Abstract
Gene therapy for the treatment of Wiskott–Aldrich syndrome (WAS) presents an alternative to the current use of allogeneic bone marrow transplantation. We describe the development of a self-inactivating lentiviral vector containing chromatin insulators for treatment of WAS and compare a gammaretroviral (MND), human cellular (EF1α), and the human WASp gene promoter for expression patterns in vivo during murine hematopoiesis using the green fluorescent protein (GFP) marker. Compared with the EF1α and the WASp promoters, expression from the MND promoter in mouse transplant recipients was much higher in all lineages examined. Importantly, there was sustained expression in the platelets of secondary recipient animals, necessary to correct the thrombocytopenia defect in WAS patients. Analysis of WAS protein expression in transduced human EBV-immortalized B-cells and transduced patient peripheral blood mononuclear cells also demonstrated stronger expression per copy from the MND promoter compared with the other promoters. In addition, when analyzed in an LM02 activation assay, the addition of an insulator to MND-promoter-containing constructs reduced transactivation of the LM02 gene. We propose a clinical trial design in which cytokine-mobilized, autologous, transduced CD34+ cells are administered after myelosuppression.
Koldej and colleagues perform preclinical evaluation of three promoters in a self-inactivating lentiviral vector being developed for ex vivo gene therapy of Wiskott Aldrich Syndrome (WAS). They demonstrate that a gammaretroviral promoter drives robust green fluorescent protein expression in multiple hematopoietic lineages in a mouse bone marrow transplant model, as well as significant WAS protein expression in patient peripheral blood cells. They further demonstrate that inclusion of insulator sequences can effectively abrogate insertional activation of the LMO2 gene, which has previously been found to drive malignant transformation of retroviral vector–transduced cells in human trials.
Introduction
Wiskott–Aldrich syndrome (WAS) is an X-linked recessive immunodeficiency that occurs at a frequency of five in every million live births. Patients present with thrombocytopenia, eczema, recurrent infections, and a high frequency of autoimmune disease and malignancy and have a shortened life expectancy (Imai et al., 2004; Bosticardo et al., 2009). Patients with mild WAS may be treated with supportive care. The only cure for WAS is bone marrow (BM) transplantation. Patients with matched sibling donors have an 88% survival rate 7 years post-treatment (Ozsahin et al., 2008). Previously, patients who received a matched unrelated donor or mismatched related donor graft have had a poorer prognosis (71% and 55%, respectively) (Filipovich et al., 2001), but recent analyses suggest that the outcome has improved (Moratto et al., 2011; Mahlaoui et al., 2012; Shin et al., 2012).
Recent clinical trials indicate that various immunodeficiencies, including WAS, can be successfully treated by retroviral-mediated gene transfer into repopulating hematopoietic cells (Cavazzana-Calvo et al., 2000; Boztug et al., 2010; Hacein-Bey-Abina et al., 2010; Gaspar et al., 2011). The WAS gene therapy trial used a gammaretroviral vector to facilitate delivery of the human WAS protein (hWASp) cDNA driven by the MMP promoter (a derivative of the myeloproliferative sarcoma virus promoter) (Klein et al., 2000) into the patient's own CD34+ cells, which were subsequently transplanted back to the patient after chemo-myelosuppression (Boztug et al., 2010). This resulted in an increase in platelet counts, emergence of corrected lymphoid cells, and remission of other disease manifestations, demonstrating the effectiveness of this treatment strategy. However, 4 of 10 patients treated to date have developed leukemia (Braun et al., 2011; Paruzynski et al., 2011). All four patients were shown to have a vector integration close to the LIM domain only two (LMO2) locus causing transactivation of this proto-oncogene, a phenomenon that has also been seen in the treatment of severe combined X-linked immunodeficiency using similar vectors (Hacein-Bey-Abina et al., 2003a,b; Hacein-Bey-Abina et al., 2008; Howe et al., 2008).
Self-inactivating (SIN) lentiviral vectors have proven less oncogenic than gammaretroviral LTR-driven vectors in a number of assays (Montini et al., 2006, 2009; Ryu et al., 2008; Modlich et al., 2009). By switching to a SIN lentiviral vector for the treatment of WAS, the therapeutic benefit to the patient should be maintained while the oncogenic potential of the delivery system is reduced. The use of insulators to transcriptionally isolate the transgene may further reduce the oncogenic potential of the vector. Originally discovered in association with DNAse I hypersensitivity sites within the β-globin locus control region (Chung et al., 1993), insulators are able to prevent histone modification and DNA methylation, which may lead to heterochromatin formation and downregulation or silencing of the transgene (Chung et al., 1997). Insulators also prevent regulatory signals in the vector (either positive or negative) from acting on nearby genes, leading to their deregulation (Gaszner and Felsenfeld, 2006).
Lentiviral vectors are currently being developed by a number of groups to deliver the hWASp cDNA into autologous BM cells (reviewed by Galy and Thrasher, 2011). Initial studies used a strong retroviral LTR promoter to drive WASp gene expression and resulted in high levels of protein expression and functional correction (Dupre et al., 2004). More recent studies have focused on the use of the endogenous WASp promoter (Martin et al., 2005; Charrier et al., 2007; Marangoni et al., 2009). Functional correction has been achieved in the mouse model using a 1.6 kb WASp promoter fragment to drive the WASp cDNA (Dupre et al., 2006; Marangoni et al., 2009; Scaramuzza et al., 2013). These data contrast with results obtained by others in which full correction of the WAS phenotype was achieved in the murine model with a vector having a retroviral promoter, whereas only partial correction was achieved with a vector having the 1.6 WAS promoter fragment (Astrakhan et al., 2010). Methodologies have also been developed for the large-scale manufacture and characterization of the lentiviral vector having the 1.6 kb WAS promoter fragment for use in a gene therapy application (Merten et al., 2011). Clinical trials have been opened at four sites over the period 2009–2011 and are currently recruiting and enrolling patients as summarized in Galy and Thrasher (2011).
