Abstract
Glioblastoma multiforme (GBM) is the most common malignant primary brain cancer in adults; it carries a dismal prognosis despite improvements in standard of care. We developed a combined gene therapy strategy using (1) herpes simplex type 1-thymidine kinase in conjunction with the cytotoxic prodrug ganciclovir to kill actively proliferating tumor cells and (2) doxycycline (DOX)-inducible Fms-like tyrosine kinase 3 ligand (Flt3L), an immune stimulatory molecule that induces anti-GBM immunity. As a prelude to a phase I clinical trial, we examined the efficacy and safety of this approach (Muhammad et al., 2010, 2012). In the present article, we investigated the efficacy and safety of the “off-label” use of the antibiotic DOX to turn on the high-capacity adenoviral vector (HC-Ad) encoding therapeutic Flt3L expression. DOX-inducible Flt3L expression in male Lewis rats was assessed using DOX doses of 30.8 mg/kg/day (low-DOX) or 46.2 mg/kg/day (high-DOX), which are allometrically equivalent (Voisin et al., 1990) to the human doses that are recommended for the treatment of infections: 200 or 300 mg/day. Naïve rats were intracranially injected with 1×109 viral particles of HC-Ad-TetOn-Flt3L, and expression of the therapeutic transgene, that is, Flt3L, was assessed using immunohistochemistry in brain sections after 2 weeks of DOX administration via oral gavage. The results show robust expression of Flt3L in the rat brain parenchyma in areas near the injection site in both the low-DOX and the high-DOX groups, suggesting that Flt3L will be expressed in human glioma patients at a DOX dose of 200 or 300 mg/day. These doses have been approved by the U.S. Food and Drug Administration to treat infections in humans and would thus be considered safe for an off-label use to treat GBM patients undergoing HC-Ad-mediated gene therapy in a phase I clinical trial.
VanderVeen and colleagues perform preclinical studies of doxycycline-mediated transgene induction for an adenovirus-based therapeutic targeting glioblastoma multiforme (GBM). They demonstrate that transgene expression is readily activated in the rat brain using doses of doxycycline equivalent to those currently approved for treating infections in humans, suggesting that transgene induction can be achieved at doses that will be well tolerated by patients.
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults, is highly invasive, and has a median survival of ∼15–21 months (Grossman et al., 2010). The current standard of care involves the resection of the tumor mass with adjuvant radiotherapy and chemotherapy; however, because of the highly infiltrative nature of GBM, total tumor resection is virtually impossible and the tumor invariably recurs. Tumor recurrence is associated with novel mutations and genetic rearrangements, which makes its treatment very challenging as patients with recurrent GBM are generally unresponsive to chemotherapy and radiotherapy. To develop a therapeutic approach for GBM that mediates improved survival—including elimination of tumor cells that infiltrate the brain parenchyma—we pursued a combined conditional cytotoxic/immune-stimulatory gene therapy approach based on high-capacity, helper-dependent adenoviral vectors (HC-Ad). We have performed rigorous preclinical studies examining the efficacy and safety of the combined HC-Ad-TK+HC-Ad-TetOn-Flt3L gene therapy in preclinical models of intracranial GBM (Muhammad et al., 2010, 2012).
The implementation of this therapy in the clinic will involve injection of both HC-Ads in the tumor bed after total tumor resection, followed by systemic administration of Valacyclovir, as the thymidine kinase (TK) prodrug (Fujita et al., 2006; Hinata et al., 2006; Shirakawa et al., 2007; Chiocca et al., 2011), and doxycycline (DOX) as the transcriptional activator of Fms-like tyrosine kinase 3 ligand (Flt3L) from the TetOn promoter (Candolfi et al., 2006; Xiong et al., 2006; Curtin et al., 2008b; Muhammad et al., 2010, 2012). Importantly, the DOX-regulatable expression of Flt3L is an added safety feature of the treatment, and allows us to turn “on” and “off” gene expression upon the addition or withdrawal of DOX, respectively (Candolfi et al., 2006; Xiong et al., 2006; Curtin et al., 2008b). In addition, the use of HC-Ads is expected to reduce the generation of anti-Ad antibodies; this may simultaneously improve efficacy and safety.
DOX is typically used in humans as an antibiotic to treat various types of infections (DRUGDEX, 2012). When testing the therapeutic efficacy of HC-Ad-TK+HC-Ad-TetOn-Flt3L in the preclinical studies, DOX was administered in rat chow at a concentration of 1,000 parts per million (ppm). Robust protein expression of Flt3L in brain tissue was shown using both ELISA and immunostaining in these studies; our data also showed high therapeutic efficacy and safety of the HC-Ad-mediated gene therapy approach (Muhammad et al., 2010, 2012).
Despite the safety and accepted use of DOX as an antibiotic to treat infections in patients, the use of DOX to activate gene transcription from the TetOn regulatable promoter system is not yet approved for use in humans. Thus, this would be an “off-label” use for this drug. Therefore, as requested by the U.S. Food and Drug Administration (FDA) before the phase I clinical trial, we assessed the dosage, route of delivery, and schedule of DOX administration that are required to turn on Flt3L expression from the TetOn promoter system encoded within HC-Ads in rodents. We demonstrate here that allometrically scaled doses of DOX, that is, equivalent doses recommended for use in humans to treat infections, can safely turn on Flt3L expression from the HC-Ad-TetOn-Flt3L vector in rodents. We conclude that our data warrant the use of DOX at doses that have been previously approved by the FDA to treat infections in humans in an off-label application of this drug for the treatment of GBM grade IV in human patients undergoing clinical trials using HC-Ad-TK+HC-Ad-TetOn-Flt3L gene therapy. Further, these data will be of high interest to other researchers aiming to implement TetOn-dependent therapeutic transgene expression within the central nervous system for the treatment of other neurological disorders.
