Antepartum depression (APD) and postpartum depression (PPD), disorders characterized by mood changes during pregnancy and after delivery, negatively impact maternal and child physical and mental health 1–4. Prevalence rates range from 4.8% to 18.4% for minor and from 5.1% to 12.7% for major depression 5–7. APD and PPD, also collectively referred to as perinatal depression, share their diagnostic criteria with major depression, but have their onset during pregnancy or within four weeks after delivery, respectively 8.
Considerable evidence indicates that women with a lifetime history of depression 9–11, high levels of stress 12–15, anxiety 16,17, and poor social support 10,11 during pregnancy are at increased risk for perinatal depression. Nevertheless, a great deal of variation in perinatal depression remains unexplained. A growing body of evidence suggests that activity of the stress-responsive hypothalamic-pituitary-adrenal axis and its end-product cortisol also may be associated with perinatal emotional well-being 17–21. Depressive symptoms have been linked to increased basal cortisol levels 22, an increased cortisol response to awakening 23–27 and poorer cortisol recovery after psychological stress 28. While increases in cortisol over the course of gestation are normative and adaptive 29, excessive elevations of maternal stress hormones have been implicated in the development of PPD 21,30–32.
A number of successful preventive intervention efforts targeting psychosocial and physiological risk factors for perinatal depression have utilized mind-body practices, which embody the idea that the mind interacts with the body to influence physical functioning, improve symptoms, and promote health 33. Yoga has provoked particular interest given its increasing acceptance in the West 34 and the growing evidence of its association with improvements in affect 35–38, decreases in depressive symptoms 39–41, and reductions in cortisol 36,42–45 in non-pregnant populations. Nascent work suggests that the psychophysiological benefits of yoga may extend to pregnancy. Evidence from non-randomized trials suggests that yoga practice is associated with reduced risk of low birth weight and preterm labor 46. Randomized controlled trials further suggest that perinatal anxiety 47,48, perceived stress 46, psychological health 49, and autonomic nervous system responses to stress 50 can be improved and the incidence of pregnancy-related hypertension alleviated 51 with yoga. Furthermore, there is preliminary evidence that yoga practice can help reduce depressive symptoms during pregnancy 52–56.
The present study contributes to the existing literature by examining the effectiveness of yoga on APD and PPD symptoms. The first aim was to evaluate the immediate effects of a prenatal yoga session on cortisol and affect at two gestational ages. We proposed that women practicing yoga during pregnancy would show greater decreases in cortisol and greater improvements in affect in response to a 90-minute yoga session relative to a “usual activity” within-subject comparison condition, and a between-subject control group. The second aim was to evaluate the effects of prenatal yoga practice on APD and PPD symptoms. We hypothesized that women practicing yoga during pregnancy would show fewer APD and PPD symptoms relative to a between-subject control group. Because psychological and physiological states have been linked to depressive symptoms, we further posited that cortisol and negative affect would be positively associated, and positive affect negatively associated with perinatal depressive symptoms.
METHOD
Participants
Women practicing yoga during pregnancy (yoga group) were recruited from two yoga studios in Southern California. Women who did not practice yoga or other relaxation techniques during pregnancy (control group) were recruited from an ongoing unrelated study of perinatal depression (data unpublished) via obstetrician referrals and community advertisements. Women were eligible to participate if they were at least 18 years old, English-speaking, nulliparous, between 12 and 19 weeks’ gestational age, and self-reported no current depressive and/or anxiety disorder diagnosis. All participants provided written informed consent. The study was approved by the Institutional Review Board of the University of California, Irvine.
Procedure
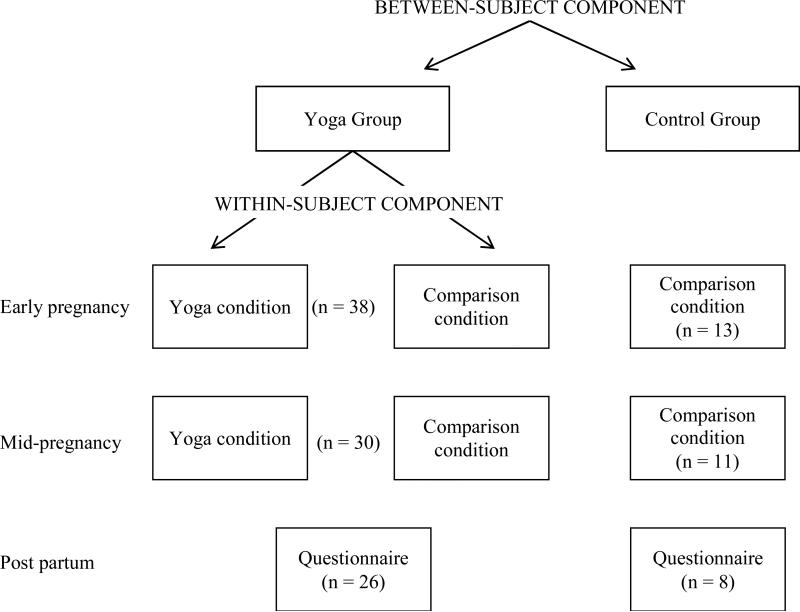
This study employed a mixed within- and between- subject design (Figure 1). Women completed assessments in early and mid-pregnancy and within two months post partum.
Figure 1.
Study Design and Participant Flow Chart
Yoga Group
Women in the yoga group completed antepartum assessments in the yoga studio and on a separate day in their typical environment (“usual activity” assessments). To account for circadian variations in cortisol secretion, all yoga studio assessments were scheduled between 3:30 p.m. and 8:30 p.m. and were matched for time with usual activity assessments. Women were instructed to abstain from eating, drinking caffeine, and engaging in strenuous physical activity for one hour prior to saliva collection.