We have compared expression levels from a retroviral promoter, the cellular EF1α promoter, and the WASp gene promoter (P500 or P1600) in insulated lentiviral vectors in hematopoietic cells with the goal of identifying a vector design to be used in a future clinical trial that has been funded by the National Heart Lung and Blood Institute (P01HL53749).
Results and Discussion
Clinical trial
General design
All patients with WAS who lack a matched related or unrelated donor will be candidates for gene therapy. Only patients who are large enough to undergo apheresis (≥12 kg) will be eligible. Patients will be screened for evidence of cells containing WASp secondary to a spontaneous correcting reversion mutation, but such patients will be eligible for enrollment if they have persistent thrombocytopenia and immunodeficiency. Cellular WASp levels will be compared by fluorescence-activated cell sorting analysis before and after gene transfer to determine efficacy in patients with residual WASp production. A preliminary apheresis after G-CSF administration will be performed to evaluate each individual patient's capacity to mobilize CD34+ cells. These cells will be used to evaluate gene transfer efficiency, and the majority will be cryopreserved as a backup for the subsequent apheresis and transduction procedures. Depending on the success of the initial mobilization, G-CSF only or G-CSF administered with AMD2100 will be used for the definitive cytapheresis mobilization. Purified CD34+ cells will be cultured under conditions previously described (Kim et al., 2010). Patients who have residual manifestations of WAS after a stem cell transplant or who fail to engraft will not be eligible for protocol enrollment.
The rationale for considering gene therapy rather than transplantation from a mismatched related or unrelated donor as the first therapeutic alternative for WAS patients lacking a matched sibling or fully matched unrelated donor is based on the poorer outcome after transplantation with a partially matched donor (Filipovich et al., 2001) and the high frequency of long-term complications (Ozsahin et al., 2008). Also, the gene therapy intervention can be implemented and, if unsuccessful, followed subsequently by an allogeneic transplantation procedure. The patients will be stratified based on age into two groups on the basis of the observation in X-linked severe combined immunodeficiency patients that the gene therapy intervention, although successful among infants, is significantly less successful in older patients (Thrasher et al., 2005; Chinen et al., 2007). Only one of five older patients had improvement in immunological function after infusion of genetically modified, primitive hematopoietic cells. The poorer outcome in older patients is thought to reflect diminished thymic function with age, a fact that may also be important in WAS patients, and therefore we propose an age stratification. We have somewhat arbitrarily chosen age 9 and younger for stratum I and age 10 and older for stratum II. Our goal is to enroll a minimum of five participants in each stratum. All patients will receive a myelosuppressive regimen consisting of Busulfan at a total dose of 8 mg/kg given at a dose of 2 mg/kg at 12 hr intervals before infusion of transduced CD34+ cells, as this dose has been used successfully in a prior gene therapy trial for WAS (Boztug et al., 2010). Trial participants will be monitored periodically after the gene transfer procedure to evaluate reconstitution and determine the proportion of genetically modified cells in peripheral blood.
Objectives
The primary objective for each of the two strata is to investigate safety and efficacy with respect to hematological and immunological reconstitution in WAS patients transplanted with autologous CD34+ cells that have been transduced with a SIN lentiviral vector expressing the WASp coding sequences. Objective endpoints by 1 year are an increase in platelet count (≥50,000) and IgM [≥lower limit of normal range for age (Orkin et al. 2009)]. The secondary objectives are (a) to estimate the incidence of, and describe serious side effects caused by lentiviral gene therapy; (b) to determine the integration site distribution of the WASp lentiviral vector in reconstituted peripheral blood cells; and (c) to observe for evidence of clonal dominance and/or the development of leukemia.
Inclusion criteria
Only patients who have a documented mutation in the WASp gene will be candidates for a gene therapy intervention. All patients who are ≥12 kg and who have significant thrombocytopenia (platelet count ≤50,000/mm3 on ≥3 occasions) and any other manifestation of WAS will be eligible. Other manifestations may include the following: (a) two or more infections requiring chronic therapy; (b) significant eczema requiring chronic therapy; (c) autoimmune disease; (d) malignancy or premalignant condition; and (e) positive family history defined by a family member with WAS who died before 10 years of age.
Exclusion criteria
Exclusion criteria include the following: (a) has a suitable, available 6/6 HLA-matched sibling donor; (b) has a suitable, available 10/10 HLA allele-matched unrelated donor identified through the National Marrow Donor Program (potential participants will become eligible for the gene therapy trial if no donor has been identified within 90 days); (c) HIV infection; (d) a medical condition that would preclude anesthesia or sedation for apheresis or a BM harvest, if needed; (e) a medical condition precluding the administration of myelosuppressive Busulfan therapy; and (f) a prior allogeneic stem cell transplant.
Objectives and study design
Our intention is to perform a comprehensive series of studies to provide preclinical data for the clinical trial described above involving the use of a SIN lentiviral vector for transfer of the WASp gene into repopulating hematopoietic stem cells from WAS patients. Murine models are being used to evaluate promoter function. In our experiments, we have utilized the GFP marker in wild-type mice, as the use of this marker provides a facile methodology for evaluating promoter function in the series of animals while testing a significant number of promoters. Our results indicate that the MND γ-retroviral promoter gives consistently high levels of expression in multiple hematopoietic lineages.
We are collaborating with the Rawlings' laboratory to evaluate promoter function in the murine WAS mouse strain. This work is supported by a subcontract from our program project grant (P01HL53749). The initial results from the Rawlings' lab have been published (Astrakhan et al., 2012). These studies demonstrated that the MND promoter gave full correction of the WAS phenotype in the murine model, whereas the vector with the 1.6 WASp gene promoter gave only partial correction. Ongoing studies provide further evaluation of the level and duration of expression of the WASp transgene in the knockout mouse model. A formal toxicity study is in the preliminary stages of planning.
