP) reconstruction with neither smoothing nor apodization. The values of the full-width at half-maximum (FWHM) of the three spatial components of the measured point-spread function (PSF) are summarized in Table II. As one can see, the VIP PSF is well inside the sub-millimeter domain.
TABLE II.
Spatial Resolution According to NEMA NU 2-2001
| Radial position (mm) | 10 | 100 |
|---|---|---|
| Radial res. (mm) | 0.694 | 0.696 |
| Tangential res. (mm) | 0.694 | 0.902 |
| Axial res. (mm) | 1.327 | 1.904 |
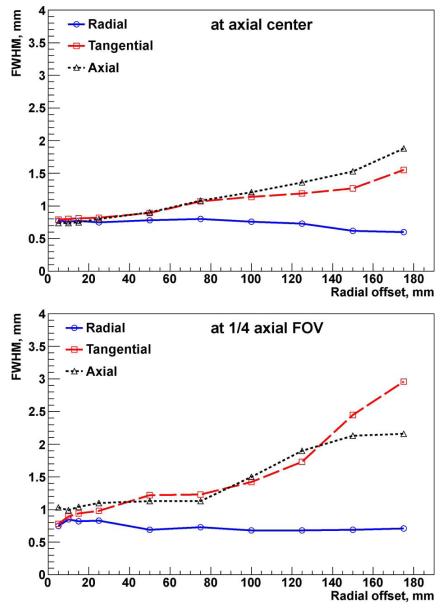
Similar results are currently achievable only with small animal PET systems for pre-clinical studies that have a very limited FOV. To compare VIP with such small scanners, the spatial resolution is also measured using the NEMA NU 4-2008 standard. For the test a 22Na point source inside the 10 × 10 × 10 mm acrylic cube is used according to the protocol. At least 105 coincidences are collected per measurement. Images are reconstructed using the SSRB and 2-D FBP reconstruction with no smoothing. The FWHM of the response PSF in all three directions is measured. The results of the radial, tangential, and axial spatial resolution in terms of FWHM are graphically presented in Fig. 7 as a function of radial and axial offsets. The slice thickness of the reconstructed image is 0.5 mm and the pixel size is 0.25 mm.
Fig. 7.
VIP radial, tangential, and axial spatial resolution as a function of radial offset reported as FWHM.
In [17] it is shown that in the case of an ideal detector with zero energy resolution, full angular coverage, infinite sensitivity, and capable of reconstructing the photon hit position within a 1 mm3 voxel, the best achievable resolution is 0.8 mm. Fig. 7 shows that the VIP perform very closely to such an ideal detector.
E. NEMA NU 4-2008 Image Quality Test
The test is based on the NEMA NU 4-2008 standard prescriptions. The document requires to place a special image quality phantom in the center of the FOV of the scanner with no surrounding material. However the VIP scanner is designed to achieve images of very high quality in challenging conditions as, for instance, the imaging of a human head. Therefore, the system is expected to be relatively immune from scattered events contamination with no deterioration of the imaging performance in the presence of a dense scattering volume between the emitting source and the scanner ring. For this reason, two different tests are performed. In the first one, the phantom is placed exactly as the protocol requires. In the second test, the same phantom is placed into a water sphere with a radius of 150 mm. A total of 1.5 million coincidence sinograms are collected to reconstruct the image in both cases for a total 3.7 MBq activity in the FOV. This number of coincidences corresponds to 19-s scan time for the case when the phantom is not surrounded by the water sphere. In both cases the image is reconstructed with the FBP algorithm. Neither attenuation, scatter, nor random corrections are applied. Since data come from simulation, no normalization is necessary. The pixel size is 0.5 mm and the slice thickness is 2 mm. A generalized Hamming window is used for filtering in order to limit the amplification of statistical noise. Fig. 8 presents the reconstructed images of the hot rods, uniform region, and cold inserts for both the cases.
Fig. 8.
