Abstract
Translational research is a lengthy, complex, and necessary endeavor in order to bring basic science discoveries to clinical fruition. The NIH offers several programs to support translational research including an important resource established specifically for gene therapy researchers—the National Heart, Lung, and Blood Institute (NHLBI) Gene Therapy Resource Program (GTRP). This paper reviews the core components of the GTRP and describes how the GTRP provides researchers with resources that are critical to advancing investigational gene therapy products into clinical testing.
Introduction
For many researchers, it might be difficult to remember a time when DNA manipulation wasn't commonplace science. In February 1975, the International Congress on Recombinant DNA Molecules was held at the Asilomar Conference Center in Pacific Grove, California. The 140 attendees met to assess the safety risks and to develop a process for going forward responsibly with this powerful new technology (Berg et al., 1975). This pivotal meeting, or “Asilomar” as it is often called, marked the beginning of an unparalleled era of biomedical discoveries and therapeutic breakthroughs. In 1977, a mere 2 years after Asilomar, somatostatin was the first human protein produced in a microorganism, and in 1982 the U.S. Food and Drug Administration (FDA) approved the world's first recombinant DNA drug—human insulin. In the intervening 30 years many other recombinant DNA technology-derived medical and agricultural products have come to the marketplace. The field of therapeutic gene transfer, which many view as the next important class of recombinant DNA technology-based therapeutics, has seen significant advances that mirror the earlier stages of development of recombinant proteins. In what may be regarded as an analogous milestone to the licensing of recombinant insulin in 1982, in July 2012 the European Medicines Agency recommended the authorisation of Glybera (alipogene tiparvovec) for marketing in the European Union, making it the first gene therapy agent approved for use in the Western world (European Medicines Agency, 2012; Kresge, 2012).
Despite these successes, and as the above timeline suggests, gene therapy investigators continue to face daunting challenges along the lengthy and broad translational pathway. What defines “translational research” can differ depending on your scientific vantage point, but the old notion that “translation” is a unidirectional flow from basic science to clinical application has been redefined. Translational research is in practice bidirectional, first from bench to preclinical testing to bedside. Then, based on lessons that often only clinical testing will reveal, researchers may go from the bedside back to preclinical testing and/or back to the bench. Several iterations of this cycle are possible as part of the overall new drug development process. Translational research with complex new biologics, such as investigational gene therapies, is an inherently complex process.
Complicating the already complex nature of translational research is the fact that the paradigm of therapeutic product development has changed significantly over the past few decades. Large pharmaceutical companies have been moving away from their traditional research and development business model and are instead licensing product candidates discovered, and at least partially de-risked by smaller corporate entities or academia. However, smaller corporations and academic investigators often face the paradox of an inability to secure adequate funding to advance their product candidate until they demonstrate some success, which they often can't do without additional funding and product development expertise. The chasm between identification of a new drug candidate and clinical testing of the investigational new drug has been termed “The Valley of Death” as many candidates fail to traverse this ground. Not only is adequate funding imperative, but effective translational research requires the coordinated interplay of many different types of expertise to bridge the knowledge gaps of any one discipline and to advance the work to a clinically meaningful endpoint. This paper examines some recent efforts on the part of the National Institutes of Health (NIH) to bridge these gaps and facilitate translational research, and it more specifically reviews the National Heart, Lung, and Blood Institute's Gene Therapy Resource Program (GTRP).