In early pregnancy, women came to the yoga studio and five minutes before the start of the yoga session provided a baseline saliva sample and completed a brief questionnaire assessing their current affect, the Derogatis Affects Balance Scale (DABS) 57. Women then participated in a 90-minute session of prenatal Hatha yoga, a type of yoga emphasizing physical, mental and breathing techniques to condition the body, focus the mind, and connect the body and mind 58. Each session, taught by studio-specific certified prenatal yoga instructors collaborating on the project, consisted of 10-15 minutes of dialogue regarding pregnancy-related concerns and gestational age-specific modifications, 60 minutes of asanas (i.e., body postures), 10 minutes of stretching, and 5-10 minutes of savasana (i.e., final relaxation), with pranayama (i.e., breathing) instruction throughout the practice. A typical class emphasized squat poses, balance poses, chest and hip openers, and restorative postures with props. Immediately after the session, women again completed the DABS and provided a saliva sample.
Approximately two days later, using materials provided earlier, women completed the DABS and provided saliva samples at times exactly matching those at the yoga studio. In the 90-minute interval between assessments, women completed questionnaires assessing sociodemographic and health information, behavioral characteristics, and depressive symptoms and then engaged in light activities of their choosing (e.g., reading, watching TV) until collection of the second saliva sample. Medication Event Monitoring System (MEMS; AARDEX, Zurich, Switzerland) caps were used to document adherence to collection times.
In mid-pregnancy, women again completed all procedures as described above and answered questionnaires assessing changes in health and behaviors since early in pregnancy. Within two months after delivery, women were mailed a questionnaire inquiring about past-week depressive symptoms. Thus, yoga group participants were assessed on five occasions: twice each in early and mid-pregnancy, and once within two months of delivery. Participating women received a 20% discount on their prenatal yoga class series.
Control Group
Control group participants completed assessments identical to those described for the yoga group only on days of usual activity at each gestational age. Timing of assessments with regard to gestational/postpartum week and time of day was matched to that of yoga group assessments. Women in the control group received a modest monetary incentive for participation.
Measures
Saliva collection and cortisol assay
Cortisol was collected with cotton swabs (Salivettes, Sarstedt, Nümbrecht, Germany). Samples were stored at room temperature (yoga days) or in a refrigerator (days of usual activity) until transported to the laboratory the following day where they were stored at -80°C until assayed. After thawing for biochemical analysis, samples were centrifuged for 10 minutes at 2,000g and 4°C. Free cortisol in saliva was determined in duplicate by a commercially available enzyme immunoassay (ELISA, IBL-America, Minneapolis, Minnesota). Inter-and intra-assay coefficients of variance are less than 4.9% and 4.1% respectively, and the sensitivity of the assay is reported at 0.012 ng/mL.
Affect
Affect was assessed with the DABS 57, which has the advantages of measuring both affective valance (positive, negative) and activation (high, low) and being suitable for use with both clinical and non-clinical populations. Participants rated to what extent (1=not at all, 5=extremely) they were currently experiencing each of 40 affect-based adjectives that constitute four positive (joy, contentment, vigor, affection) and four negative (anxiety, depression, guilt, hostility) affects. A positive affects total and a negative affects total score were computed. Internal consistency of the DABS ranges from α = .79 to α = .92 and test-retest correlations range from r = .78 to r = .84 59.
Depressive symptoms
Depressive symptoms were assessed with the 9-item Center for Epidemiologic Studies Depression Scale (CES-D) 60. Participants were asked how frequently they experienced a set of feelings and engaged in certain behaviors in the last week on a scale ranging from 0 = rarely or none of the time (less than 1 day) to 3 = most or all of the time (5-7 days). The internal consistency of the 9-item CES-D has been demonstrated (α = .87) 60.
Yoga, relaxation technique, and exercise practice
Researcher-created questions assessed the timing, frequency, and duration of lifetime and prenatal practice of relaxation techniques, including yoga. Additional questions assessed the frequency of exercise in the year prior to the woman's pregnancy and the average weekly frequency of strenuous, moderate, and mild intensity exercise during the current pregnancy. Participants’ activity levels between cortisol samples in the usual activity comparison condition and the control group were assessed by self-report.
Statistical analyses
Cortisol and negative affect values were positively skewed and thus were log transformed. To evaluate the immediate effects of a prenatal yoga session on cortisol and affect relative to a usual activity comparison condition and a control group (Aim 1), Generalized Estimating Equations (GEEs) were performed. A Gaussian family distribution, identity link and exchangeable correlation structure were specified to evaluate the effects of time (pre-to-post), condition (yoga, usual activity) or group (yoga, control), and gestational age (early, mid).To evaluate the effects of prenatal yoga practice on APD and PPD symptoms (Aim 2), one-way between-groups analyses of covariance (ANCOVA) were performed comparing symptoms in yoga and control groups. To evaluate the bivariate relationships of cortisol and affect with perinatal depressive symptoms, average values and pre-post difference scores were computed. Pearson product-moment correlations were performed when data were normally distributed and Spearman rank correlations when data were not normally distributed. Multiple regression examined the relative contribution of selected summary measures to perinatal depressive symptoms. Data were analyzed using Stata 12 (StataCorp, College Station, Texas). Unstandardized beta coefficients (B) and two-tailed p-values are reported.
RESULTS
Participants
Fifty-one pregnant women enrolled in this study at a mean of 15.16 weeks’ gestational age (SD = 1.29, range = 12 to 19). At the mid-pregnancy assessment (M = 25.88 weeks’ gestational age, SD = 1.95, range = 22 to 31), 43 women were retained; 34 women completed the postpartum questionnaire (retention rate: 64%) (Figure 1).