Transverse images of the image quality phantom reconstructed using FBP algorithm with 1.5 · 106 coincidences. Top row: the phantom is in air; bottom row: phantom is in the water sphere; left: hot rods; middle: cold inserts; right: uniform region.
The NEMA NU 4-2008 protocol requires to calculate several parameters to assess image quality, namely the recovery coefficients (RC) for each rod, the minimum, the maximum and the mean uniformity values of the center part of the phantom, and the spill-over ratio (SOR) of the two cold regions. Results are summarized in Table III. The image quality parameter obtained for the standard test, the one with the phantom in air, is compatible with the results generally achieved by current small animal PETs. Nevertheless, one should keep in mind that the simulated VIP ring is larger than currently available small animal PETs and that the results are obtained with a dataset that is 60 times smaller than the one prescribed by NEMA (19 s instead of the required 20 min data taking). This is due to the combination of the high purity of the VIP data, the seamless geometry, and a stopping power of 4 cm CdTe.
TABLE III.
Image Quality Parameters Comparison for NEMA NU 4-2008 Phantom Placed in Air and in Water
| Parameter | In air | In water |
|---|---|---|
| RC(%STD) 1 mm | 0.106(36.2%) | 0.0929(26.5%) |
| RC(%STD) 2 mm | 0.352(16.7%) | 0.323(20%) |
| RC(%STD) 3 mm | 0.621(16.4%) | 0.578(18.4%) |
| RC(%STD) 4 mm | 0.821(18.7%) | 0.797(15.9%) |
| RC(%STD) 5 mm | 0.939(17.1%) | 0.924(16.9%) |
| Uniformity max. | 1.13 | 1.2 |
| Uniformity min. | 0 | 0 |
| Uniformity mean | 0.764 | 0.798 |
| Uniformity %STD | 15.9% | 15.6% |
| SOR(%STD) water | 0.184(24.3%) | 0.168(22.9%) |
| SOR(%STD) air | 0.211(26.4%) | 0.207(25.9%) |
The comparison of the two images in Fig. 8 and the values of the parameters in Table III show no significant deterioration of the VIP image quality in the presence of a dense scattering volume. This is a unique feature of the VIP scanner, not achievable by standard devices based on scintillating crystals and it is again due to the excellent energy resolution of CdTe and the resulting very narrow energy acceptance window.
IV. Discussion
The VIP project is under development and the results presented here are based on simulation using GAMOS software package. We are aware that it is not completely fair to compare the VIP simulation results with the current state of the art PET systems. Nevertheless, to convince ourselves on how good GAMOS is in mimicking reality, we have used the ECAT HRRT [18] geometry and simulated it with GAMOS. The results of this exercise are summarized in Table IV. Over all one can see that GAMOS prediction is very realistic. For example, the spatial resolution of HRRT in the center of the FOV is predicted within an error of 7%, and about 20% at 10 cm from the center of the FOV. The error in predicting the scatter fraction is about 15%, while the error in predicting total sensitivity is on average around 25%. Therefore we can say with more confidence that what we have claimed is not far from the reality given that we have used as an input to GAMOS the pixel detector response that has been measured in the lab for both energy resolution and time coincidence.
TABLE IV.
Comparison of the Characteristics of the Simulated ECAT HRRT Scanner With GAMOS and the Data Published in [18]
| Our simulated data | Published data | ||
|---|---|---|---|
| Transverse radial | 2.16 | 2.3 | |
| Spatial resolution (mm) near the center of the FOV | Transverse tangential | 2.16 | 2.3 |
| Axial | 2.78 | 2.5 | |
| Transverse radial | 2.24 | 3.2 | |
| Spatial resolution (mm) at 10 cm radius from the center of the FOV | Transverse tangential | 2.23 | 3.2 |
| Axial | 3.6 | 3.4 | |
| Scatter fraction | 52% | 45% | |
| Total sensitivity | 3.9% | 2.5% - 3.3% |
At the same time we are aware of the intrinsic limitations of Cd(Zn)Te detectors. For example, such detectors cannot be used for TOF because of slow charge collections. Moreover the detector suffers polarization effects and we need to cycle the HV bias every 5 min to ensure the FWHM is 1.6% at 511 keV. Nevertheless, successful small scale implementations of CdTe PET systems have already been achieved as documented in [3].