Results and Discussion
Clinical trial
Malignant gliomas (GBM) have a very poor prognosis with a median survival that is measured in months rather than years (Wen and Kesari, 2008; Grossman et al., 2010). Such an abysmal disease prognosis compels novel therapeutic approaches. We propose that the combination approach incorporating the crucial immune-stimulating component through HC-Ad-mediated gene transfer (i.e., Flt3L) will provide the next advancement in the development of an effective treatment for GBM. In our combined approach, the HSV1-TK does not play the pivotal role, as its aim is to kill sufficient tumor cells to provide tumor antigens for incoming immune cells (Curtin et al., 2009). The pivotal role of our approach is played by Flt3L, which we have shown in many different models to recruit antigen presenting immune cells to the brain (Curtin et al., 2006, 2008a, 2009). The primary objective of the phase I trial will be to evaluate a range of doses and identify a maximum tolerated dose (MTD) of HC-Ad-TK+HC-Ad-TetOn-Flt3L when administered into the peritumoral region of resected malignant gliomas (GBM WHO grade IV). The secondary objective will be to determine early therapeutic benefits of HC-Ad-TK+HC-Ad-TetOn-Flt3L gene therapy in primary malignant glioma patients. The subject population will comprise 18 evaluable adult male and female subjects with malignant gliomas who in the opinion of the clinical investigator are candidates for surgical treatment and who meet all inclusion and exclusion criteria defined for this trial (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/humc) to be treated in one of five escalating dose cohorts.
As study agents, two HC-Ad vectors will be used; each vector will encode one therapeutic transgene. Both vectors are human serotype 5, replication-defective, high-capacity, helper-dependent adenoviral vectors deleted in all viral genes with the exception of the viral packaging sequences. HC-Ad-TK expresses herpes-simplex thymidine kinase under the constitutive mouse cytomegalovirus promoter; when administered together with the oral prodrug valacyclovir, highly proliferating tumor cells are preferentially killed. The other vector, HC-Ad-TetOn-Flt3L, expresses human soluble Flt3L under the control of the DOX-inducible rtTA2SM2/tTSKid promoter system; transcription of the immunostimulatory Flt3L is turned on with oral administration of DOX (Candolfi et al., 2006; Xiong et al., 2006; Curtin et al., 2008b; Muhammad et al., 2010, 2012).
Both HC-Ad vectors will be manufactured at the vector production facility at Baylor College of Medicine Center for Cell and Gene Therapy. The HC-Ad vector particles will be formulated in 20 mM Tris/25 mM sodium chloride/2.5% glycerol (w/w) (pH 8.0). For dose levels requiring dilution before administration, the vialed product will be diluted with sterile saline by the hospital pharmacist.
As the prodrug for the TK cytotoxic vector, patients will be treated with valacyclovir, which has received marketing approval by FDA as an orally administered antiherpetic drug (FDA-NDA#020487, 2010; Baker, 1999; Tyring et al., 2012). Valacyclovir is a valine-ester of acyclovir that is quickly converted to acyclovir by first-pass intestinal and/or hepatic metabolism. Clinical studies comparing intravenous acyclovir to oral valacyclovir delivery have shown that 10 mg/kg of intravenous acyclovir given over 1 hr every 8 hr and 2 g of valacyclovir given every 8 hr have a similar concentration over time profile as acyclovir (AUC; hr-μg/ml) (Aguilar-Cordova, 2001). On the basis of the data from this study, this protocol will use a dose of 2 g three times daily for 14 days.
For transcription activation of Flt3L from the inducible TetOn promoter system, DOX will be administered orally. DOX is approved for the treatment of a number of different types of bacterial infections. The typical dose is 100 mg/day, but 200 or 300 mg/day is also indicated for more serious infections. For the clinical trial, we will propose to use a DOX dose of 200 mg/day for 1 month. A brief overview of the treatment plan follows: subjects meeting all inclusion and exclusion criteria (Supplementary Table S1) and providing informed consent will be enrolled in the sequential dosing cohorts. This protocol will be structured as a dose escalation study of HC-Ad-TK+HC-Ad-TetOn-Flt3L administered at the time of surgery after the tumor has been resected. The oral prodrug, valacyclovir, will be started 1–3 days after vector administration, and continued for 14 days. DOX will also be delivered orally, twice a day, and continued for 1 month. Temozolomide (Temodar) will begin 15–17 days after surgery and administered at 75 mg/m2 daily for 42 days concomitant with focal radiotherapy (60 Gy administered in 30 fractions) followed by maintenance Temodar for 12 cycles. Intensive prospective safety monitoring will take place. Systematic clinical assessments will occur over a 24-month period postoperatively. The experimental cohorts will be as follows—cohort 1: 1×1010 viral particles (vp) HC-Ad-TK; cohort 2: 1×1010 vp HC-Ad-TK+1×109 vp HC-Ad-TetOn-Flt3L; cohort 3: 1×1011 vp HC-Ad-TK+1×109 vp HC-Ad-TetOn-Flt3L; cohort 4: 1×1011 vp HC-Ad-TK+1×1010 vp HC-Ad-TetOn-Flt3L; cohort 5: 1×1011 vp HC-Ad-TK+1×1011 vp HC-Ad-TetOn-Flt3L. The first patient will be assigned to the first dose (TK alone), and the dose for all future patients will be sequentially determined from the TITE-CRM algorithm (Cheung and Chappell, 2000; Zhao et al., 2011); MTD will be determined after 15 patients are observed. The TITE-CRM method assumes a model for the time of occurrence of a toxic response as a function of dose, and allows information from all patients enrolled in the trial to be employed when allocating a new patient to a dose level. Subjects will be continuously recruited throughout the trial—without recruitment pauses—as long as patients are treated at a dose consistent with the current safety profile. It has been shown that TITE-CRM designs are more efficient (Goodman et al., 1995; Normolle and Lawrence, 2006; Zhao et al., 2011) than traditional 3+3 designs. They identify MTD more accurately and provide phase II information, but do not expose patients to additional risk.
For the surgical procedure, a craniotomy with resection of the tumor will be performed. At the neurosurgeon's discretion, this may involve stereotactic methods and/or intraoperative navigational guidance and/or intraoperative magnetic resonance imaging or other radiologic guidance. Tumor resection will be performed per routine. After the tumor resection has been completed and adequate hemostasis obtained, two tuberculin syringes will be used to deliver the HC-Ad-TK+HC-Ad-TetOn-Flt3L vectors in 100 μl at each of 20 sites (the total volume infused will be 2 ml). The assignment of dosages will have been determined preoperatively as per protocol. Injections will take place over 2 min for each syringe. The vectors will be brought from the pharmacy in sterile vials, and the filling of the syringes will occur on a sterile table next to the main operating room table. If needed, each vial and/or syringe could be filled with an additional volume of sterile, preservative-free saline to bring up the volume to 100 μl/site. The vials will not be in the operating room at room temperature for more than 4 hr before use. The injection sites will be selected by the surgeon to avoid injections into adjacent motor or speech cortex, the cerebral ventricle, or seepage into the subarachnoid space. After the injections are complete, the needle will be slowly withdrawn, and the remainder of the surgery will consist of routine wound closure. Staples will not be used because of interference with subsequent radiation therapy. It is expected that the delivery of the viral vectors will require an additional 40 min to the time of surgery. It is expected that each subject will stay up to four nights in the hospital after surgery. Before discharge, the subject must be independent at baseline (preoperative status) to be able to walk, speak, and go to the bathroom. If the patient does not meet the aforementioned criteria, yet he/she is stable from a cardiopulmonary standpoint, the subject will be discharged to a rehabilitation facility. Chemotherapy and radiation therapy will be performed according to current clinical practice (Stupp et al., 2005, 2007).