Promoter function in the studies described in this article was also evaluated in human B-cells (EBV immortalized B-cells) from normal individuals and WAS patients using a vector encoding WASp. Similar studies were done with peripheral blood mononuclear cells from both normal and individuals with WAS. Our studies are designed to provide a comprehensive evaluation of promoter function in an effort to identify the appropriate promoter for a clinical vector. We have also tested insulator elements, as the use of the MND promoter will almost certainly occur in the context of a vector with insulator elements.
Toxicity studies are being performed in a variety of assays. Described in this article are data using targeted integration to recreate vector insertions observed in patients with immunodeficiency treated with gene therapy, who then developed leukemia. Cre-mediated cassette exchange is used to place candidate vector genomes into these targeted integration sites. We have compared a vector with the MND promoter without insulator to comparable vectors having insulator elements.
Summary of data
Vector construction and promoter activity during hematopoiesis
Gene therapy for WAS requires that the promoter be active in all hematopoietic lineages except erythrocytes. This can be achieved relatively easily in B lymphocytes and T lymphocytes, where there is positive selective pressure on hWASp-expressing cells because of restoration of responses to proliferative signals (Klein et al., 2003; Strom et al., 2003; Westerberg et al., 2008). However, hWASp-expressing cells of the myeloid lineage (megakaryocytes, monocytes, granulocytes, macrophages) do not have such an advantage, and therefore in these cells the promoter utilized must drive sufficient hWASp expression independent of selection pressure. In addition, it has been shown by two groups recently that if the T lymphocyte defect is corrected (which requires a low amount of hWASp) in WAS mice without correcting the B lymphocyte defect (which requires a higher amount of hWASp), a state of autoimmunity develops (Becker-Herman et al., 2011; Recher et al., 2012).
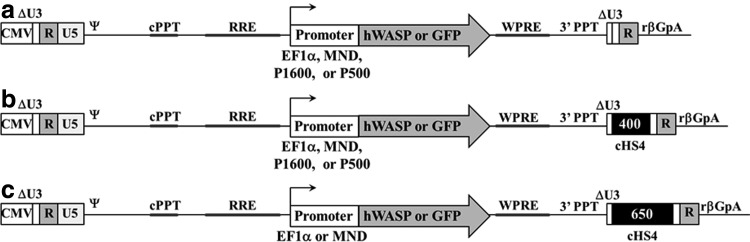
To compare the effect of promoters and insulators on hWASp (or a GFP marker) expression in lentiviral vectors, a series of vectors were constructed (Fig. 1) (Hanawa et al., 2002, 2009). The insulators were derived from the canonical 1.2 kb chicken hypersensitive site four (cHS4) fragment from the β-globin locus (Emery et al., 2000; Gaszner and Felsenfeld, 2006; Arumugam et al., 2007). The 400 bp cHS4 insulator includes the 250 bp core element and adjacent 150 bp of sequences (Aker et al., 2007), while the 650 includes the 250 bp core and 400 bp from the 3′ end of the 1.2 kb insulator fragment (Arumugam et al., 2009). It has previously been shown that the 400 bp cHS4 insulator placed in the reverse orientation within the LTR, combined with a 46 bp deletion that removes the residual nef sequence, reduces the frequency of polyadenylation read-through from a lentiviral LTR (Hanawa et al., 2009), and therefore this configuration was used for our vectors. Similarly, the 650 bp cHS4 insulator was placed in the reverse orientation. For both the EF1α and MND promoters, expression in the context of a 400 bp, 650 bp, or noninsulated lentivirus (400EF1α, 650EF1α, NIEF1α, 400MND, 650MND, and NIMND) was tested, while for the endogenous hWASp promoter vectors (P500 and P1600), the 400 bp and noninsulated backbones were utilized (400P500, NIP500, 400P1600, and NIP1600) (Fig. 1). All vectors were based on the pCL20cw series of lentiviral vectors and contain a mutated WPRE (Hanawa et al., 2009).
FIG. 1.
Schematic of vectors used in this study. (a) Noninsulated vectors expressing hWASp cDNA or GFP from the EF1α, P500, P1600, or MND promoters. (b) 400 bp cHS4 insulated vectors expressing hWASp cDNA or GFP from the EF1α, P500, P1600, or MND promoters. (c) 650 bp insulated vectors expressing hWASp cDNA or GFP from the EF1α or MND promoters. 3′PPT, 3′ polypurine tract; βGpA, β-globin polyadenylation signal; cHS4, chicken hypersensitive site four; CMV, cytomegalovirus promoter; cPPT, central polypurine tract; hWASp, human Wiskott–Aldrich syndrome protein; RRE, Rev response element; WPRE, Woodchuck hepatitis post-transcriptional regulatory element.