Translational Research and the National Institutes of Health
It is only within the past decade that the NIH significantly intensified efforts to reengineer the national clinical research enterprise, including fostering multidisciplinary efforts to ultimately facilitate the translation of basic science discoveries into clinical applications. Under the leadership of then-NIH Director Elias Zerhouni, M.D., the NIH Roadmap for Medical Research was launched in 2003 (Zerhouni, 2003). In October 2006 the Clinical and Translational Science Awards (CTSA) Program was launched to create academic homes for clinical and translational research and to provide integrated support services for researchers (Zerhouni, 2005; Zerhouni and Alving, 2006). Shortly after his August 2009 appointment as director of the NIH, Francis S. Collins, M.D., Ph.D., delineated five areas of research opportunity for the NIH (Collins, 2010). “Translational Medicine” was one of these five areas that Dr. Collins identified as holding particular promise for major advances and under his leadership the National Center for Advancing Translational Sciences (NCATS) was established on December 23, 2011 (Collins, 2011; NIH News, 2011). NCATS became the home for many of the NIH translational support programs such as the CTSAs; the Small Business Innovation Research (SBIR)/Small Business Technology Transfer (STTR) program; the Therapeutics for Rare and Neglected Diseases (TRND) program; and the Bridging Interventional Development Gaps (BrIDGs) program, formerly the NIH Rapid Access to Intervention Development (RAID) program. All of these programs can provide gene therapy researchers with valuable resources and, along with other important resources, can be found on the program index page of the NCATS website at http://www.ncats.nih.gov/about/program-index/program-index.html.
Three important translational resource programs established specifically for gene therapy researchers, and not housed under NCATS, are the National Gene Vector Biorepository (NGVB); the Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases (www.cfmgt.ucdavis.edu); and the National Heart, Lung, and Blood Institute's (NHLBI) Gene Therapy Resource Program (GTRP) (www.gtrp.org). The NGVB (www.NGVBCC.org) provides basic researchers with access to novel reagents; information about gene therapy pharmacology and toxicology studies on file with the FDA; and assistance with FDA post-trial monitoring requirements. The Center for Fetal Monkey Gene Transfer (CFMGT) conducts gene transfer studies in monkeys to evaluate safety and efficiency, and provides NHLBI-supported investigators with the expertise, resources, and services necessary to actively pursue gene transfer approaches in monkeys in their research programs. The reader is referred to the respective websites for more information about the NGVB and the CFMGT programs. The remainder of this paper focuses on the NHLBI Gene Therapy Resource Program (GTRP).
Background of the NHLBI Gene Therapy Resource Program
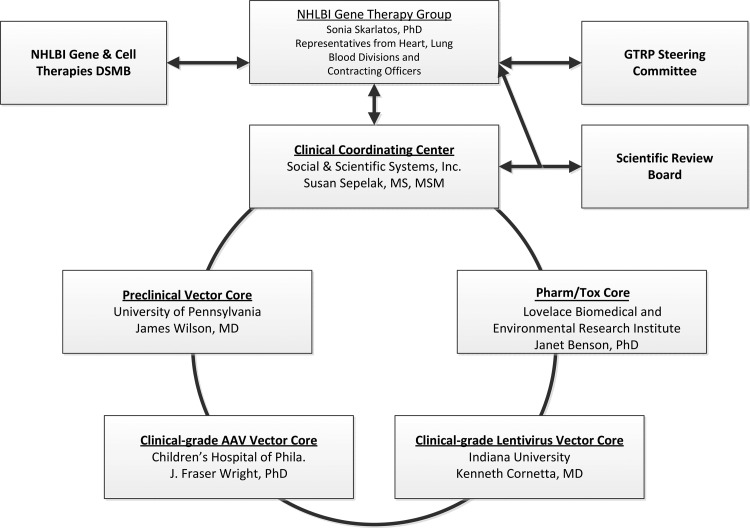
On the basis of recommendations from a previously convened expert Working Group (Woo et al., 2006) the NHLBI launched the 5-year, $29.5M GTRP in March 2007. The GTRP was established with the express purpose of facilitating translational research in gene therapy focused primarily on heart, lung, and blood disorders by providing U.S.-based researchers with resources critical to advancing investigational gene transfer products into clinical testing. The services of the GTRP, ranging from production of preclinical vectors to support of early-phase clinical trials, and the many relevant activities in-between, are offered at no cost to the investigator. The structure and oversight of the GTRP is illustrated in Fig. 1. Details about each of these program components, and links to their respective websites, can be found on the GTRP website at www.gtrp.org. By all accounts, the first 5 years (2007–2012) of the GTRP were successful, and in June 2012 the GTRP was renewed for another 5 years with a new total budget of $40M.