The primary objective of this phase I trial is to evaluate the safety and tolerability of intratumor cavity HC-Ad-mediated gene therapy as assessed by adverse events, laboratory safety parameters, general physical/neurological examination, and the presence of circulating antiadenovirus antibodies. Toxicity will be assessed and graded by the NCI Common Terminology Criteria for Adverse Events v4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/About.html). Once the MTD is determined, or after all subjects have been evaluated and found to tolerate the highest dose, the phase 1 study will end and the results will be reviewed. The appropriate dose for a future phase 2 study will be the highest administered dose or MTD from this study. The clinical and tumor responses will include the following: functional status, medication log, medical and neurological examinations, magnetic resonance imaging, survival from date of operation, survival with respect to pre-existing neutralizing anti-adenovirus antibodies, and progression-free survival. Ad-hCMV-TK with concomitant valacyclovir administration in various cancers has been well tolerated in previous and ongoing clinical trials using the same dosages that will be evaluated in this study. Additionally, the use of the HC-Ad virus is expected to further decrease the toxicity to the scaffolding of the normal brain parenchyma as seen in myriad animal models. Results from previous and on-going clinical studies in various cancers with Ad-hCMV-TK followed by valacyclovir indicate that the treatment is well tolerated within the dose range to be evaluated in this study, and has been associated with minimal discomfort. In addition, there have been reports of partial responses in some patients (Sandmair et al., 2000; Immonen et al., 2004; Chiocca et al., 2011). The standard of care for the patients who are eligible for this protocol will not be altered by their participation in receiving this experimental therapy. On the basis of the collective body of information gleaned from nonclinical and clinical studies with very similar approaches, the expectation is that there will be minimal risk for the individual patients participating in this protocol.
Objectives and study design
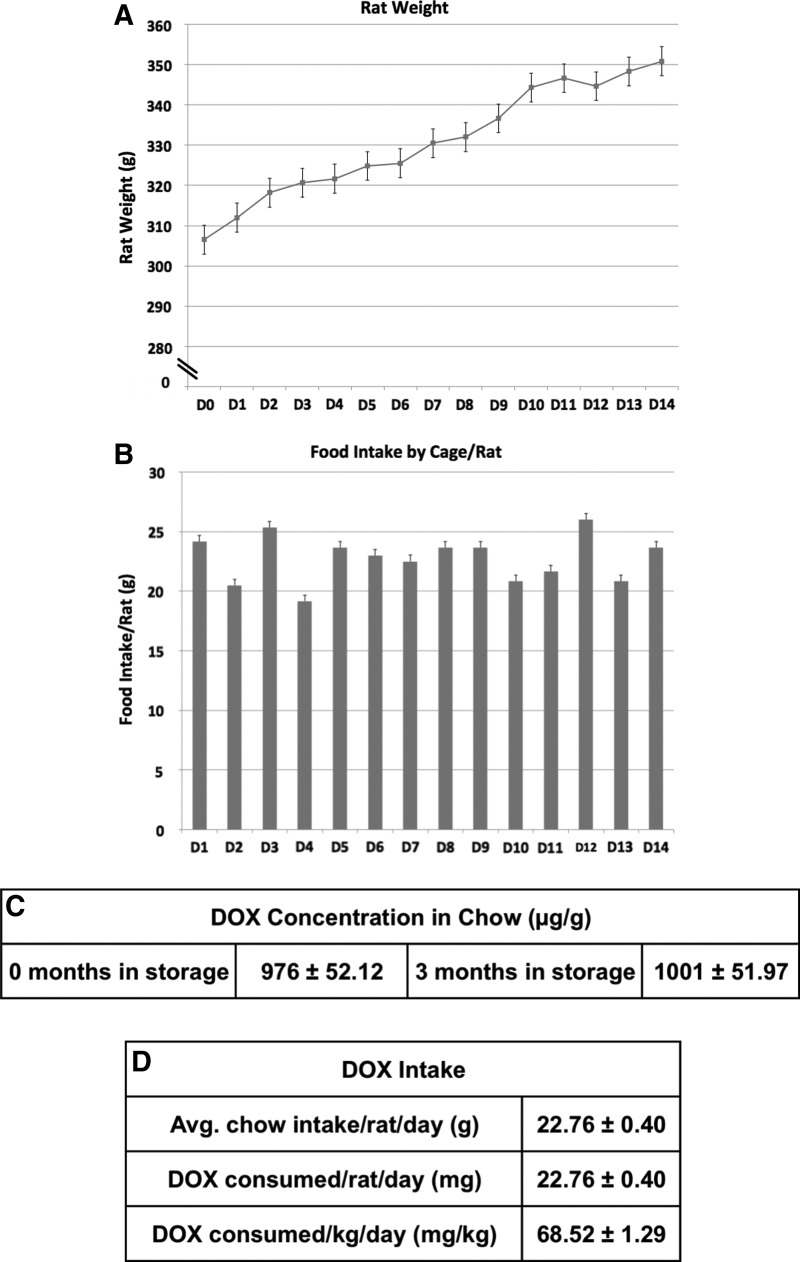
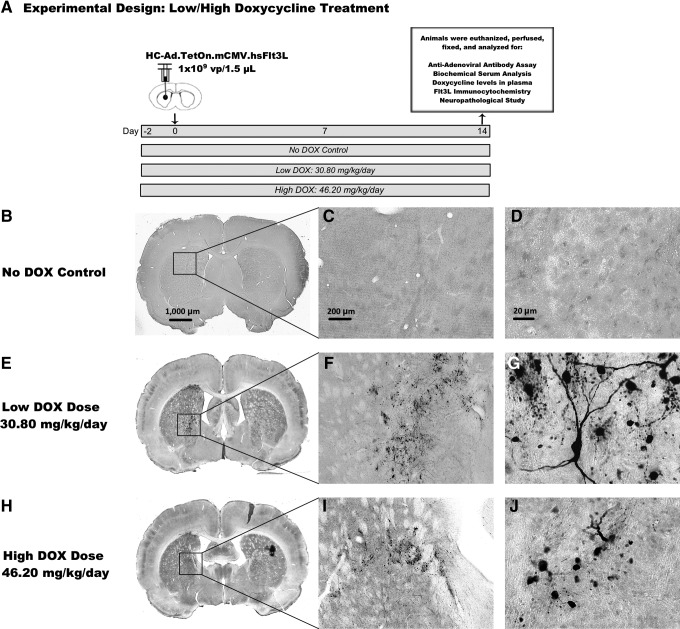
The primary objective of the present work was to evaluate whether allometrically scaled doses of DOX are able to activate Flt3L gene expression from the HC-Ad-TetOn-Flt3L in rat brains. Safety assessments, including complete blood count, serum biochemistry, neuropathology, and circulating neutralizing antibody titer, were included as secondary outcomes of the study. To assess if DOX doses previously used by us (Muhammad et al., 2010) were compatible with the FDA-approved doses of DOX used in the clinic to treat infections in humans, we performed a feeding trial (Fig. 1). The aim was to determine the amount of DOX consumed by rats when given ad libitum access to 1,000 ppm DOX-containing chow (Fig. 1C). Rats were given free access to DOX chow for 2 weeks, and the amount of DOX consumed per rat was calculated (Fig. 1D). We determined that the previously used dose of DOX to turn on transgene expression in Lewis rats in vivo was higher than the levels approved for treating infections in humans. Thus, to progress toward the clinical implementation of this combined gene therapy in a phase I clinical trial for GBM, it was critical to determine whether allometrically scaled doses of DOX were able to induce robust Flt3L expression in the brain. To this end, HC-Ad-TetOn-Flt3L vector was injected into the striatum of naïve Lewis rats, and 30.8 or 46.2 mg/kg/day of DOX was administered by oral gavage for 2 weeks. Tissues were harvested at the end of the study for Flt3L expression, neuropathology, neutralizing antibody titer, and serum chemistry; a schematic of the experimental design is shown in Fig. 2A.