Most yoga group participants (87%) had previous experience with yoga, ranging from several months to 10 years. A majority of women reported practicing yoga at least once a week in early (92%) and mid- (66%) pregnancy. Neither differences in change in cortisol nor affect were observed as a function of yoga studio (all n.s.). Data from both studios were therefore combined.
Control group participants were younger, less likely to be White, less educated, had lower income, and were less likely to be married at the time of recruitment than yoga group participants (all ps < .05), but did not differ from yoga group participants with regard to gestational age at each assessment (Table 1). Importantly, there were no group differences in depressive symptoms at study onset, affect on days of usual activity during pregnancy, and frequency of exercise participation (all n.s.). Women who did not complete the study did not differ from those who remained in the study with regard to sociodemographic characteristics or depressive symptoms at study onset (all n.s.).
Table 1.
Baseline Sociodemographic and Obstetric Characteristics by Yoga and Control Group
| Yoga Group n = 38a | Control Group n = 13 | ||
|---|---|---|---|
| n(%) | n(%) | ||
| Race* | Asian | 3(8) | 0(0) |
| White | 28(74) | 5(39) | |
| Other (e.g., Mixed, Hispanic or Latino) | 7(18) | 8(61) | |
| Marital status* | Single | 1(3) | 4(31) |
| Married | 34(90) | 9(69) | |
| Divorced/Separated | 1(3) | 0(0) | |
| Education** | Elementary/ Junior/High School | 5(13) | 7(58) |
| Associates Degree | 3(8) | 2(17) | |
| Bachelor's Degree | 13(34) | 2(17) | |
| Graduate Degree | 17(45) | 1(8) | |
| Annual household income* | ≤$15,000 | 0(0) | 1(8) |
| $15,000- $35,000 | 4(11) | 5(39) | |
| $35,000- $50,000 | 2(5) | 2(15) | |
| $50,000- $100,000 | 6(16) | 2(15) | |
| $100,000- $150,000 | 13(34) | 2(15) | |
| ≥ $150,000 | 13(34) | 1(8) | |
| M(SD) | M(SD) | ||
|---|---|---|---|
| Gestational age (weeks) | Early pregnancy | 15.16(1.18) | 15.16(1.63) |
| Mid-pregnancy | 25.87(1.98) | 25.91(1.97) | |
| Post partum | 8.91(4.29) | 8.67(1.86) | |
| Chronological age** | 32.95(5.51) | 26.85(5.13) |
Two participants in the yoga group dropped out of the study after the first yoga session and demographic information is not available for these participants.
Note. p-values are associated with Fisher's exact tests for categorical variables or t-tests for continuous variables, as appropriate.
p <.05
p <.01
Compliance with data collection in the usual activity condition and the control group
Among the 34 participants for whom these data were available in early pregnancy, MEMS cap openings occurred 17.79 (SD = 30.14) and 16.03 (SD = 35.91) minutes from the scheduled times of saliva collection, indicating good compliance. Among the 21 participants for whom these data were available in mid-pregnancy, MEMS cap openings occurred 33.05 (SD = 60.35) and 23.76 (SD = 49.88) minutes from the scheduled saliva collection times in mid- pregnancy, confirming acceptable compliance.
During the 90-minute interval between cortisol samplings in early and mid-pregnancy, approximately 75% of women reported not being physically active and 25% reported engaging in light activity. Both groups reported spending approximately 30 minutes completing study questionnaires and the remaining time watching television, talking on the phone, browsing the internet, or doing light housework.
Effects of prenatal yoga on cortisol and affect
Cortisol
Means and standard deviations for cortisol and affect across conditions and groups are reported in Table 2. Within the yoga group, cortisol levels were lower on yoga days relative to days of usual activity (B = .28, SE = .10, p = .005) and, as expected, were lower in early compared to mid-pregnancy (B = .32, SE = .11, p = .003). Cortisol levels decreased over the 90-minute time interval (B = -.29, SE = .10, p = .004); however, there was no indication of a more pronounced decrease in cortisol in response to a yoga session relative to usual activity (B = -.06, SE = .14, n.s.).
Table 2.
Comparison of Affect and Cortisol Between Conditions and Groups (Mean ± SD)
| Yoga Group (n = 38)a | Control Group (n = 12)a | |||||
|---|---|---|---|---|---|---|
| Yoga | Usual Activity | |||||
| Pre | Post | Pre | Post | Pre | Post | |
| Cortisol (ng/mL)bcde | ||||||
| Early Pregnancyf | 0.79 ± .98 | 0.47 ± .55 | 1.25 ± 1.34 | 0.77 ± 1.02 | 1.65 ± 1.62 | 1.46 ± 2.71 |
| Mid-Pregnancy | 1.66 ± 2.43 | 1.35 ± 2.20 | 1.66 ± 1.54 | 1.05 ± .78 | – | – |
| Total Positive Affectcdei | ||||||
| Early Pregnancy | 59.66 ± 12.65 | 64.03 ± 15.28 | 50.49 ± 17.86 | 50.26 ± 16.79 | 53.75 ± 12.14 | 53.00 ± 10.51 |
| Mid-Pregnancyg | 54.28 ± 13.46 | 63.38 ± 12.88 | 52.32 ± 14.91 | 52.30 ± 14.63 | 50.50 ± 16.73 | 48.00 ± 15.61 |
| Contentmentcdegh | ||||||
| Early Pregnancy | 16.53 ± 3.37 | 19.79 ± 3.30 | 14.49 ± 4.90 | 14.66 ± 4.44 | 15.50 ± 3.42 | 15.75 ± 2.56 |
| Mid-Pregnancy | 14.78 ± 4.04 | 19.50 ± 3.44 | 14.19 ± 4.38 | 14.83 ± 4.04 | 14.70 ± 4.14 | 14.50 ± 4.88 |
| Total Negative Affectcgh | ||||||
| Early Pregnancy | 24.58 ± 5.87 | 20.74 ± 1.18 | 24.37 ± 5.41 | 23.03 ± 4.48 | 22.83 ± 5.25 | 22.75 ± 5.21 |
| Mid-Pregnancy | 23.84 ± 3.15 | 20.56 ± 1.11 | 24.58 ± 8.80 | 21.67 ± 2.80 | 26.10 ± 9.70 | 24.70 ± 7.85 |
In mid-pregnancy, Yoga Group n= 32, Control Group n= 10.