FIG. 1.
Structure and Oversight of the GTRP. The operations of the GTRP are overseen by the NHLBI Gene Therapy Group (GTG) and coordinated by the Clinical Coordinating Center (CCC). The GTG receives program advice from the GTRP Steering Committee (SC) composed of outside experts, ex officio representatives from FDA and NIH Office of Biotechnology Activities (OBA), and the PIs from the Core Laboratories and the CCC. The Scientific Review Board (SRB), a panel composed of experts in various aspects of gene therapy and/or disease-specific fields, provides written reviews on particular funding requests on an ad hoc basis at the request of the GTG. Clinical trials receiving funding assistance from the GTRP are overseen by the NHLBI Gene and Cell Therapies Data and Safety Monitoring Board.
Access to the GTRP Program
United States-based investigators working on heart, lung, and blood diseases and sleep disorders who wish to request program services must first register online at http://www.gtrp.org/invest_reg.aspx. Once approved by the NHLBI for registration, the investigator needs to complete a “Request for Service Application” (RSA) in order to apply for any of the services offered by the GTRP. An investigator may submit more than one type of RSA. Personnel from the corresponding Core may assist the investigator in completing the RSA.
Researchers supported by other NIH Institutes and Centers, or programs such as BrIDGs, may also be eligible for GTRP Core Laboratory services if funds can be transferred directly to the NHLBI. Investigators are encouraged to discuss this with their respective Program Officials, or contact the GTRP Clinical Coordinating Center (CCC) via the website.
How the GTRP Core Components Facilitate Translational Research
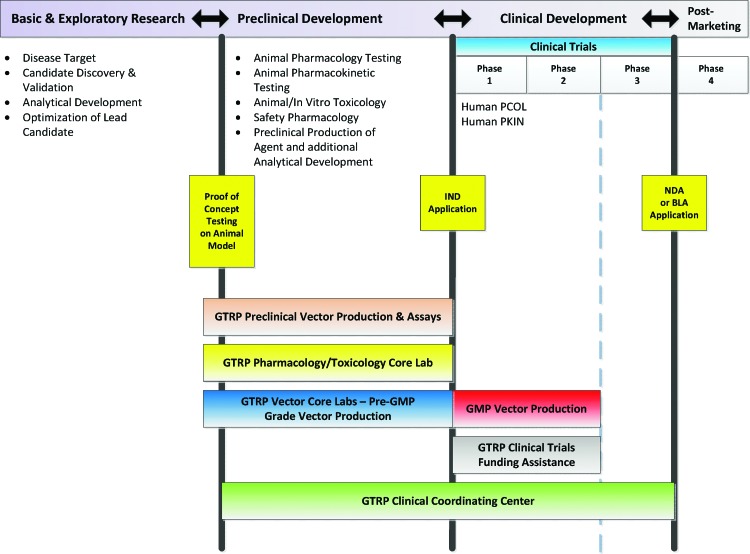
Figure 2 is a somewhat oversimplified representation of the translational pathway, with the major development milestones for a new drug or therapeutic biological product divided into four general stages: Basic and Exploratory Research; Preclinical Development; Clinical Development; and the Post-Marketing stage. Each stage requires a complex and coordinated interplay of wide-ranging expertise and can represent years of work. Even a well-trained researcher would not be expected to be familiar with the details of the entire process in a single stage of development, let alone the entire translational pathway. Let's now examine in more detail some of the successes of the inaugural program and how each component of the GTRP can help researchers advance the translation of gene therapy discoveries toward the clinic.
FIG. 2.