FIG. 1.
Feeding trial in male Lewis rats consuming DOX chow. Six male Lewis rats were housed three to a cage (two cages total) and were fed DOX chow for 2 weeks ad libitum, while the daily weight of each animal (A) was measured. (B) DOX chow consumption per cage was also measured. (C) DOX concentration in the rat chow (TestDiet, cat. no. 5001) was determined at the University of Michigan College of Pharmacy Pharmacokinetics Core facility using a liquid chromatography and dual-mass spectrometry (LC-MS/MS) system; samples were tested upon arrival (0 months in storage), and after being refrigerated for 3 months (3 months in storage). (D) Using the aforementioned data, we were able to calculate the average chow intake/rat/day to then determine the amount of DOX that rats undergoing the preclinical testing consumed (mg/kg/day). DOX, doxycycline.
FIG. 2.
Determination of effective dose of DOX to turn on therapeutic transgene expression from HC-Ad-TetOn-Flt3L. (A) Diagram of experimental design used. Adult naïve Lewis rats were injected stereotactically into the right striatum with 1×109 viral particles of HC-Ad-TetOn-Flt3L. All rats received DOX or water (control group) via oral gavage twice daily 2 days before the injection and for 14 days after the injection. One group received 30.80 mg/kg/day (low-DOX), while the second group received 46.20 mg/kg/day (high-DOX). The control group received equal volumes of water (No DOX control). Rats were evaluated 14 days after the injection for circulating levels of anti-adenovirus-neutralizing antibodies in the plasma, serum biochemistry, circulating DOX levels, transgene expression, and neuropathology. The brains were processed for immunocytochemistry using primary antibodies against Flt3L to detect expression of the therapeutic transgene in the striatum. Transgene expression of Flt3L in (B–D) the absence of DOX, (E–G) low-DOX dose of 30.80 mg/kg/day (human equivalent: 200 mg), and (H–J) the high-DOX dose of 46.20 mg/kg/day (human equivalent: 300 mg) are shown within the striatum of all HC-Ad-injected animals. Scale bar: 1,000 μm for full brain sections, 200 μm for 5× images, and 20 μm for 40× images. Flt3L, Fms-like tyrosine kinase 3 ligand; HC-Ad, high-capacity, helper-dependent adenoviral vectors.
Summary of data
Feeding trial in Lewis rats
To address the need to develop novel therapeutics for GBM, we tested the efficacy and safety of a combined high-capacity adenoviral gene transfer therapy, HC-Ad-TK+HC-Ad-TetOn-Flt3L, in preclinical studies (Muhammad et al., 2010, 2012). The therapy combines the use of HSV1-TK+ganciclovir, a conditional cytotoxic paradigm to induce tumor cell killing with the induction of immune stimulation elicited by HC-Ad-mediated Flt3L expression within the tumor microenvironment (Ali et al., 2004, 2005). This combined gene therapy strategy results in tumor regression and the induction of an antitumor immune response (Curtin et al., 2006; King et al., 2008a, 2008b, 2011; Ghulam Muhammad et al., 2009; Muhammad et al., 2010). As an added safety feature, the expression of Flt3L is under the control of the TetOn regulatable promoter system (Xiong et al., 2006, 2008), in which Flt3L transcription is turned on in the presence of DOX and is turned off in the absence of DOX (Muhammad et al., 2010).