To make our results more robust, in addition to the ECAT HRRT exercise, we repeated the VIP simulation assuming considerably worse values for some critical parameters. For example, we smeared the energy resolution from 1.6% to 5%, increased the amount of passive material (kapton and electronic) by 100%, that is doubling it, and we assumed a 1% randomly distributed dead channels. From Table V we realize that the biggest impact is on the SF, that has increased by a factor of 3, and on the total sensitivity, that has dropped by 25%, but overall the VIP scanner in such a worst case scenario is still performing considerably well.
TABLE V.
Comparison of the Simulated VIP Scanner Performance With Standard and Smeared Parameters
| Standard | Smeared | ||
|---|---|---|---|
| Transverse radial | 0.694 | 0.799 | |
| Spatial resolution (mm) near the center of the FOV | Transverse tangential | 0.694 | 0.799 |
| Axial | 1.3 | 1.5 | |
| Transverse radial | 0.696 | 0.739 | |
| Spatial resolution (mm) at 10 cm radius from the center of the FOV | Transverse tangential | 0.902 | 1.024 |
| Axial | 1.904 | 2.688 | |
| Scatter fraction | 3.95% | 11.26% | |
| Total sensitivity (cps/kBq) | 14.37 | 11.41 | |
| NEC peak (kcps) | 122 | 88 | |
| NEC peak activity (MBq) | 56.2 | 31.6 |
Marketwise, we are aware that the VIP is rather an expensive PET scanner when compared with typical PET based on scintillating crystals. However, the price is comparable to the CdTe PET described in [3], with the exception that the pixel resolution of VIP is better, it has less dead material, and it is more compact in size. The main cost of the VIP is dominated by the price of the CdTe detector, which we expect to drop significantly in the future when there is a large market (supply and demand) which is emerging because CdTe is becoming a future material for the new generation of CT scanners.
V. Conclusion
The VIP project aims to build a prototype of a compact PET with high spatial and energy resolution, optimized for brain metabolism study. The sensitivity and imaging performances of the new design are evaluated by computer simulation following the recommendations of the NEMA NU 2-2001 and the NEMA NU 4-2008 standards.
In this work it is shown that the VIP novel PET design, initially presented in [19], has the potential to provide a clinical head scanner with combination of excellent spatial and energy resolutions fused with high detection sensitivity, that are currently achievable only by small animal PET employed in research [17].
The crack-free geometry of the VIP scanner with a stopping power of 4 cm CdTe provides a sensitivity of 14.37 cps/kBq (according to NEMA NU 2-2001 standard). A very low scatter fraction is achieved due to the good energy resolution provided by the CdTe detectors. The high number of channels in the VIP scanner (450 channels/cm3) makes the full system less affected by the dead time of the individual detector voxels. This effect, together with the good energy resolution, leads to a very good NEC rate. The calculated NEC peak values are 908 kcps at 1.6 MBq/mL for the NEMA NU 4-2008 mouse phantom, and 122 kcps at 5.3 kBq/mL for the NEMA NU 2-2001 test. The high sensitivity together with the virtually noise-free data acquisition allows to dramatically reduce the scan time and thereby reduce the image blurring due to the motion of the patient. On the other hand, the patient dose can be significantly reduced while keeping the time of the screening the same. It permits to perform a large number of consecutive low-dose scans in order to control and accurately follow the disease treatment or the drug development.