Translational Pathway for Investigational Therapeutic Product Development. This figure illustrates the major stages of investigational new drug or biologic development and some of the types of testing that must be done in those stages. The spectrum of coverage by each of the GTRP components is illustrated. In general, the GTRP does not cover the basic and exploratory stage, but some optimization of the lead candidate (i.e., the vector) or analytical development may be conducted by the GTRP laboratories. The GTRP does not fund Phase 3 clinical trials, but the CCC may provide some selected support services. Color images available online at www.liebertpub.com/humc
Preclinical Vector Core Laboratory (University of Pennsylvania)
The Preclinical Vector Core provides high-quality research-grade viral vectors based on adeno-associated virus (AAV), adenovirus, lentivirus, and nonviral vectors intended for basic research directed toward clinical application. The Preclinical Core also offers consultation services on vector construction and experimental design, quality control services, immunology testing, and preclinical biodistribution analysis. Optimization of vector-mediated gene expression can be an important first step in advancing the investigational agent. This Core offers full characterization of source materials used for vector production; assists investigators in analyzing sequence information, as well as in identifying and redesigning constructs not yet optimized for gene expression; and develops vectors for improved gene delivery. By providing approved investigators with access to advanced vector technologies and vector-related services offered by the Preclinical Core, the GTRP facilitates proof-of-concept studies as well as studies that transition the work from small to large animal models.
With the transition of research to large animal model systems, an understanding of host immune responses to vector-mediated gene delivery is critical. The presence of pre-existing neutralizing antibodies (NAbs) and the induction of T-cell responses are of greater concern in large animals than in small animal model systems. Careful screening of animals prior to vector administration and the appropriate analysis of immune responses post vector administration are pivotal for success and advancement to further stages of product development. The Preclinical Core offers services for the detection of pre-existing and vector-induced circulating NAbs, as well as assays to detect vector-induced T-cell responses that can result in elimination of transduced cells and reduced expression of the intended therapeutic genes.
Perhaps the biggest contribution of the GTRP Preclinical Core laboratory thus far has been the development of novel vectors with transduction profiles superior to previous generations. The Core can provide AAV vectors based on serotypes 1, 2, 5, 6, 6.2, 7, 8, 9, rh10, and others for mediating gene delivery to heart, liver, lung, and blood tissues. The GTRP Preclinical Vector Core works closely with key laboratories to transition new vector technologies and make them available to the scientific community.
Pharmacology/Toxicology Testing Core Laboratory (Lovelace Biomedical and Environmental Research Institute)
The mission of the Pharmacology/Toxicology (Pharm/Tox) Core is to aid investigators in the design and implementation of Investigational New Drug (IND)-enabling preclinical safety and biodistribution studies. This is often one of the most challenging and costly steps in the translational process, as many researchers have little to no experience in designing IND-enabling trials. If needed, staff from the Pharm/Tox Core will aid in the development of pre-pre-IND and pre-IND information packages for FDA review and will participate in FDA review meetings of these materials. The Pharm/Tox Core scientists work closely with the Investigator(s), the GTRP Core providing the vector (if applicable), and the CCC to facilitate development of the IND packages and the preclinical study protocol.
The GTRP Pharm/Tox Core provides test article dosing and subsequent endpoint evaluations including, but not limited to: clinical observations, clinical pathology, histopathology, safety pharmacology, vector biodistribution (qPCR), immunology endpoints, and assessments of transgene expression. In most cases, endpoint assays can be transferred from investigators to the Core for qualification and implementation in studies. If the GTRP Pharm/Tox Core cannot provide the requested service (e.g., cardiac surgeries in large animal models), the Core will still serve as the lead laboratory and assist investigators in identifying suitable alternative facilities that can be subcontracted to perform the work. In all cases, studies are conducted as much as is feasible in compliance with the Code of Federal Regulations Title 21, Part 58 (Good Laboratory Practice for Nonclinical Laboratory Studies) with any exceptions clearly stated in the Study Protocol. Upon completion of the study, the Pharm/Tox Core staff prepares an audited final report for inclusion in the IND submission to the FDA.