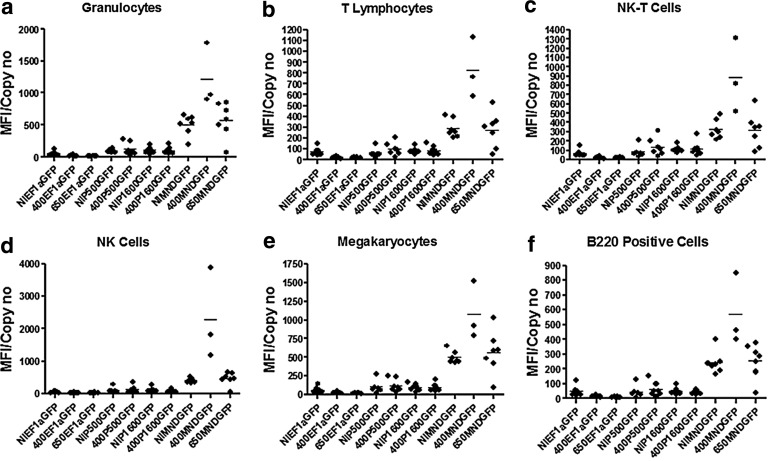
GFP vectors were used to examine promoter activity during murine hematopoiesis. Concentrated lentiviral vector stocks were produced for each vector and used to transduce lin− CD45.1+ BM cells at a multiplicity of infection (MOI)=20 and transplanted into lethally irradiated CD45.2+ mice. BM from primary recipients was harvested at 22 weeks, and the cells from four mice having the highest engraftment and GFP expression (based on a 16-week peripheral blood analysis) were each transplanted into two secondary recipients. Secondary recipients were analyzed at 28–36 weeks post-transplant. The blood and BM were examined from both primary and secondary recipients for vector copy number (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/humc) and for GFP expression (Fig. 2 and Supplementary Figs. S2 and S3) and for vector marking of methylcellulose culture colony forming units (CFU-C) (Supplementary Table S1). The inguinal, auxiliary, cervical, and mesenteric lymph nodes and spleen were pooled to examine expression in lymphoid tissues (Supplementary Fig. S4).
FIG. 2.
GFP expression in various lineages from primary bone marrow as determined by flow cytometry. (a) Granulocytes (CD45.1+/CD11b+/GR1+/GFP). (b) T lymphocytes (CD45.1+/CD3+/NK1.1-/GFP). (c) NK-T cells (CD45.1+/CD3+/NK1.1+/GFP). (d) NK cells (CD45.1+/CD3-/NK1.1+/GFP). (e) Megakaryocytes (CD45.1+/CD41+/GFP). (f) B220+ cells (CD45.1+/B220+/GFP). A two-tailed, heteroscedastic variance t-test was used to calculate p-values between the groups shown. Student's t-tests were used throughout to assess significance. p-Values less than or equal to 5% or 0.05 were deemed significant.
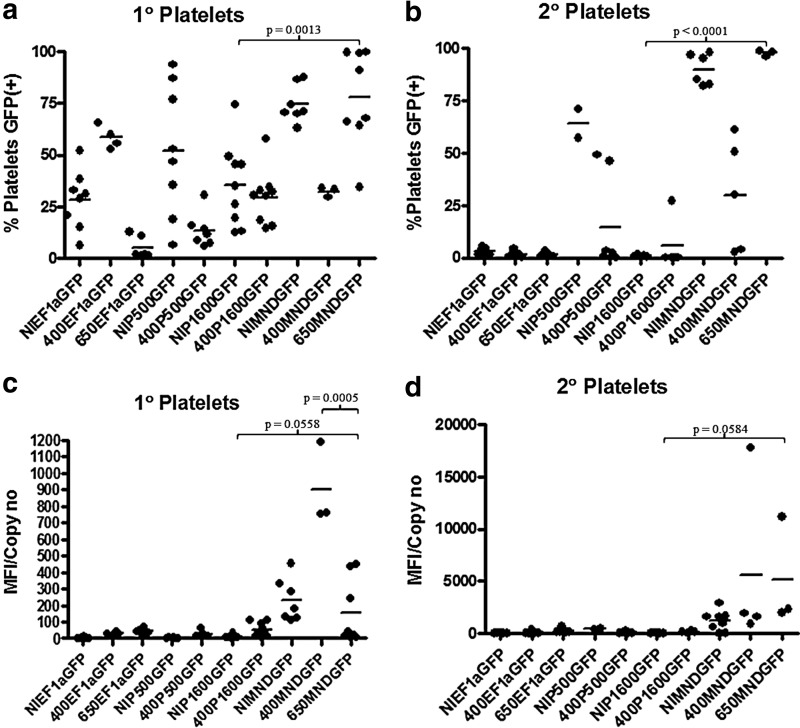
All of the promoters examined in this study did express in all relevant hematopoietic lineages. The details are provided in Supplementary Data. The incorporation of insulators had a relatively minor impact on virus titers (<1 log drop with the 650 bp and no drop with the 400 bp; Supplementary Table S2). The effect of insulators on expression from the individual promoters, including the MND promoter, was modest. However, there were great differences in promoter activity. The endogenous WASp promoter fragments showed weak expression in the mouse model. In general, expression the from P500 and P1600 promoters was reduced in secondary transplants and raises concerns as to whether either of the promoters would provide sufficient expression for long-term correction. In addition, long-term expression in the platelets and megakaryocytes, needed to correct the thrombocytopenia defect, was not seen with the WASp gene promoters (Fig. 3 and Supplementary Table S3). Expression from EF1α promoter was also very low in the mouse model, showing a lack of long-term expression in megakaryocytes and platelets. In contrast, expression from the MND promoter was much stronger and that expression level was maintained in all lineages 22 weeks after transplantation, even when challenged with a second repopulation/engraftment event and evaluated over 28–36 weeks after the secondary transplantation.
FIG. 3.
Platelet analysis in transplant recipients. (a) GFP marking in the platelets of primary recipients. (b) GFP marking in the platelets of secondary recipients. (c) GFP expression per copy in the platelets of primary recipients. (d) GFP expression per copy in the platelets of secondary recipients. Copy number was determined by analysis of bone marrow.
Vectors in which the WASp coding sequences were under the control of a retroviral promoter have been shown to correct human hematopoietic cell defects in vitro (Dewey et al., 2006; Charrier et al., 2007) and indeed have been used in the initial clinical trial in which WAS patients were shown to benefit from the gene transfer procedure (Boztug et al., 2010). Our results contrast with those of Charrier et al. (2007), in which vectors using the endogenous promoters of the WASp gene were shown to give similar expression levels to that observed with vectors utilizing the EF1α promoter or a γ-retroviral LTR. Most studies have been done in murine cells, which may account for some of the discrepancy compared with human cells. Recent studies have also shown that the EF1α promoter gives normal levels of WASp from a vector in which the cDNA had been codon-optimized in both murine and human cells (Avedillo Diez et al., 2011).