Tests based on both NEMA NU 2-2001 and NEMA NU 4-2008 standards show excellent spatial resolution (~ 1 mm) in the FOV center. Nowadays, such a good spatial resolution is achievable only by small pre-clinical state-of-the-art PET scanners. Moreover, simulated images acquired with the VIP are generally characterized by high contrast and low noise, and are obtained with a very small number of coincidences. The system is shown in simulations to be capable to detect down to 1-mm-diameter hot rods without background activity with only few seconds scan time needed to get good quality images. No deterioration of the image quality is observed in the presence of a dense scattering volume between the imaged source and the detector ring. This effect is again due to the excellent energy resolution of CdTe and the resulting very narrow energy acceptance window. This is a unique feature of the VIP scanner, not achievable by standard devices based on scintillating crystals.
Though the authors are aware of the big engineering challenge of translating the simulation findings into a real working device, the results of this study are very promising and show the high potential of the VIP concept for the future PET generation.
Acknowledgments
This work has been supported by FP7-ERC-Advanced Grant 250207.
Contributor Information
Gianluca De Lorenzo, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Mokhtar Chmeissani, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Machiel Kolstein, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Mario Cañadas, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT), 28040 Madrid, Spain..
Pedro Arce, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT), 28040 Madrid, Spain..
Yonatan Calderón, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Dilber Uzun, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Gerard Ariño, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
José Gabriel Macias-Montero, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Ricardo Martinez, Centro Nacional de Microelectrónica, IMB-CNM(CSIC), Campus Universidad Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Carles Puigdengoles, Institut de Física d’Altes Energies (IFAE), Edifici Cn, Universitat Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
Enric Cabruja, Centro Nacional de Microelectrónica, IMB-CNM(CSIC), Campus Universidad Autònoma de Barcelona (UAB), E-08193 Bellaterra, Spain..
References
- [1].Voxel Imaging PET (VIP) pathfinder project [Online] Available: www.vip-erc.com.
- [2].Shiga T, Morimoto Y, Kubo N, Katoh N, Katoh C, Takeuchi W, Usui R, Hirata K, Kojima S, Umegaki K, Shirato H, Tamaki N. A new PET scanner with semiconductor detectors enables better identification of intratumoral inhomogeneity. J. Nucl. Med. 2009;50(1):148–155. doi: 10.2967/jnumed.108.054833. [DOI] [PubMed] [Google Scholar]
- [3].Morimoto Y, Ueno Y, Takeuchi W, Kojima S, Matsuzaki K, Ishitsu T, Umegaki K, Kiyanagi Y, Kubo N, Katoh C, Shiga T, Shirato H, Tamaki N. Development of a 3D brain PET scanner using CdTe semiconductor detectors and its first clinical application. IEEE Trans. Nucl. Sci. 2011 Oct.58(5):2181–2189. [Google Scholar]
- [4].Vaska P, Kim DH, Southekal S, Pratte JF, Fried J, Krishnamoorthy S, Stoll S, Bolotnikov A. Ultra-high resolution PET: A CZT-based scanner for the mouse brain. J. Nucl. Med. 2009;50(2):293–293. [Google Scholar]
- [5].Vaska P, Dragone A, Lee W, Kim DH, Pratte JF, Cui YG, Fried J, Krishnamoorthy S, Bolotnikov A, Park SJ, O’Connor P, Dilmanian FA, James RB. A prototype CZT-based PET scanner for high resolution mouse brain imaging. IEEE Nucl. Sci. Symp. Conf. Rec. 2007;5:3816–3819. [Google Scholar]