Because pharm/tox testing employs animal models, is often quite costly, and involves a lengthy time commitment, the GTRP requires that these “Request for Service Applications” (RSAs) be reviewed by the GTRP Scientific Review Board and the Steering Committee before final review and funding approval by the NHLBI Gene Therapy Group. In addition, the GTRP will not fund the pharm/tox RSA until the study protocol has been reviewed by the FDA and the investigator has responded to FDA comments. Therefore, in order to facilitate this critical step in translation, it is important that the investigator work closely with the GTRP Pharm/Tox Core and CCC in completing the RSA, as well as in preparing for the pre-pre-IND and pre-IND meetings with the FDA (unless the investigator has independent support for preparing the regulatory materials).
Clinical AAV Vector Core Laboratory (The Children's Hospital of Philadelphia)
The GTRP Clinical AAV Vector Core Laboratory is a purpose-built and fully qualified clinical production facility at The Children's Hospital of Philadelphia (CHOP). This Core Laboratory manufactures and certifies customized adeno-associated virus (AAV)-based vectors meeting high safety, purity, and potency standards in compliance with current Good Manufacturing Practice (cGMP) Guidelines. The Laboratory is set up to manufacture custom vector constructs packaged within varying AAV serotypes (including 1, 2, 5, 6, 8, and 9) at two grade levels. The more cost- and time-effective GMP “process-comparable”-grade AAV vectors are suitable and recommended for use in critical IND-enabling preclinical studies (e.g., pharmacology and toxicology studies), and the GMP clinical-grade AAV vectors are for use in IND clinical trials. Both grades of vectors are made using the same sequence of process steps, performed at the same scale, and are each extensively characterized to ensure purity, potency, and safety. However, the GMP clinical-grade vector is subject to an additional series of tests and controls necessary for human-use products.
The management and staff of the AAV Vector Core have extensive translational research experience and can offer investigators services beyond vector manufacture and testing. Some of the other services offered to GTRP-funded investigators include: consultation regarding vector plasmid design; preparation of reference vectors as standards to ensure continuity throughout the translational process; preparation of the requisite chemistry, manufacturing, and controls (CMC) information for pre-pre-IND and pre-IND meetings with the FDA; and preparation of the CMC regulatory documentation required for the actual IND submission. Additionally this Core offers support related to the administration of the investigational agent, such as assistance with the design and performance of vector-compatibility studies for investigational product administration devices, and long-term investigational product stability study design and performance.
The AAV Core Laboratory team has participated in several ground-breaking clinical trials in the United States using recombinant AAV-based investigational products for conditions including hemophilia, Leber congenital amaurosis, and Parkinson disease, as well as participating in translational research programs in the European Union. The breadth of expertise within the AAV Vector Core Laboratory team provides GTRP-funded researchers with valuable assistance along the translational research pathway, from optimizing their vector design to facilitating navigation through the complex steps needed to successfully initiate promising new gene transfer clinical trials.
Clinical Lentivirus Vector Core Laboratory (Indiana University)
The GTRP Clinical Lentivirus Vector Core Laboratory at the Indiana University Vector Production Facility (IU VPF) has collaborated with GTRP-funded investigators on the manufacture and certification of a variety of lentiviral vector products. Manufacturing is performed in a 4258-square foot cleanroom dedicated solely to the production of viral vector products. The facility is BL3-capable and contains four ISO Class 7 production suites along with additional space for media preparation and product storage. Current manufacturing methods use transient transfection of third generation HIV-1-based lentiviral plasmid into HEK293T cells. Material is harvested and processed by a step-filtration method followed by ion exchange purification, then diafiltration and concentration by tangential flow filtration. Average vector lots before concentration are between 10 and 40 liters, but can be increased in scale to 100-liter batch sizes.
The GTRP Clinical Lentivirus Vector Core has also developed a number of release assays for retroviral and lentiviral vectors and can perform the majority of these assays in-house. This latter service decreases the time to vector release and allows the development of a certification plan that is tailored to the specific investigator's needs. Similar to the GTRP AAV Core Laboratory, manufacturing and testing procedures performed at the Clinical Lentiviral Vector Core Laboratory are conducted at GMP standards to comply with FDA regulations.