Production of hWASp in human B-cells
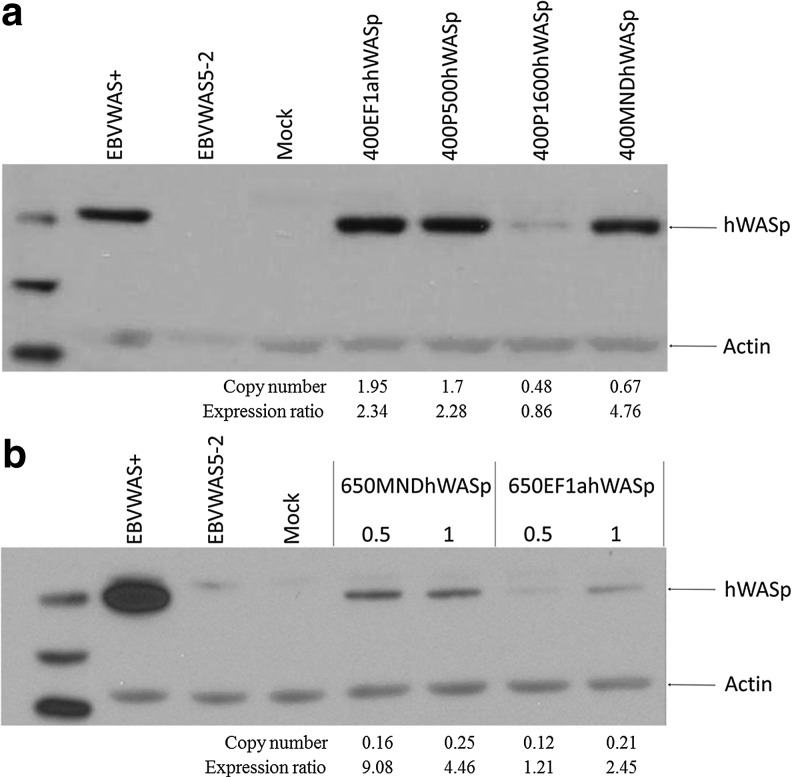
EBV immortalized B-cells were used to analyze hWASp expression in human B-cells in vitro. The cells were transduced with the 400EF1αhWASp, 400P500hWASp, 400P1600hWASp, or 400MNDhWASp vector at an MOI=7.5 and hWASp expression was determined 21 days post-transduction by Western blotting. When copy number was taken into consideration, expression from 400MNDhWASp was higher than that from the other vectors (Fig. 4). Densitometry was performed and the density value of the WASp bands corrected for both the relative intensity of the actin band and also for the copy number as determined by polymerase chain reaction (PCR). An expression ratio is now provided in Figure 4 to allow the direct comparisons of the various vectors. Expression from 400EF1αhWASp and 400P500hWASp was strong, but this reflected that the vector copy number was two- to three-fold higher. The cells transduced with the 400P1600hWASp vector had a comparable copy number to cells transduced with the 400MNDhWASp, but hWASp expression was much weaker with the WASp promoter. In a separate experiment, the 650MNDhWASp and 650EF1αhWASp vectors were compared for expression in EBVWAS5-2 cells at low MOIs (0.5 and 1). Expression levels at both MOIs clearly showed that the 650MNDhWASp vector produced more hWASp per copy than the 650EF1αhWASp vector (Fig. 4).
FIG. 4.
hWASp expression in EBVWAS5-2 cells, a patient-derived WASp-negative B-cell line. (a) EBVWAS5-2 cells were transduced with either the 400EF1αhWASp, 400P500hWASp, 400P1600hWASp, or 400MNDhWASp vectors at an MOI=7.5, and hWASp expression was determined 21 days post-transduction by Western blotting. (b) EBVWAS5-2 cells were transduced with the 650MNDhWASp or 650EF1αhWASp vector at various MOIs, and hWASp expression was determined 21 days post-transduction by Western blotting. MOI, multiplicity of infection.
Production of hWASp in primary human WAS T cells
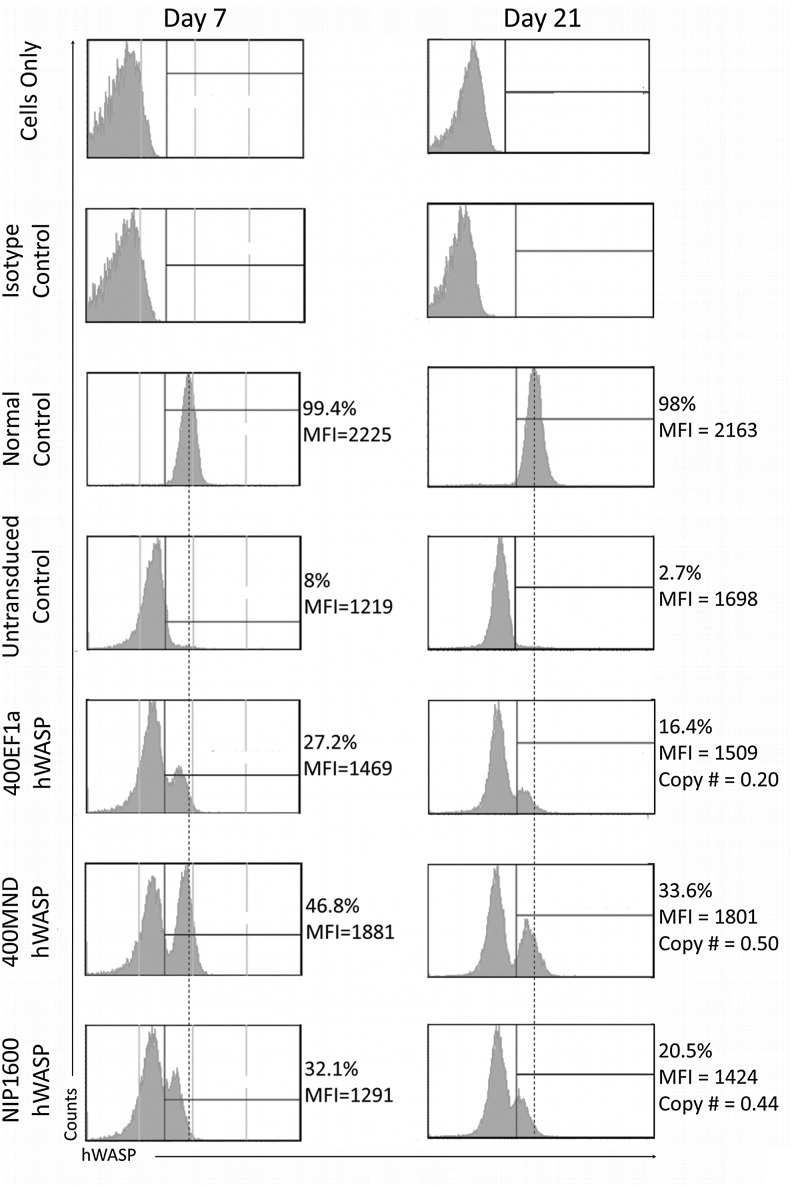
Expression in transduced WAS patient peripheral blood mononuclear cells (PBMCs) was determined using flow cytometry (Fig. 5). PBMCs were harvested from the blood of WAS patients and normal controls and underwent T-cell stimulation followed by transduction with either the 400EF1αhWASp, NIP1600hWASp, or 400MNDhWASp vector at an MOI=40. Transduction with the various vectors was approximately equivalent with a net over background at 7 days, ranging from 19% to 28%. Copy number was not performed at this early time point because of the likelihood of the presence of episomal genomes. hWASp expression was measured at 7 and 21 days post-transduction. NIP1600hWASp was included in this experiment as it is equivalent to a lentiviral vector currently in clinical trials for WAS gene therapy (Scaramuzza et al., 2013). While all three samples showed hWASp expression at both