- [6].De Lorenzo G, Chmeissani M, Uzun D, Kolstein M, Ozsahin I, Mikhaylova E, Arce P, Cañadas M, Ariño G, Calderón Y. Pixelated CdTe detectors to overcome intrinsic limitations of crystal based positron emission mammographs. J. Instrum. 2013 Jan.8:pii C01030. doi: 10.1088/1748-0221/8/01/C01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Calderon Y, Kolstein M, Uzun D, De Lorenzo G, Chmeissani M, Arce P, Arino G, Cabruja E, Canadas M, Macias-Montero JG, Martinez R, Mikhaylova E, Ozsahin I, Puigdengoles C. Modeling, simulation, and evaluation of a Compton camera based on a pixelated solid-state detector. IEEE Nucl. Sci. Symp. Conf. Rec. 2011:2708–2715. [Google Scholar]
- [8].Ariño G, Chmeissani M, Puigdengoles C, De Lorenzo G, Diener R, Arce P, Cabruja E, Calderon Y, Canadas M, Kolstein M, Macias-Montero JG, Martinez R, Mikhaylova E, Ozsahin I, Uzun D. Characterization of CdTe detector for use in PET. IEEE Nucl. Sci. Symp. Conf. Rec. 2011:4598–4603. [Google Scholar]
- [9].Karp JS, Freifelder R, Geagan MJ, Muehllehner G, Kinahan PE, Lewitt RM, Shao L. Three-dimensional imaging characteristics of the HEAD PENN-PET scanner. J. Nucl. Med. 1997;38(4):636–643. [PubMed] [Google Scholar]
- [10].Macias-Montero JG, Sarraj M, Chmeissani M, Puigdengoles C, De Lorenzo G, Martinez R. Toward VIP-PIX: A low noise readout ASIC for pixelated CdTe gamma-ray detectors for use in the next generation of PET scanners. IEEE Trans. Nucl. Sci. 2013 Aug.60(4):2898–2904. doi: 10.1109/TNS.2013.2270115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arce P, Lagares JI, Harkness L, Perez-Astudillo D, Cañadas M, Rato P, Prado MD, Abreu Y, De Lorenzo G, Kolstein M, Dìaz A. GAMOS: A framework to do Geant4 simulations in different physics fields with an user-friendly interface. Nucl. Inst. Meth. Phys. Res. A. 2013 [Google Scholar]
- [12].Sellin PJ, Davies AW, Lohstroh A, Ozsan ME, Parkin J. Drift mobility and mobility-lifetime products in CdTe:Cl grown by the travelling heater method. IEEE Trans. Nucl. Sci. 2005 Dec.52(6):3074–3078. [Google Scholar]
- [13].Ariño G, Chmeissani M, De Lorenzo G, Puigdengoles C, Cabruja E, Calderòn Y, Kolstein M, Macias-Montero JG, Martinez R, Mikhaylova E, Uzun D. Energy and coincidence time resolution measurements of CdTe detectors for PET. J. Instrum. 2013 Feb.8:pii C02015. doi: 10.1088/1748-0221/8/02/C02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nat. Electr. Manufact. Assoc. Performance measurements of positron emission tomographs. (NEMA Standards Pub. NU 2-2001). [Google Scholar]
- [15].Nat. Electr. Manufact. Assoc. Performance measurements for small animal positron emission tomographs. (NEMA Standards Pub. NU 4-2008). [Google Scholar]
- [16].Daube-Witherspoon ME, Muehllehner G. Treatment of axial data in three-dimensional PET. J. Nucl. Med. 1987 Nov.28:1717–1724. [PubMed] [Google Scholar]
- [17].Stickel JR, Cherry SR. High-resolution PET detector design: Modeling components of intrinsic spatial resolution. Phys. Med. Biol. 2005 Jan.50:179–195. doi: 10.1088/0031-9155/50/2/001. [DOI] [PubMed] [Google Scholar]
- [18].de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA. Performance evaluation of the ECAT HRRT: An LSO-LYSO double layer high resolution, high sensitivity scanner. Phys. Med. Biol. 2007;52:1505. doi: 10.1088/0031-9155/52/5/019. [DOI] [PubMed] [Google Scholar]
- [19].Chmeissani M, Arce PP, Cañadas M. Modeling and simulation of PET scanner based on pixelated solid-state detector. IEEE Nucl. Sci. Symp. Conf. Rec. 2009:3496–3502. [Google Scholar]