The Core maintains a Drug Master File with the FDA that facilitates regulatory submissions by providing a letter of cross-reference. By providing this letter to the FDA, the investigator does not need to submit the extensive Standard Operating Procedures (SOPs) and facility information within their IND applications, saving the investigator time and allowing them to focus their submission on the clinically relevant issues.
The Core team work has ranged from well-known vector systems, such as an HIV-1-based vector that the Core was able to rapidly complete because the methodology and procedures are well established, to more challenging cases such as large-scale production of a vector based on the feline leukemia virus. The Core has also collaborated with the GTRP Pharm/Tox Core to provide a retroviral vector positive control for animal toxicology studies and the Core developed and qualified a replication-competent lentivirus (RCL) assay for the successful certification and release of the first clinical product to be generated using a novel lentiviral packaging cell system. As these examples illustrate, the GTRP Lentivirus Vector Core team will closely collaborate with investigators to develop whatever is needed for the next step in the translational process.
Clinical Coordinating Center (Social & Scientific Systems, Inc.)
The Clinical Coordinating Center (CCC) is the linchpin of the GTRP and works directly with the NHLBI Gene Therapy Group. The CCC coordinates the logistics of the program and interfaces with all of the GTRP Core laboratories, investigators requesting and receiving program services, the GTRP Steering Committee, and with the GTRP Scientific Review Board (SRB).
The CCC manages a web-based system for investigator registrations and the process for submitting Request for Service Applications (RSAs) to the GTRP. This system assigns unique identifiers to each RSA and its associated documents and review forms. The system employs extensive auto-email feedback and an event tracking history to maximize timely communications with the submitting investigators, a key to the achievement of a successful RSA.
In addition to developing and maintaining the program infrastructure, ensuring process management, and coordinating the program logistics, the CCC provides two vital services to investigators on behalf of the GTRP. First, the CCC offers a wide array of regulatory assistance to GTRP-approved investigators. For many researchers, navigating through the regulatory processes is one of the more challenging aspects of translational research. Some examples of regulatory support services offered by the CCC include:
• Assistance with pre-pre-IND and pre-IND meeting scheduling and package compilation for submission to the FDA
• Assistance with IND preparation and submission to the FDA
• Assistance with the preparation of materials for meetings of oversight bodies such as the FDA, Institutional Review Board (IRB), Institutional Biosafety Committee (IBC), National Institutes of Health Office of Biotechnology Activities (NIH OBA), and the Data and Safety Monitoring Board (DSMB)
• Arranging for outside expert scientific advice on preclinical and clinical study designs
• Assistance in securing an IBC for oversight of a protocol to be conducted at an institution without a standing IBC
• Assistance with the preparation of a study-specific Manual of Procedures (MOP) and a Regulatory Binder, including tips on organization and content
• Assistance with the development and review of required study/site-specific forms such as Case Report Forms (CRFs)
-
• And other services specifically offered to investigators whose clinical trial is partially funded by the GTRP include:
○ Guidance on trial site selection, site readiness assessments, Good Clinical Practice (GCP) training, and guidance on clinical trial site monitoring
The second vital service provided by the CCC is the development and management of the subcontract(s) for the disbursement of GTRP funds for approved gene therapy clinical trials. The GTRP provides partial funding for early-stage (Phase I/II) clinical trials that are within the Mission of the NHLBI. Investigators are expected to have additional funding sources, such as institutional support, grant support, Public–Private Partnerships, or other sources to ensure sufficient resources for trial completion. Currently, the GTRP is funding three clinical trials and others are in development. Investigators are encouraged to submit multiple RSAs for a single research project as service needs are identified. For example, during the initial 5-year period of the GTRP one investigator maximized the utility of the GTRP by submitting multiple regulatory assistance RSAs, including assistance with IND submission, assistance with the required DSMB presentation, and assistance with clinical site readiness. This investigator also requested and received clinical trial funding assistance from the GTRP and that trial is nearing completion. This example well represents the original goals and intention of the GTRP—to provide resources to investigators to facilitate the advancement of investigational gene transfer products into clinical testing.