time points, the proportion of cells expressing at a wild-type level of hWASp was greatest with the 400MNDhWASp vector (Fig. 5 and Supplementary Fig. S5). In a subsequent experiment, the noninsulated, 400 bp-insulated, and 650 bp-insulated versions of EF1α and MND were compared under the same conditions. The presence/absence of an insulator had no effect on hWASp expression levels per cell, which is not surprising given the relatively short culture time (data not shown). As in the previous experiment, there were a higher proportion of cells expressing hWASp from the MND promoter compared with the EF1α promoter.
FIG. 5.
hWASp expression from 400MNDhWASp, 400EF1αhWASp, or NIP1600hWASp in patient peripheral blood mononuclear cells. Cells were prestimulated with T cell activation beads and transduced with virus at an MOI of 40. hWASp expression was determined at 7 and 21 days by intracellular staining for hWASp and flow cytometry.
Acute in vivo toxicity
No acute in vivo toxicity was observed in these studies other than the complications related to radiation and a transient period of myelosuppression in the murine model.
Biodistribution
Since repopulated hematopoietic stem cells were transduced, the biodistribution of the vector reflects the distribution of the progeny of transduced stem cells. Thus, vector sequences and expression were found in the BM, spleen, and lymph nodes as predicted. No formal biodistribution studies were performed.
Genotoxicity
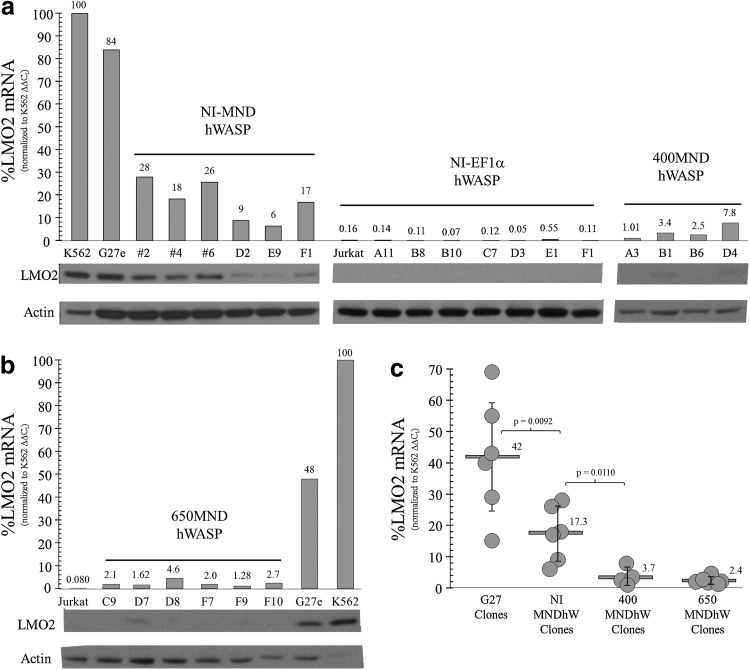
We utilized Jurkat cell lines having a targeted integration site within the promoter (Zhou et al., 2012) or the first intron (Ryu et al., 2008; Zhou et al., 2010) of the LM02 gene to test our vectors for the potential for proto-oncogene activation. The targeted integrations were obtained by homologous recombination of an LTR-GFP or mCherry vector with LoxP sites followed by Cre-mediated cassette exchange (Ryu et al., 2008; Zhou et al., 2010; Zhou et al., 2012). The methodology for deriving the new clones with an insertion upstream from the distal promoter is shown in Supplementary Figure S6. As shown in Figure 6a and Supplementary Figure S7a, the vector without the insulator having the MND promoter (NIMNDhWASp) activated the LM02 gene in several clones although the level of activation was significantly lower than that observed with clones containing the original LTR fluorescent targeting cassette (G27e). Addition of the 400 or 650 bp insulator elements in combination with the MND promoter reduced proto-oncogene activation compared with MND vector without an insulator, with statistical significance with both vectors (Fig. 6b and c, and Supplementary Fig. S7b and c). The 650 bp insulator was somewhat more effective than the 400 bp insulator fragment in reducing LM02 activation compared with the noninsulated vector (Fig. 6 and Supplementary Fig. S7).