Yoga group sample size for cortisol varies due to missing data (n = 29-38). Cortisol values for the control group are not reported due to limited sample size (n = 6).
Significant main effect of time (pre, post)
Significant main effect of GA (early, mid)
Significant main effect of condition (yoga vs. usual activity)
Significant main effect of group (yoga vs. control)
Significant condition × time interaction
Significant group × time interaction
Significant condition × GA interaction
Note. Sensitivity analyses were performed with and without extreme outlying values. No substantial differences were observed and results are therefore presented with extreme outliers included.
Lower cortisol levels were also observed in the yoga relative to the control group in early pregnancy (B = -.50, SE = .23, p = .029). Cortisol levels did not change over time (B = -.28, SE = .18, n.s.) and no differences in trajectories were observed between groups (B = -.01, SE = .20, n.s.). Due to the small number of valid (i.e., uncontaminated, containing sufficient saliva) cortisol samples available for control group participants in mid-pregnancy (n = 6), differences in cortisol between yoga and control groups were not tested.
Positive affect
Positive affect increased over time (B = 4.37, SE = 2.20, p = .047), however this increase was not different across yoga days and days of usual activity (B = -4.59, SE = 3.11, n.s.). Similar to the cortisol findings, positive affect was higher on yoga days relative to days of usual activity (B = -9.17, SE = 2.20, p < .001) and in early compared to mid-pregnancy (B = -6.15, SE = 2.32, p = .008). These main effects were qualified by an interaction between condition and gestational age (B = 7.65, SE = 3.27, p = .019) such that the difference in overall positive affect between the two conditions was greater in early than mid-pregnancy. GEEs therefore were run separately by gestational age and revealed that positive affect was higher on yoga days relative to days of usual activity only in early pregnancy (B = -9.17, SE = 2.05, p < .001). Comparisons of yoga and control groups revealed no change in positive affect over time or across gestation, and no differences between groups (all n.s.).
GEEs were subsequently performed including only the contentment subscale (low-activation positive affect), for which the largest effects were hypothesized. Within the yoga group, contentment increased over time (B = 3.26, SE = .66, p < .001), was higher in early than mid-pregnancy (B = -2.01, SE = .70, p = .004), and was overall higher on yoga days compared to days of usual activity (B = -2.04, SE = .66, p = .002). As expected, contentment increased more in response to a yoga session relative to usual activity (condition x time interaction, B = -3.09, SE = .93, p = .001). Comparisons of yoga and control groups revealed no change in contentment over time or across gestation, and no group differences (all n.s.). However, contentment increased to a greater extent in the yoga relative to the control group (condition x time interaction, B = 3.01, SE = 1.31, p = .021).
Negative affect
Negative affect decreased more in response to a yoga session relative to usual activity (condition x time interaction, B = .04, SE = .02, p = .035); however, a main effect of condition was not detected (B = -.004, SE = .01, n.s.). Between-subject comparisons suggest a similar pattern with negative affect decreasing more in the yoga compared to the control group (B = -.06, SE = .02, p = .007). Significant main effects of group, gestational age or group x gestational age interactions were not detected (all n.s.).
Perinatal depressive symptoms
As shown in Table 3, women in the yoga group reported fewer depressive symptoms than women in the control group in the postpartum period, after adjusting for APD symptoms in early [F (1, 32) = 5.20, p = .030, partial η2= .14] or mid-pregnancy [F (1, 31) = 5.89, p = .021, partial η2= .16]. In contrast, differences in APD symptoms between groups were not detected at either gestational age.
Table 3.
Depressive Symptoms by Gestational Age
| Yoga Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Outcome variable | n | M | SD | n | M | SD |
| Early pregnancy | 38 | 5.29 | 3.14 | 13 | 4.54 | 3.43 |
| Mid-pregnancy | 31 | 5.87 | 3.52 | 10 | 5.00 | 3.62 |
| Post partum* | 26 | 3.92 | 2.68 | 8 | 6.63 | 2.83 |
p < .05
Exploratory one-way ANOVAs and Pearson correlations indicated that depressive symptoms were not dependent on sociodemographic characteristics (all n.s.) and t-tests indicated that APD symptoms were not dependent on frequency of yoga practice (all n.s.). However, women who practiced yoga twice a week or more in the weeks prior to the first assessment reported fewer PPD symptoms (M = 2.13, SD = 1.55) than women who practiced yoga once a week or less (M = 4.72, SD = 2.72; t (24) = 2.51, p < .05).
No significant associations among cortisol and affect with APD symptoms were found. Neither positive nor negative affect were significantly associated with PPD symptoms. The change in cortisol over time on yoga days and average cortisol levels on days of usual activity in mid-pregnancy were significantly and positively associated with PPD symptoms (Table 4). However, when all cortisol summary measures in mid-pregnancy were simultaneously included in a regression model none emerged as significant predictors of PPD symptoms (all n.s.), suggesting that cortisol did not explain the presence of PPD symptoms.
Table 4.