DOX is a broad-spectrum tetracycline antibiotic widely used to treat infections in humans. The typical dosing regime is 200 mg on the first day followed by 100 mg/day (Vibramycin product labeling, Pfizer; Monodox product labeling, Aqua Pharmaceuticals), while severe infections require 200 mg/day. Either as a single dose or continued for 10 days, 300 mg/day has also been used. For example, the recommended adult treatment regimen for Acinetobacter, acne vulgaris, bartonellosis, Clostridia, Escherichia coli, and listeriosis, among others, is 200 mg orally on day 1 followed by 100 mg/day. For more severe infections with these organisms, 200 mg/day is recommended (DRUGDEX, 2012). Certain types of serious infections warrant increased dose. For example, a dose of 200 mg DOX/day for 2 months in conjunction with additional antibiotics is recommended for anthrax inhalation (DRUGDEX, 2012). For late latent syphilis, 200 mg/day for 1 month is recommended (DRUGDEX, 2012). Doses of 300 mg are rare; however, product labeling for Monodox indicates treatment with 300 mg/day for at least 10 days for primary and secondary syphilis, and the DRUGDEX database occasionally indicates the use of a 300 mg dose for cholera. The dosing duration is typically 7–10 days, but for some indications the recommended duration is 3–4 weeks or more. It is important to note that these doses are recommended for the treatment of infection in humans. The use of DOX to activate gene transcription from a regulatable TetOn promoter in a gene therapy clinical trial will therefore constitute off-label use of DOX. Thus, the FDA required further characterization related to the use of DOX for this purpose.
Preclinical studies using tumor-bearing male Lewis rats have shown that the combined HC-Ad therapy can effectively and safely treat an intracranial, syngeneic model of glioma (Muhammad et al., 2010, 2012). In these previously published studies, DOX was administered ad libitum orally in rat chow. In preparation for a phase I clinical trial for GBM, it will be important to estimate the DOX dose necessary to turn on Flt3L expression in humans, and assess if this dose is compatible to doses of DOX currently used to treat infections in humans. The use of DOX to turn on therapeutic gene expression from HC-Ad-TetOn-Flt3L will be an off-label use of this drug. In response to FDA requirements to enable filing of an Investigational New Drug for this approach, we needed to determine the dose of DOX that would turn on therapeutic transgene expression for the HC-Ad-TetOn-Flt3L and assess if the required DOX doses would be compatible to the currently used doses of DOX in humans. To this end, we performed a feeding trial with DOX chow in male Lewis rats to quantify the average amount of DOX consumed by each rat per day. (See Supplementary Materials and Methods.) The results of the 2-week trial are shown in Fig. 1. Figure 1A shows that the average weight of the rats (n=6) increased from approximately 310 to 350 g over the course of the trial. Figure 1B shows the chow intake on each day, which remained relatively constant over the 2-week period. Using the estimated value of 1,000 ppm for the DOX concentration in the rat chow (Fig. 1C) and an average chow intake of 22.76 g/day (Fig. 1D), we calculated the average rat daily DOX intake to be 68.52 mg/kg/day over the course of the study ([22.76 mg/day]/0.330 kg=68.52 mg/kg/day).
DOX-mediated Flt3L expression after HC-Ad-TetOn-Flt3L injection
To compare the dose of DOX previously used in the rodent preclinical efficacy and safety data of the HC-Ad-mediated gene therapy approach with the doses of DOX administered to humans to treat infections, we converted the rat DOX dose to a human dose by dividing the rat dose of DOX by the allometric conversion factor, that is, 7 (Voisin et al., 1990), and multiplying by 45.45 kg, an accepted minimum weight for an adult human (DRUGDEX, 2012). This resulted in an estimated human dose of 445 mg/day. Since this DOX dose is higher than the dose typically used to treat infections in humans, we performed an additional study in rats to assess whether Flt3L can be expressed from the HC-Ad-TetOn-Flt3L vector using DOX doses allometrically equivalent to those currently used in humans.
In humans, a typical dose of DOX administered for infections is 100 mg/day; however, for more serious infections, 200 mg/day, or even 300 mg/day, may be recommended (DRUGDEX, 2012). Therefore, we performed a study to assess Flt3L transgene expression in rats orally gavaged with DOX doses allometrically equivalent to two of the doses used in humans, that is, 200 and 300 mg/day.
To do this, we allometrically converted the two human doses for rats using the human-to-rat conversion factor of 7 (Voisin et al., 1990). Assuming a human weight of 45.45 kg, an accepted minimum weight for an adult human (DRUGDEX, 2012), we calculated the 200 mg/day human dose to be equivalent to a rat dose of 30.8 mg/kg/day (low-DOX group). Similarly, we calculated the 300 mg/day human dose to be equivalent to a rat dose of 46.2 mg/kg/day ([(300 mg/day)/45.45 kg]×7=46.2 mg/kg/day: high-DOX group). As shown in the study timeline in Fig. 2A, these two doses of DOX were administered to male Lewis rats via oral gavage starting on day −2 and lasting until day 14. The HC-Ad-TetOn-Flt3L vector containing the DOX-inducible Flt3L gene was injected into the striatum on day 0. On the final day of the experiment, day 14, the rats were transcardially perfused with Tyrode's solution and fixed with 4% paraformaldehyde. To assess Flt3L expression, we performed immunohistochemistry on free-floating fixed brain sections. As shown in Fig. 2E–G and Fig. 2H–J, robust Flt3L expression was observed in both the low-DOX and the high-DOX groups, respectively, compared with the no DOX control, which showed no expression of Flt3L (Fig. 2B–D). These data suggest that the allometrically equivalent human doses will be able to activate Flt3L expression in the clinical setting with doses currently used to treat bacterial infections.