FIG. 6.
The cHS4 insulator diminishes LMO2 activation in proviral MNDhWASP cell clones with the vector-integrated upstream of Exon 1. (a) Jurkat cell clones were screened by polymerase chain reaction (PCR) to identify CRE-mediated, MNDhWASP exchange clones, in an anti-sense orientation, upstream of Exon 1 of LMO2, identical to the site mapped for an X-SCID patient containing a γc retroviral insertion (Hacein-Bey-Abina et al., 2003). G27e was one of six AAV-targeted clones that contained an MFG mCherry expression cassette, anti-sense to LMO2, and was used for all CRE-mediated cassette exchange reactions. Positive exchange clones were identified by PCR, expanded, and validated by Southern blot. LMO2 mRNA (quantitative real-time [qRT]-PCR) and protein (Western blot) were measured, relative to that in K562 cells (positive control) and Jurkat cells (negative control). NI-MNDhWASP and NI-EF1αhWASP exchange clones served as relevant, proviral positive and negative controls, respectively, to assess the effect of the cHS4 400 bp insulator on LMO2 activation for 400MNDhWASP exchange clones. (b) LMO2 mRNA and protein levels of CRE-mediated 650MNDhWASP exchange clones. (c) The relative amount of LMO2 mRNA levels for all CRE-mediated, proviral MNDhWASP exchange clones, as assessed by qRT-PCR. Six independently targeted, LMO2-MFG mCherry G27 clones, including subclone G27e, were used as positive controls to assess the ability of cHS4 insulator elements to diminish LMO2 activation in MNDhWASP clones containing a strong retroviral LTR promoter, upstream of Exon 1. LMO2, LIM domain only two; PCR, polymerase chain reaction.
Conclusions
The characteristics of an ideal lentiviral vector for WAS gene therapy are as follows: Expression at a therapeutic level at a low copy number, long-term maintenance of expression in all hematopoietic lineages (except erythrocytes) and insulation to prevent transactivation of nearby genes. On the basis of the data presented here, the 650MNDhWASp vector meets these criteria and is undergoing further testing and development for clinical application.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Marguerite Evans-Galea and Byuong Ryu for the provision of plasmids and the flow cytometry unit for analysis of samples, and Pat Streich for her editorial assistance. This work was supported by the National Heart, Lung, and Blood Institute (Grants P01HL 53749 and R21HL111804).
Author Disclosure Statement
No competing financial interests exist.
References
- Aker M. Tubb J. Groth A.C., et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum. Gene. Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Scholes J. Perelman N., et al. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol. Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Urbinati F. Velu C.S., et al. The 3′ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PLoS One. 2009;4:e6995. doi: 10.1371/journal.pone.0006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrakhan A. Sather B.D. Ryu B.Y., et al. Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich Syndrome. Blood. 2012;119:4395–4407. doi: 10.1182/blood-2011-03-340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avedillo Diez I. Zychlinski D. Cocci E.G., et al. Development of novel efficient SIN vectors with improved safety features for Wiskott-Aldrich Syndrome stem cell based gene therapy. Mol. Pharm. 2011;8:1525–1537. doi: 10.1021/mp200132u. [DOI] [PubMed] [Google Scholar]
- Becker-Herman S. Meyer-Bahlburg A. Schwartz M.A., et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 2011;208:2033–2042. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosticardo M. Marangoni F. Aiuti A., et al. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288–6295. doi: 10.1182/blood-2008-12-115253. [DOI] [PubMed] [Google Scholar]
- Boztug K. Schmidt M. Schwarzer A., et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. Boztug K. Schmidt M., et al. Efficacy of gene therapy for Wiskott-Aldrich-syndrome. Blood (ASH Annual Meeting Abstracts) 2011;118:165. [Google Scholar]
- Cavazzana-Calvo M. Hacein-Bey S. de Saint Basile G., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Challita P.-M. Skelton D. El-Khoueiry A., et al. Multiple modification in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J. Virol. 1995;69:748–755. doi: 10.1128/jvi.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier S. Dupre L. Scaramuzza S., et al. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Ther. 2007;14:415–428. doi: 10.1038/sj.gt.3302863. [DOI] [PubMed] [Google Scholar]
- Chinen J. Davis J. De Ravin S.S., et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood. 2007;110:67–73. doi: 10.1182/blood-2006-11-058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.H. Whiteley M. Felsenfeld G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- Chung J.H. Bell A.C. Felsenfeld G. Characterization of the chicken beta-globin insulator. Proc. Natl. Acad. Sci. U. S. A. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R.A. Avedillo Diez I. Ballmaier M., et al. Retroviral WASP gene transfer into human hematopoietic stem cells reconstitutes the actin cytoskeleton in myeloid progeny cells differentiated in vitro. Exp. Hematol. 2006;34:1161–1169. doi: 10.1016/j.exphem.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Dupre L. Trifari S. Follenzi A., et al. Lentiviral vector-mediated gene transfer in T cells from Wiskott-Aldrich syndrome patients leads to functional correction. Mol. Ther. 2004;10:903–915. doi: 10.1016/j.ymthe.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Dupre L. Marangoni F. Scaramuzza S., et al. Efficacy of gene therapy for Wiskott-Aldrich syndrome using a WAS promoter/cDNA-containing lentiviral vector and nonlethan irradiation. Hum. Gene Ther. 2006;17:303–313. doi: 10.1089/hum.2006.17.303. [DOI] [PubMed] [Google Scholar]