Correlations between PPD symptoms and Cortisol and affect average and change scores
| Early pregnancy | Mid-pregnancy | |||||
|---|---|---|---|---|---|---|
| Cortisol | Affect | Cortisol | Affect | |||
| Positive (Total/Contentment) | Negative | Positive (Total/Contentment) | Negative | |||
| Avg yoga | .12 | .03/.17 | −.11 | −.18 | −.03/.05 | −.10 |
| Diff yoga | −.05 | .24/.30 | .00 | .43* | .12/.16 | −.05 |
| Avg nat env | .10 | −.13/−.15 | .13 | .47* | −.06/−.03 | .17 |
| Diff nat env | .26 | −.12/.21 | −.23 | −.03 | .02/.13 | −.21 |
Note. Avg= average of pre- and post- values; Diff= post value – pre value
p < .05
DISCUSSION
The present study examined whether women practicing yoga during pregnancy would show acute health benefits reflected in more adaptive cortisol and affective responses to a single 90-minute yoga session, and longer-term benefits as reflected by reduced perinatal depressive symptoms. Findings in part support these hypotheses. Women who practiced yoga during pregnancy showed lower mean cortisol levels and higher positive affect on days of yoga practice relative to days of usual activity, greater immediate improvements in contentment and negative affect but not cortisol in response to yoga relative to a usual activity comparison condition and to a control group, and fewer PPD symptoms but not APD symptoms relative to a control group. These findings build on a small but growing body of research suggesting that yoga may confer psychophysiological benefits during pregnancy, and highlight the potential importance of prenatal yoga for postpartum well-being.
The first aim of this study was to investigate the immediate effects of a prenatal yoga session on changes in cortisol and affect. Greater improvements in response to a yoga session than to a usual activity comparison condition and a control group were not detected for cortisol; however, average cortisol levels were lower on days that yoga participants engaged in a yoga session compared to days of usual activity. Overall lower cortisol levels on yoga days may reflect women's expectation of a relaxing, beneficial activity. These expectations may have led to anticipatory reductions in cortisol before the start of the yoga session, thereby reducing the potential of improvement in response to the yoga session itself. In line with this argument, a previous study of pregnant women indicated that the simple instruction to reduce stressors and increase relaxation on the following day was associated with significantly decreased cortisol levels 45 minutes after awakening 61.
As predicted, greater improvements in negative affect and contentment in response to a yoga session relative to both a comparison condition and a control group were observed. Differences in change in positive affect were not observed; however, positive affect was higher on days that yoga participants engaged in a yoga session compared to days of usual activity. These findings corroborate previous studies showing significant decreases in acute negative affect in response to a yoga session 36,62 and suggest that yoga can buffer against negative affect, perhaps by decreasing rumination and changing affective appraisals of and coping with stress 63. Congruent with the essence of yoga – to focus the mind and cultivate unity of the body and mind – these findings also suggest that Hatha yoga may confer immediate advantage for low- (e.g., contentment) rather than high-activation positive affect. Similar to the findings for cortisol, overall higher positive affect on yoga days but no evidence of greater increases in positive affect in response to a yoga session also may reflect women's expectations and associated anticipatory increases in positive affect before the start of the yoga session.
The second aim was to investigate the effects of prenatal yoga practice on APD and PPD symptoms. Our data suggest that regular yoga practice during pregnancy, while not associated with concurrent depressive symptoms, is, in fact, associated with fewer PPD symptoms experienced several months later. This finding is in line with other prospective reports that suggest yoga-related improvements in depressive symptoms 64 and rumination 65 with time in non-pregnant individuals, and has important implications for the timing of interventions because it suggests that prenatal interventions may yield postpartum benefits. The lack of group differences for APD symptoms is inconsistent with several recent reports of greater reductions in APD symptoms in yoga relative to control groups 53,54,56 in women who were either clinically depressed or at high risk for developing perinatal depression. Our study sample involved women with no self-reported depressive and/or anxiety disorder diagnosis at study onset. As such, it may be that differences in APD symptoms between groups are less pronounced among non-clinically depressed women. We did not assess postpartum yoga practice and, therefore, cannot comment on whether similar improvements in PPD symptoms can be achieved with yoga practice in the postpartum period. In sum, this pattern of findings suggests that the effects of yoga practice during pregnancy may not be immediate, but develop over the course of pregnancy and post partum.
Lastly, we did not find evidence for an association of PPD symptoms with cortisol and affect. It is possible that the relatively narrow range of depressive symptoms exhibited by participants in our study could have weakened the associations of cortisol and affect with PPD symptoms. Furthermore, it may be that other physiological or psychosocial factors are responsible for the antidepressant effects of yoga. Recent studies have proposed autonomic responses to stress, social support, pregnancy mindfulness, and mother-infant attachment as underlying mechanisms of the beneficial impact of yoga on depressive symptoms 55,63.
The present study improved on methodological limitations of previous studies by employing both within- and between-subject controls and both psychological and physiological outcome measures. Nevertheless, certain limitations should be noted. The main limitation is the potential presence of selection bias as yoga group participants were recruited among pregnant women already enrolled in prenatal yoga classes. Yoga group participants differed sociodemographically from control group participants, which poses a threat to the internal validity of the findings and impacts the ability to generalize findings to the broader population of pregnant women. These sociodemographic group differences, however, also point to the lower availability, affordability, and/or appeal of yoga to pregnant women of lower SES, and thus highlight the need for prenatal yoga programs targeted toward diverse populations. An additional limitation is the small sample size of the study, and of the control group in particular, which may have limited the statistical power to detect meaningful differences between yoga and control groups and did not allow for adjustment of baseline sociodemographic differences between them. Of importance, that differences in APD symptoms between groups were not observed suggests that group differences in PPD symptoms can tentatively be interpreted in favor of intervention effects. Future randomized controlled trials with larger, more diverse samples and other mind-body comparison intervention programs are needed to corroborate the findings of the present study.