Summary
Tremendous strides have been made in the field of therapeutic gene transfer and one can expect even more advances in the near future, but translational research is a challenging process that requires myriad expertise and extensive resources. The NHLBI Gene Therapy Resource Program can provide heart, lung, and blood disease and sleep disorder researchers with the expertise and resources necessary to facilitate the realization of a clinical gene therapy agent. Researchers are encouraged to learn more about the GTRP, and apply for services, at the website www.gtrp.org.
Acknowledgments
The authors recognize and thank the other members of the GTRP Core teams and the NHLBI Gene Therapy Group: Ms. Jenee Bevett, Mr. Eric Daniels, Ms. Nora Rivera, and Drs. Ray Ebert, Pankaj Qasba, Rita Sarkar, and Susan Schlegel. Sources of funding: Each GTRP Core component is supported under a contract with the NHLBI as follows: AAV Core (HHSN2682012000041); Clinical Coordinating Center (HHSN2682012000021); Lentivirus Core (HHSN2682012000051); Pharmacology/Toxicology Core (HHSN2622012000031); Preclinical Core (HHSN268200041C).
Author Disclosure Statement
K.C. is a shareholder and founder of Rimedion Inc., but he is not employed by the company and there is no financial conflict of interest with the information presented. J.F.W. is coauthor of patents relating to AAV technologies; serves on the scientific advisory board of Avalanche Therapeutics; and has consulted for Tacere Therapeutics, Genzyme, Novartis, and Genetix Inc. J.M.W. is an inventor on patents licensed to various biopharmaceutical companies and is a founder of, consultant to, and a grant recipient from ReGenX Holdings.
References
- Berg P. Baltimore D. Brenner S., et al. Summary statement of the Asilomar Conference on Recombinant DNA Molecules. Proc. Natl. Acad. Sci. U.S.A. 1975;72:1981–1984. doi: 10.1073/pnas.72.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.S. Research agenda: Opportunities for research and NIH. Science. 2010;327:36–37. doi: 10.1126/science.1185055. [DOI] [PubMed] [Google Scholar]
- Collins F.S. Reengineering translational science: The time is right. Sci. Transl. Med. 2011;3:90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. Glybera: Opinion. 2012. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002145/smops/Positive/human_smop_000235.jsp&mid=WC0b01ac058001d127 http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002145/smops/Positive/human_smop_000235.jsp&mid=WC0b01ac058001d127
- Kresge N. “UniQure's Glybera Wins EU Backing as First Gene Therapy.”. Businessweek. 2012. Jul 20, 2012. http://www.businessweek.com/news/2012-07-20/uniqure-s-glybera-wins-eu-backing-as-first-gene-therapy http://www.businessweek.com/news/2012-07-20/uniqure-s-glybera-wins-eu-backing-as-first-gene-therapy
- NIH News. “NIH Establishes National Center for Advancing Translational Sciences.” Press release. 2011. Dec 23, 2011. http://www.nih.gov/news/health/dec2011/od-23.htm http://www.nih.gov/news/health/dec2011/od-23.htm
- Woo S.L. Skarlatos S.I. Joyce M.M., et al. Heart, Lung, Blood Diseases Working Group. Critical resources for gene therapy in Heart, Lung, and Blood Diseases Working Group. Mol. Ther. 2006;13:641–643. doi: 10.1016/j.ymthe.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Zerhouni E. Medicine: The NIH roadmap. Science. 2003;302:63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- Zerhouni E.A. US biomedical research: Basic, translational and clinical sciences. JAMA. 2005;294:1352–1358. doi: 10.1001/jama.294.11.1352. [DOI] [PubMed] [Google Scholar]
- Zerhouni E.A. Alving B.M. Clinical and Translational Science Awards: A framework for a national research agenda. Translational Res. 2006;148:4–5. doi: 10.1016/j.lab.2006.05.001. [DOI] [PubMed] [Google Scholar]