- Emery D.W. Yannaki E. Tubb J. Stamatoyannopoulos G. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovich A.H. Stone J.V. Tomany S.C., et al. Impact of donor type on outcome of bone marrow transplantation for Wiskott-Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood. 2001;97:1598–1603. doi: 10.1182/blood.v97.6.1598. [DOI] [PubMed] [Google Scholar]
- Galy A. Thrasher A.J. Gene therapy for the Wiskott-Aldrich syndrome. Curr. Opin. Allergy Clin. Immunol. 2011;11:545–550. doi: 10.1097/ACI.0b013e32834c230c. [DOI] [PubMed] [Google Scholar]
- Gaspar H.B. Cooray S. Gilmour K.C., et al. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 2011;3:97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- Gaszner M. Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M., et al. LM02-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003a;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. von Kalle C. Schmidt M., et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003b;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Garrigue A. Wang G.P., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Hauer J. Lim A., et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H. Kelly P.F. Nathwani A.C., et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Yamamoto M. Zhao H., et al. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol. Ther. 2009;17:667–674. doi: 10.1038/mt.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe S.J. Mansour M.R. Schwarzwaelder K., et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K. Morio T. Zhu Y., et al. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- Kim Y.S. Wielgosz M.W. Hargrove P., et al. Transduction of human primitive repopulating hematopoietic cells with lentiviral vectors pseudotyped with various envelope proteins. Mol. Ther. 2010;18:1310–1317. doi: 10.1038/mt.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. Bueler H. Mulligan R.C. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J. Exp. Med. 2000;191:1699–1708. doi: 10.1084/jem.191.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. Nguyen D. Liu C.H., et al. Gene therapy for Wiskott-Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood. 2003;101:2159–2166. doi: 10.1182/blood-2002-05-1423. [DOI] [PubMed] [Google Scholar]
- Mahlaoui N. Pellier I. Magnot C., et al. Characteristics and outcome of early-onset, severe forms of Wiskott-Aldrich syndrome. Blood. 2012;121:1510–1516. doi: 10.1182/blood-2012-08-448118. [DOI] [PubMed] [Google Scholar]
- Marangoni F. Bosticardo M. Charrier S., et al. Evidence for long-term efficacy and safety of gene therapy for Wiskott-Aldrich syndrome in preclinical models. Mol. Ther. 2009;17:1073–1082. doi: 10.1038/mt.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. Toscano M.G. Blundell M., et al. Lentiviral vectors transcriptionally targeted to hematopoietic cells by WASP gene proximal promoter sequences. Gene Ther. 2005;12:715–723. doi: 10.1038/sj.gt.3302457. [DOI] [PubMed] [Google Scholar]
- Merten O.W. Charrier S. Laroudie N., et al. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum. Gene Ther. 2011;22:343–356. doi: 10.1089/hum.2010.060. [DOI] [PubMed] [Google Scholar]
- Modlich U. Navarro S. Zychlinski D., et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M., et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Montini E. Cesana D. Schmidt M., et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratto D. Giliani S. Bonfim C., et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980–2009: an international collaborative study. Blood. 2011;118:1675–1684. doi: 10.1182/blood-2010-11-319376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S.H. Nathan D.G. Ginsburg D., et al. Nathan and Oski's Hematology of Infancy and Childhood: Expert Consult. 7th. Saunders Elsevier Inc.; Philadelphia, PA: 2009. p. 1793. [Google Scholar]
- Ozsahin H. Cavazzana-Calvo M. Notarangelo L.D., et al. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111:439–445. doi: 10.1182/blood-2007-03-076679. [DOI] [PubMed] [Google Scholar]
- Paruzynski A. Boztug K. Ball C., et al. Molecular follow-up of the German WAS clinical gene therapy trial. Mol. Ther. (ASGCT Annual Meeting Abstracts) 2011;19:133. [Google Scholar]
- Recher M. Burns S.O. de la Fuente M.A., et al. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819–2828. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B.Y. Evans-Galea M.V. Gray J.T., et al. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramuzza S. Biasco L. Ripamonti A., et al. Preclinical safety and efficacy of human CD34(+) cells transduced with lentiviral vector for the treatment of Wiskott-Aldrich syndrome. Mol. Ther. 2013;21:175–184. doi: 10.1038/mt.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C.R. Kim M.O. Li D., et al. Outcomes following hematopoietic cell transplantation for Wiskott-Aldrich syndrome. Bone Marrow Transplant. 2012;47:1428–1435. doi: 10.1038/bmt.2012.31. [DOI] [PubMed] [Google Scholar]
- Strom T.S. Gabbard W. Kelly P.F., et al. Functional correction of T cells derived from patients with the Wiskott-Aldrich syndrome (WAS) by transduction with an oncoretroviral vector encoding the WAS protein. Gene Ther. 2003;10:803–809. doi: 10.1038/sj.gt.3301950. [DOI] [PubMed] [Google Scholar]
- Thrasher A.J. Hacein-Bey-Abina S. Gaspar H.B., et al. Failure of SCID-X1 gene therapy in older patients. Blood. 2005;105:4255–4257. doi: 10.1182/blood-2004-12-4837. [DOI] [PubMed] [Google Scholar]
- Westerberg L.S. de la Fuente M.A. Wermeling F., et al. WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood. 2008;112:4139–4147. doi: 10.1182/blood-2008-02-140715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. Mody D. DeRaven S.S., et al. A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LM02 expression in human T cells. Blood. 2010;16:900–908. doi: 10.1182/blood-2009-10-250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. Lu T. Zhijun M., et al. A novel Jurkat-LM02 assay system for vector safety testing and insulator screening. Mol. Ther. 2012;20(Suppl 1):S136. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.