In summary, the present study provides preliminary evidence for immediate benefits of yoga on negative affect and on feelings of contentment, as well as overall benefits on cortisol and positive affect during pregnancy. Moreover, the more favorable PPD symptom outcomes in yoga relative to control group participants are promising and suggest that Hatha yoga may have the potential to improve maternal postpartum well-being.
ACKNOWLEDGMENTS
The authors wish to thank the pregnant women who participated in this study for their time and dedication. The invaluable assistance of Dr. JoAnn Prause with data analysis and the help of numerous research assistants with data management are also gratefully acknowledged.
Funding source
This study was supported, in part, by grant MH082270 from the National Institutes of Health and by a Faculty Research Grant from the University of California, Irvine to Dr. Ilona S. Yim. These funding sources had no involvement in the design, data collection, writing, or article submission of the present study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no competing interests to report. In the interest of full disclosure, Linda Trumpfheller is a yoga instructor at YogaWorks and Holly Beck Kimble is a yoga instructor at and Diana Pipaloff owner of Yoga Shakti, the sites of data collection in the present study.
REFERENCES
- 1.Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007;20(3):189–209. doi: 10.1080/14767050701209560. doi:10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- 2.Field T. Prenatal depression effects on early development: A review. Infant Behav Dev. 2011;34(1):1–14. doi: 10.1016/j.infbeh.2010.09.008. doi: http://dx.doi.org/10.1016/j.infbeh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Lilja G, Edhborg M, Nissen E. Depressive mood in women at childbirth predicts their mood and relationship with infant and partner during the first year postpartum. Scand J Caring Sci. 2012;26(2):245–53. doi: 10.1111/j.1471-6712.2011.00925.x. doi: http://dx.doi.org/10.1111/j.1471-6712.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 4.Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry. 2007;48(3-4):245–61. doi: 10.1111/j.1469-7610.2006.01714.x. doi: http://dx.doi.org/10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. doi:10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 6.Gaynes BN, Gavin N, Meltzer-Brody S, et al. AHRQ Evidence Report Summaries; 1998-2005. Vol. 119. Agency for Healthcare Research and Quality (US); Rockville (MD): 2005. [December 9, 2012]. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes: Summary. http://www.ncbi.nlm.nih.gov/books/NBK11838/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melville JL, Gavin A, Guo Y, Fan M-Y, Katon WJ. Depressive Disorders During Pregnancy. Obstet Gynecol. 2010;116(5):1064–70. doi: 10.1097/AOG.0b013e3181f60b0a. doi:10.1097/AOG.0b013e3181f60b0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association Committee on Nomenclature and Statistics . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Fourth Edition. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 9.Dudas RB, Csatordai S, Devosa I, et al. Obstetric and psychosocial risk factors for depressive symptoms during pregnancy. Psychiatry Res. 2012;200(2-3):323–8. doi: 10.1016/j.psychres.2012.04.017. doi:10.1016/j.psychres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 10.O'Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65(12):1258–69. doi: 10.1002/jclp.20644. doi:10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- 11.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–95. doi: 10.1016/j.genhosppsych.2004.02.006. doi:10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50(5):275–85. doi: 10.1097/00006199-200109000-00004. doi:10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5–14. doi: 10.1016/j.ajog.2009.09.007. doi:10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngai F-W, Chan SW-C. Psychosocial factors and maternal wellbeing: An exploratory path analysis. Int J Nurs Stud. 2011;48(6):725–31. doi: 10.1016/j.ijnurstu.2010.11.002. doi: http://dx.doi.org/10.1016/j.ijnurstu.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues OMPR, Schiavo R de A. Stress in pregnancy and puerperium: a correlation with postpartum depression. Rev Bras Ginecol Obstet. 2011;33(9):252–7. doi: 10.1590/s0100-72032011000900006. doi:10.1590/S0100-72032011000900006. [DOI] [PubMed] [Google Scholar]
- 16.Austin M-PV, Tully L, Parker G. Examining the relationship between antenatal anxiety and postnatal depression. J Affect Disord. 2007;101(1-3):169–74. doi: 10.1016/j.jad.2006.11.015. doi:10.1016/j.jad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Parcells DA. Women's mental health nursing: depression, anxiety and stress during pregnancy. J Psychiatr Ment Health Nurs. 2010;17(9):813–20. doi: 10.1111/j.1365-2850.2010.01588.x. doi:10.1111/j.1365-2850.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 18.Brummelte S, Galea LAM. Depression during pregnancy and postpartum: Contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):766–76. doi: 10.1016/j.pnpbp.2009.09.006. doi:10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Halbreich U. Postpartum disorders: multiple interacting underlying mechanisms and risk factors. J Affect Disord. 2005;88(1):1–7. doi: 10.1016/j.jad.2005.05.002. doi:10.1016/j.jad.