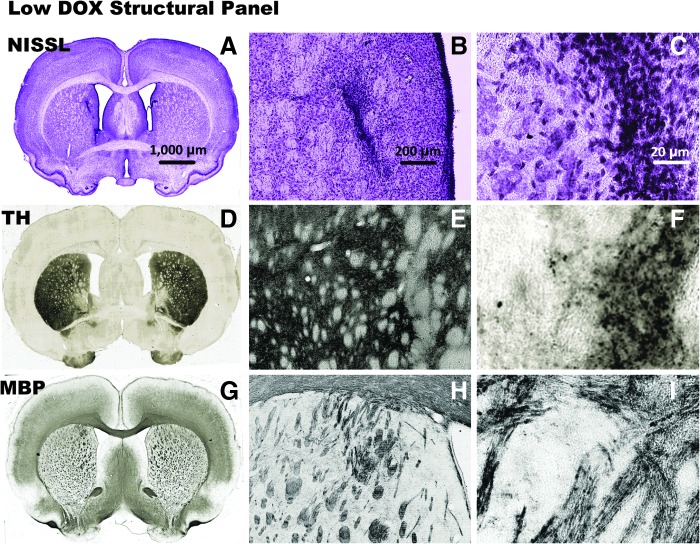
Neuropathology analysis
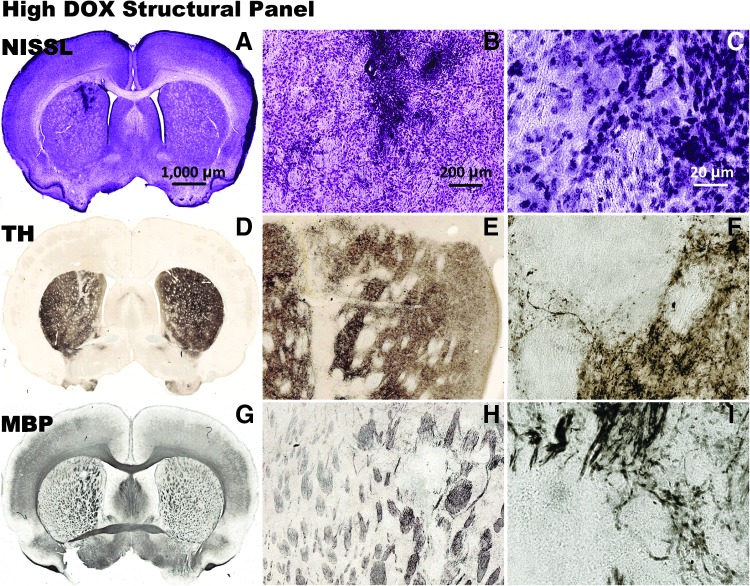
To investigate the effects of HC-Ad-TetOn-Flt3L injection and DOX administration on the architecture and immune cellular infiltrates in the low DOX groups (Fig. 3 and Fig. 4) and the high DOX groups (Fig. 5 and Fig 6) within the brain, we performed a detailed neuropathology analysis of brain sections at the end of the study. In the no-DOX control (Supplementary Fig. S1A–C), the low-DOX (Fig. 3A–C), and the high-DOX (Fig. 5A–C) groups, NISSL staining demonstrated largely unaffected gross tissue morphology with an area of increased cell density near the needle track. Immunostaining for tyrosine hydroxylase (Supplementary Fig. S1D–F, Fig. 3D–F, and Fig. 5D–F), indicative of dopaminergic nerve terminals, showed no gross morphological changes; decreased staining intensity was present in the vicinity of the needle track. Immunostaining for myelin basic protein (Supplementary Fig. S1G–I, Fig. 3G–I, and Fig. 5G–I), which indicates myelinated axons, also showed no gross morphological changes.
FIG. 3.
Neuropathological analysis after injection with HC-Ad-TetOn-Flt3L is shown after 14 days of low-DOX (30.80 mg/kg/day) treatment. The brains were processed for NISSL staining to show gross morphology (A–C), and immunocytochemistry using primary antibodies against TH to label striatal dopaminergic fibers (D–F), and MBP to label oligodendrocytes and myelin sheaths (G–I). Scale bar: 1,000 μm for full brain sections, 200 μm for 5× images, and 20 μm for 40× images. MBP, myelin basic protein; TH, tyrosine hydroxylase.
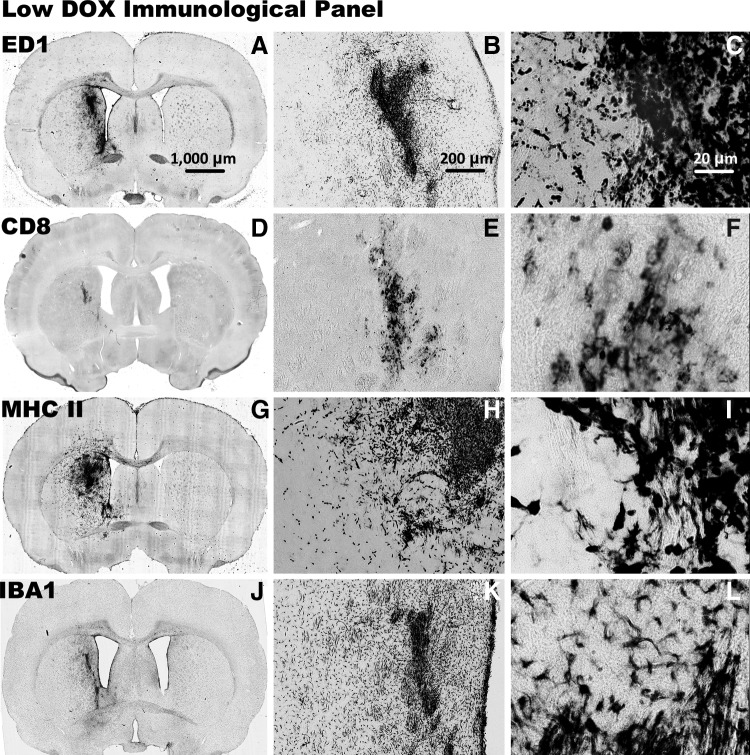
FIG. 4.
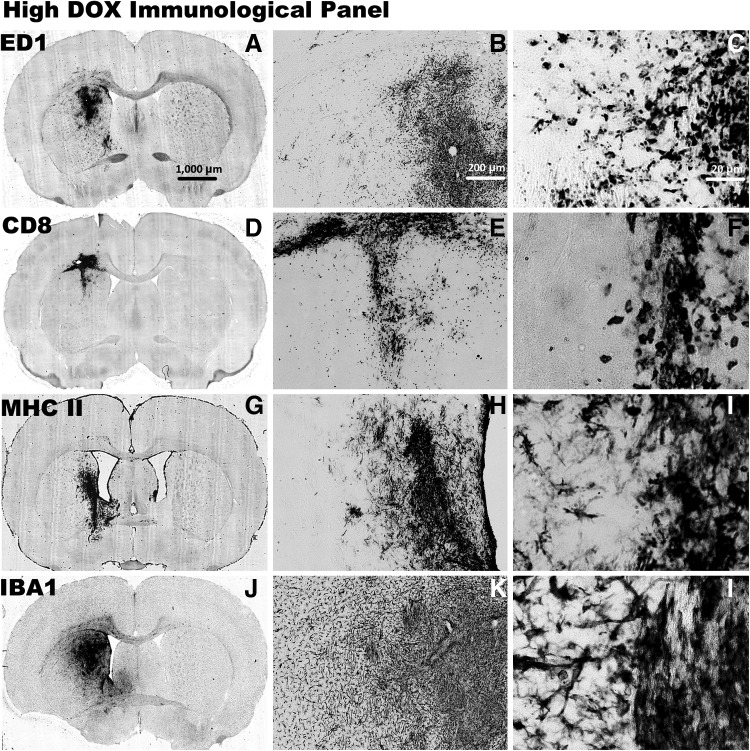
Immune cellular infiltrates after injection with HC-Ad-TetOn-Flt3L are shown after 14 days of low-DOX (30.80 mg/kg/day) treatment. The brains were processed with immunocytochemistry using primary antibodies against ED1 for labeling macrophages and activated microglia (A–C), CD8+T cells (D–F), MHC-II to label MHC-II+macrophages, microglia, and immune cells (G–I), and IBA1 to label activated macrophages and microglia (J–L). Scale bar: 1,000 μm for full brain sections, 200 μm for 5× images, and 20 μm for 40× images.