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95(1):1–14. doi: 10.1159/000327017. doi: http://dx.doi.org/10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66(2):162–9. doi: 10.1001/archgenpsychiatry.2008.533. doi:10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stetler C, Miller GE. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research. Psychosom Med. 2011;73(2):114–26. doi: 10.1097/PSY.0b013e31820ad12b. doi:10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 23.Bhagwagar Z, Hafizi S, Cowen P. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182(1):54–7. doi: 10.1007/s00213-005-0062-z. doi:10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- 24.Harris TO, Borsanyi S, Messari S, et al. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br J Psychiatry. 2000;177:505–10. doi: 10.1192/bjp.177.6.505. doi:10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- 25.Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-Reported Depressive Symptoms and Stress Levels in Healthy Young Men: Associations With the Cortisol Response to Awakening. Psychosom Med. 2003;65(1):92–9. doi: 10.1097/01.psy.0000040950.22044.10. doi:10.1097/01.PSY.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 26.Vreeburg SA, Hoogendijk WJG, van Pelt J, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–26. doi: 10.1001/archgenpsychiatry.2009.50. doi:10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 27.Wardenaar KJ, Vreeburg SA, van Veen T, et al. Dimensions of depression and anxiety and the hypothalamo-pituitary-adrenal axis. Biol Psychiatry. 2011;69(4):366–73. doi: 10.1016/j.biopsych.2010.09.005. doi:10.1016/j.biopsych.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrino. 2005;30(9):846–56. doi: 10.1016/j.psyneuen.2005.02.010. doi:10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Lagercrantz H, Slotkin TA. The “stress” of being born. Sci Am. 1986;254(4):100–7. doi: 10.1038/scientificamerican0486-100. doi:10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- 30.Handley SL, Dunn TL, Waldron G, Baker JM. Tryptophan, cortisol and puerperal mood. Br J Psychiatry. 1980;136:498–508. doi: 10.1192/bjp.136.5.498. doi:10.1192/bjp.136.5.498. [DOI] [PubMed] [Google Scholar]
- 31.Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. 2006;68(6):931–7. doi: 10.1097/01.psy.0000244385.93141.3b. doi:10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- 32.O'Keane V, Lightman S, Patrick K, et al. Changes in the maternal hypothalamic-pituitary-adrenal axis during the early puerperium may be related to the postpartum “blues”. J Neuroendocrinol. 2011;23(11):1149–55. doi: 10.1111/j.1365-2826.2011.02139.x. doi: http://dx.doi.org/10.1111/j.1365-2826.2011.02139.x. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Complementary and Alternative Medicine What Is Complementary and Alternative Medicine? [May 23, 2013];2007 http://nccam.nih.gov/health/whatiscam#definingcam.
- 34.Yoga Journal [May 22, 2013];Yoga in America study. 2008 http://www.yogajournal.com/press/yoga_in_america.
- 35.Berger BG, Owen DR. Mood alteration with yoga and swimming: aerobic exercise may not be necessary. Percept Mot Skills. 1992;75(3 Pt 2):1331–43. doi: 10.2466/pms.1992.75.3f.1331. doi:10.2466/pms.1992.75.3f.1331. [DOI] [PubMed] [Google Scholar]
- 36.West J, Otte C, Geher K, Johnson J, Mohr D. Effects of hatha yoga and african dance on perceived stress, affect, and salivary cortisol. Ann Behav Med. 2004;28(2):114–8. doi: 10.1207/s15324796abm2802_6. doi:10.1207/s15324796abm2802_6. [DOI] [PubMed] [Google Scholar]
- 37.Narasimhan L, Nagarathna R, Nagendra H. Effect of integrated yogic practices on positive and negative emotions in healthy adults. Int J Yoga. 2011;4(1):13–9. doi: 10.4103/0973-6131.78174. doi:10.4103/0973-6131.78174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredrickson BL. Cultivating positive emotions to optimize health and well-being. Prevention & Treatment. 2000;3(1):1a. doi:10.1037/1522-3736.3.1.31a. [Google Scholar]
- 39.Ernst E, Lee MS. How effective is yoga? A concise overview of systematic reviews. Focus Alternat Complement Ther. 2010;15(4):274–9. doi:10.1111/j.2042-7166.2010.01049.x. [Google Scholar]
- 40.Mehta P, Sharma M. Yoga as a Complementary Therapy for Clinical Depression. Complement Health Pract Rev. 2010;15(3):156–70. doi:10.1177/1533210110387405. [Google Scholar]
- 41.Uebelacker LA, Epstein-Lubow G, Gaudiano BA, Tremont G, Battle CL, Miller IW. Hatha yoga for depression: critical review of the evidence for efficacy, plausible mechanisms of action, and directions for future research. [July 30, 2012];J Psychiatr Pract. 2010 16(1):22. doi: 10.1097/01.pra.0000367775.88388.96. http://journals.lww.com/practicalpsychiatry/Abstract/2010/01000/Hatha_Yoga_for_Depression__Critical_Review_of_the.4.aspx. [DOI] [PubMed] [Google Scholar]
- 42.Kamei T, Toriumi Y, Kimura H, Ohno S, Kumano H, Kimura K. Decrease in serum cortisol during yoga exercise is correlated with alpha wave activation. Percept Mot Skills. 2000;90(3 Pt 1):1027–32. doi: 10.2466/pms.2000.90.3.1027. doi:10.2466/pms.2000.90.3.1027. [DOI] [PubMed] [Google Scholar]
- 43.Michalsen A, Grossman P, Acil A, et al. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive yoga program. [October 28, 2012];Med Sci Monit. 2005 11(12):CR555–61. http://www.ncbi.nlm.nih.gov/pubmed/16319785. [PubMed] [Google Scholar]
- 44.Smith JA, Greer T, Sheets T, Watson S. Is there more to yoga than exercise? [October 28, 2012];Altern Ther Health Med. 2011 17(3):22–9. http://www.ncbi.nlm.nih.gov/pubmed/22164809. [PubMed] [Google Scholar]