FIG. 5.
Neuropathological analysis after injection with HC-Ad-TetOn-Flt3L is shown after 14 days of high-DOX (46.20 mg/kg/day) treatment. The brains were processed for NISSL staining to show gross morphology (A–C), and immunocytochemistry using primary antibodies against TH to label striatal dopaminergic fibers (D–F), and MBP to label oligodendrocytes and myelin sheaths (G–I). Scale bar: 1,000 μm for full brain sections, 200 μm for 5× images, and 20 μm for 40× images.
FIG. 6.
Immune cellular infiltrates after injection with HC-Ad-TetOn-Flt3L are shown after 14 days of high-DOX (46.20 mg/kg/day) treatment. The brains were processed with immunocytochemistry using primary antibodies against ED1 for labeling macrophages and activated microglia (A–C), CD8+T cells (D–F), MHC-II to label MHC-II+macrophages, microglia, and immune cells (G–I), and IBA1 to label activated macrophages and microglia (J–L). Scale bar: 1,000 μm for full brain sections, 200 μm for 5× images, and 20 μm for 40× images.
Immune cellular infiltrates were present in the no-DOX control, the low-DOX, and the high-DOX groups surrounding the injection site. Immunostaining for ED1 (Supplementary Fig. S1J–L, Fig. 4A–C, and Fig. 6A–C), which is a marker for activated macrophages, microglia, and monocytes, showed immunoreactive cells in the vicinity of the injection site. Immunostaining for CD8+ T-cells (Supplementary Fig. S1M–O, Fig. 4D–E, and Fig. 6D–E) showed cellular infiltration near the injection site, as did immunostaining for MHC-II (Supplementary Fig. S1P–R, Fig. 4G–I, and Fig. 6G–I), a marker of antigen presenting cells, and IBA1 (Supplementary Fig. S1S–U, Fig. 4J–L, and Fig. 6J–L), which is a marker for activated microglia. As shown in Figs. 4 and 6, CD8+ staining and IBA1+ staining were less intense in the low-DOX group compared with the high-DOX group, with even less staining in the no-DOX control group (Supplementary Fig. S1). Immune cells were occasionally observed along the lining of the ventricles, but no positive staining for ED1, CD8, MHC-II, or IBA1 was present in the contralateral hemisphere. There was no loss of tissue or enlargement of the ventricles in either group, confirming the safety of the HC-Ad-TetOn-Flt3L vector in the presence of the two doses of DOX tested in this study.
Circulating neutralizing antiadenovirus antibodies
To measure the levels of circulating neutralizing adenovirus antibodies (NAABs), blood was collected from each animal during euthanasia via cardiac puncture (see Supplementary Materials and Methods). Antibody titers of 64 or below were considered negative, and titers of 128 or above were considered positive (Nwanegbo et al., 2004; Puntel et al., 2013). As shown in Supplementary Fig. S2, the results demonstrate that plasma levels of NAABs in both the low-DOX and the high-DOX groups were similar to that found in the saline-injected control group, and all were below the positive-titer threshold of 128. This indicates that the HC-Ad vectors delivered into the brain parenchyma do not induce a systemic immune response to the Ad capsid.
DOX concentration in rat plasma
Analysis of the rat plasma after 2 weeks of DOX administration showed a DOX concentration in the low-DOX treated group of 1.27±0.295 mg/liter (mean±SEM) and a DOX concentration of 3.06±0.254 mg/liter in the high-DOX treated group (see Supplementary Materials and Methods, Supplementary Table S1). These levels are comparable to those measured in humans. Product labeling for Vibramycin capsules notes that after a 200 mg oral dose of DOX, normal adults had an average serum concentration of 2.6 mg/liter after 2 hr and 1.5 mg/liter after 24 hr. Product labeling for another formulation, Monodox, cites an average maximum serum concentration of 3.61±0.18 mg/liter approximately 2.6 hr after a 200 mg DOX dose. In a review of studies examining the plasma pharmacokinetics of DOX after a 200 mg single dose, maximum concentration of 5 mg/liter has been measured (Agwuh and MacGowan, 2006). These data suggest that the plasma DOX concentrations achieved with DOX doses currently approved for treating infections in humans are compatible with DOX levels required to achieve therapeutic transgene expression from HC-Ad-TetON-Flt3L.
Analysis of clinical laboratory parameters
To evaluate any potential systemic toxicity, we measured several clinical parameters in the plasma, including indicators of renal and liver function (see Supplementary Materials and Methods, Supplementary Table S2). Both the low-DOX and the high-DOX groups were shown to have normal liver and renal parameters with respect to naïve Lewis rats, although ALT (alanine aminotransferase) levels were slightly elevated in both the low-DOX and the high-DOX groups. Normal renal function was confirmed with analysis of blood urea nitrogen (BUN), creatinine (CRE), and albumin (ALB). These results on the safety of the HC-Ad therapy with DOX administration are consistent with those demonstrated in other studies by our group (Muhammad et al., 2010, 2012).
An issue related to the off-label use of DOX to activate Flt3L expression in glioma patients is to assess its safety profile at the doses required to turn on therapeutic Flt3L gene expression. In this respect, the safety of DOX in humans has been studied previously. A pharmacology and toxicology study for a previously marketed DOX product (Periostat; NDA # 50-744) tested increasing doses of DOX in rats. In a 2-week study in rats that used DOX doses ranging from 50 to 400 mg/kg/day, which included analysis of food intake, body weight, clinical symptoms, organs, and organ weights, slight side effects, including excess salivation and reduced food intake, were seen only in the highest dose group of 400 mg/kg/day. In line with these data, we show in the present report that Flt3L can be expressed at both 30.8 and 46.2 mg/kg/day of DOX with no signs of toxicity; these two DOX doses are well below the 400 mg/kg/day dose identified as toxic in the Periostat toxicology review.
Conclusions
In the present study, we show that Flt3L is expressed in rat brain tissue using allometrically scaled doses of DOX equivalent to those recommended to treat infections in humans. Importantly, the DOX concentrations in rat plasma measured in this study are quantitatively similar to those seen in human serum after treatment using a 200 mg dose of DOX in adults. In this study, no toxicities were observed in either the low-DOX or the high-DOX treatment groups both within the central nervous system or systemically. In conclusion, this report supports the off-label use of DOX to turn on FLt3L gene expression after injection of HC-Ad-TetOn-Flt3L into the tumor cavity in human glioma patients. These data will be of critical importance for the Investigational New Drug filing to the FDA in order to enable the clinical implementation of the combined HC-Ad-mediated gene therapy strategy to treat GBM in a phase I clinical trial.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants U01-NS052465, U01-NS052465-S1, R01-NS074387, and R01-NS057711 to M.G.C.; NIH/NINDS Grants R01-NS054193, R01-NS061107, and R01-NS082311 to P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH UL1-TR000433, and MICHR U040007; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; and the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863. We are grateful to Dr. Karin Murasko for her academic leadership; to M. Dahlgren, D. Tomford, and S. Napolitan for superb administrative support; and to R. Lemons and M. Dzaman for outstanding technical assistance.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- Aguilar-Cordova E. Methods for treatment of solid tumors and metastasis by gene therapy. 2001. Patent no. WO2001024684A2.
- Agwuh K.N. MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006;58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- Ali S. Curtin J.F. Zirger J.M., et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol. Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. King G.D. Curtin J.F., et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.A. The use of antiviral medications in the treatment of herpes simplex virus infections of women. Int. J. Fertil. Womens Med. 1999;44:227–233. [PubMed] [Google Scholar]
- Candolfi M. Curtin J.F. Xiong W.D., et al. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol. Ther. 2006;14:371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y.K. Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- Chiocca E.A. Aguilar L.K. Bell S.D., et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J. Clin. Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.F. King G.D. Barcia C., et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J. Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.F. Candolfi M. Fakhouri T.M., et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008a;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.F. Candolfi M. Xiong W., et al. Turning the gene tap off: implications of regulating gene expression for cancer therapeutics. Mol. Cancer Ther. 2008b;7:439–448. doi: 10.1158/1535-7163.MCT-07-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.F. Liu N. Candolfi M., et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUGDEX. Thomson Reuters. 2012. http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/79C165/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/F53C9B/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToFullDocumentLink/docId/0523/contentSetId/31/title/DOXYCYCLINE/servicesTitle/DOXYCYCLINE. [Aug;2013 ]. http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/79C165/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/F53C9B/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToFullDocumentLink/docId/0523/contentSetId/31/title/DOXYCYCLINE/servicesTitle/DOXYCYCLINE publication date 2012.
- FDA-NDA#0204877. 1995. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=search.DrugDetails. [Sep;2013 ]. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=search.DrugDetails
- Fujita T. Teh B.S. Timme T.L., et al. Sustained long-term immune responses after in situ gene therapy combined with radiotherapy and hormonal therapy in prostate cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:84–90. doi: 10.1016/j.ijrobp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Ghulam Muhammad A.K. Candolfi M. King G.D., et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin. Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S.N. Zahurak M.L. Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat. Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- Grossman S.A. Ye X. Piantadosi S., et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinata N. Shirakawa T. Terao S., et al. Progress report on phase I/II clinical trial of Ad-OC-TK plus VAL therapy for metastatic or locally recurrent prostate cancer: initial experience at Kobe University. Int. J. Urol. 2006;13:834–837. doi: 10.1111/j.1442-2042.2006.01418.x. [DOI] [PubMed] [Google Scholar]
- Immonen A. Vapalahti M. Tyynela K., et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- King G.D. Muhammad A.K. Curtin J.F., et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro. Oncol. 2008a;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G.D. Muhammad A.K. Xiong W., et al. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J. Virol. 2008b;82:4680–4684. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G.D. Muhammad A.K. Larocque D., et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol. Ther. 2011;19:1793–1801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A.K. Puntel M. Candolfi M., et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin. Pharmacol. Ther. 2010;88:204–213. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A.K. Xiong W. Puntel M., et al. Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: implications for a glioma phase 1 clinical trial. Hum. Gene Ther. Methods. 2012;23:271–284. doi: 10.1089/hgtb.2012.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normolle D. Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J. Clin. Oncol. 2006;24:4426–4433. doi: 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- Nwanegbo E. Vardas E. Gao W., et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntel M. Muhammad A.K.M.G. Farrokhi C., et al. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol. Appl. Pharmacol. 2013;268:318–330. doi: 10.1016/j.taap.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmair A.M. Loimas S. Puranen P., et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Shirakawa T. Terao S. Hinata N., et al. Long-term outcome of phase I/II clinical trial of Ad-OC-TK/VAL gene therapy for hormone-refractory metastatic prostate cancer. Hum. Gene Ther. 2007;18:1225–1232. doi: 10.1089/hum.2007.074. [DOI] [PubMed] [Google Scholar]
- Stupp R. Mason W.P. Van Den Bent M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Stupp R. Hegi M.E. Gilbert M.R. Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J. Clin. Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- Tyring S.K. Plunkett S. Scribner A.R., et al. Valomaciclovir versus valacyclovir for the treatment of acute herpes zoster in immunocompetent adults: a randomized, double-blind, active-controlled trial. J. Med. Virol. 2012;84:1224–1232. doi: 10.1002/jmv.23329. [DOI] [PubMed] [Google Scholar]
- Voisin E.M. Ruthsatz M. Collins J.M. Hoyle P.C. Extrapolation of animal toxicity to humans: interspecies comparisons in drug development. Regul. Toxicol. Pharmacol. 1990;12:107–116. doi: 10.1016/s0273-2300(05)80052-2. [DOI] [PubMed] [Google Scholar]
- Wen P.Y. Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Xiong W. Goverdhana S. Sciascia S.A., et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W. Candolfi M. Kroeger K.M., et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol. Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. Lee J. Mody R. Braun T.M. The superiority of the time-to-event continual reassessment method to the rolling six design in pediatric oncology Phase I trials. Clin. Trials. 2011;8:361–369. doi: 10.1177/1740774511407533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.