- 45.Raghavendra RM, Vadiraja HS, Nagarathna R, et al. Effects of a Yoga Program on Cortisol Rhythm and Mood States in Early Breast Cancer Patients Undergoing Adjuvant Radiotherapy: A Randomized Controlled Trial. Integr Cancer Ther. 2009;8(1):37–46. doi: 10.1177/1534735409331456. doi:10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 46.Babbar S, Parks-Savage A, Chauhan S. Yoga during Pregnancy: A Review. Am J Perinatol. 2012;29(06):459–64. doi: 10.1055/s-0032-1304828. doi:10.1055/s-0032-1304828. [DOI] [PubMed] [Google Scholar]
- 47.Khalajzadeh M, Shojaei M, Mirfaizi M. The effect of yoga on anxiety among pregnant women in second and third trimester of pregnancy. [January 9, 2013];Eur J Sport Sci. 2012 1(3):85–9. http://scholarsresearchlibrary.com/EJSES-vol1-iss3/EJSES-2012-1-3-85-89.pdf. [Google Scholar]
- 48.Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: Results of a pilot study. Arch Womens Ment Health. 2008;11(1):67–74. doi: 10.1007/s00737-008-0214-3. doi: http://dx.doi.org/10.1007/s00737-008-0214-3. [DOI] [PubMed] [Google Scholar]
- 49.Rakhshani A, Maharana S, Raghuram N, Nagendra HR, Venkatram P. Effects of integrated yoga on quality of life and interpersonal relationship of pregnant women. Qual Life Res. 2010;19(10):1447–55. doi: 10.1007/s11136-010-9709-2. doi:10.1007/s11136-010-9709-2. [DOI] [PubMed] [Google Scholar]
- 50.Satyapriya M, Nagendra HR, Nagarathna R, Padmalatha V. Effect of integrated yoga on stress and heart rate variability in pregnant women. Int J Gynaecol Obstet. 2009;104(3):218–22. doi: 10.1016/j.ijgo.2008.11.013. doi:10.1016/j.ijgo.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Rakhshani A, Nagarathna R, Mhaskar R, Mhaskar A, Thomas A, Gunasheela S. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: A randomized controlled trial. Prev Med. 2012;55(4):333–40. doi: 10.1016/j.ypmed.2012.07.020. doi:10.1016/j.ypmed.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 52.Doran F, Hornibrook J. Women's experiences of participation in a pregnancy and postnatal group incorporating yoga and facilitated group discussion: A qualitative evaluation. Women Birth. 2012;26(1):82–6. doi: 10.1016/j.wombi.2012.06.001. doi:10.1016/j.wombi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Field T, Diego M, Hernandez-Reif M, Medina L, Delgado J, Hernandez A. Yoga and massage therapy reduce prenatal depression and prematurity. J Bodyw Mov Ther. 2012;16(2):204–9. doi: 10.1016/j.jbmt.2011.08.002. doi:10.1016/j.jbmt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell J. Yoga Reduces Prenatal Depression Symptoms. Psychology. 2012;03(29):782–6. doi:10.4236/psych.2012.329118. [Google Scholar]
- 55.Muzik M, Hamilton SE, Rosenblum KL, Waxler E, Hadi Z. Mindfulness yoga during pregnancy for psychiatrically at-risk women: Preliminary results from a pilot feasibility study. Complement Ther Clin Pract. 2012;18(4):235–40. doi: 10.1016/j.ctcp.2012.06.006. doi:10.1016/j.ctcp.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Field T, Diego M, Delgado J, Medina L. Tai chi/yoga reduces prenatal depression, anxiety and sleep disturbances. Complement Ther Clin Pract. 2013;19(1):6–10. doi: 10.1016/j.ctcp.2012.10.001. doi:10.1016/j.ctcp.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derogatis LR. The affects balance scale. Baltimore, Clinical Psychometric Research. 1975 [Google Scholar]
- 58.Raub JA. Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med. 2002;8(6):797–812. doi: 10.1089/10755530260511810. doi:10.1089/10755530260511810. [DOI] [PubMed] [Google Scholar]
- 59.Derogatis LR, Rutigliano PJ. Derogatis Affects Balance Scale: DABS. In: Spiker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Lippincott-Rave; Philadelphia: 1996. [October 29, 2012]. pp. 107–18. http://www.statisticssolutions.com/resources/directory-of-survey-instruments/derogatis-affect-balance-scale-dabs. [Google Scholar]
- 60.Santor D, Coyne J. Shortening the CES-D to improve its ability to detect cases of depression. Psychol Assess. 1997;9(3):233–43. doi:10.1037/1040-3590.9.3.233. [Google Scholar]
- 61.Urizar GG, Jr., Milazzo M, Le H-N, Delucchi K, Sotelo R, Muñoz RF. Impact of stress reduction instructions on stress and cortisol levels during pregnancy. Biol Psychol. 2004;67(3):275–82. doi: 10.1016/j.biopsycho.2003.11.001. doi:10.1016/j.biopsycho.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Woolery A, Myers H, Sternlieb B, Zeltzer L. A yoga intervention for young adults with elevated symptoms of depression. [December 12, 2012];Altern Ther Health Med. 2004 10(2):60–3. http://www.ncbi.nlm.nih.gov/pubmed/15055096. [PubMed] [Google Scholar]
- 63.Kinser PA, Goehler L, Taylor AG. How Might Yoga Help Depression? A Neurobiological Perspective. Explore (NY) 2012;8(2):118–26. doi: 10.1016/j.explore.2011.12.005. doi:10.1016/j.explore.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simard A-A, Henry M. Impact of a short yoga intervention on medical students’ health: a pilot study. Med Teach. 2009;31(10):950–2. doi: 10.3109/01421590902874063. doi:10.3109/01421590902874063. [DOI] [PubMed] [Google Scholar]
- 65.Kinser PA, Bourguignon C, Whaley D, Hauenstein E, Taylor AG. Feasibility, Acceptability, and Effects of Gentle Hatha Yoga for Women With Major Depression: Findings From a Randomized Controlled Mixed-Methods Study. Arch Psychiatr Nurs. 2013;27(3):137–47. doi: 10.1016/j.apnu.2013.01.003. doi:10.1016/j.apnu.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]