Abstract
Ligament and tendon repair is an important topic in orthopedic tissue engineering; however, the cell source for tissue regeneration has been a controversial issue. Until now, scientists have been split between the use of primary ligament fibroblasts or marrow-derived mesenchymal stem cells (MSCs). The objective of this study was to show that a co-culture of anterior cruciate ligament (ACL) cells and MSCs has a beneficial effect on ligament regeneration that is not observed when utilizing either cell source independently. Autologous ACL cells (ACLcs) and MSCs were isolated from Yorkshire pigs, expanded in vitro, and cultured in multiwell plates in varying %ACLcs/%MSCs ratios (100/0, 75/25, 50/50, 25/75, and 0/100) for 2 and 4 weeks. Quantitative mRNA expression analysis and immunofluorescent staining for ligament markers Collagen type I (Collagen-I), Collagen type III (Collagen-III), and Tenascin-C were performed. We show that Collagen-I and Tenascin-C expression is significantly enhanced over time in 50/50 co-cultures of ACLcs and MSCs (p≤0.03), but not in other groups. In addition, Collagen-III expression was significantly greater in MSC-only cultures (p≤0.03), but the Collagen-I-to-Collagen-III ratio in 50% co-culture was closest to native ligament levels. Finally, Tenascin-C expression at 4 weeks was significantly higher (p≤0.02) in ACLcs and 50% co-culture groups compared to all others. Immunofluorescent staining results support our mRNA expression data. Overall, 50/50 co-cultures had the highest Collagen-I and Tenascin-C expression, and the highest Collagen-I-to-Collagen-III ratio. Thus, we conclude that using a 50% co-culture of ACLcs and MSCs, instead of either cell population alone, may better maintain or even enhance ligament marker expression and improve healing.
Introduction
Injuries to the anterior cruciate ligament (ACL) represent one of the most common sport-related injuries and the most common ligament injury in the knee.1,2 In the United States, there are at least 100,000 ACL repairs performed each year.3 For these reasons, the development of techniques that promote the healing and repair of ligaments and tendons is of increased importance in orthopedics.
Tissue-engineering principles have recently been applied to the development of novel ligament regeneration techniques; the ideal cell source, however, has been a controversial topic within orthopedic tissue engineering. Initial studies, focused on defining the optimal individual cell source for ligament regeneration, are divided between the use of primary ACL cells (ACLcs) and mesenchymal stem cells (MSCs).1,4,5 However, new evidence suggests that there are advantages to the use of a co-culture system. Recent reports have established that indirect co-culture of MSCs and ligament/tendon cells results in an increased expression of ligament/tendon markers Collagen type I (Collagen-I), Collagen type III (Collagen-III), and Tenascin-C in the MSCs.6–8 It was also observed that cell proliferation, DNA content, and collagen production were all increased in MSCs as compared to non-co-cultured controls; the induction of differentiation of MSCs toward a ligament lineage was also noted.6–9 Direct cell–cell contact between MSCs and a variety of other differentiated cell populations has proven to be a key determinant of the fate and effect of MSCs in culture.10,11 It has been observed that cell–cell contact between MSCs and certain fibroblast populations can induce phenotypic changes in the fibroblast.10,11 Furthermore, recent studies have shown that MSCs have anti-inflammatory and immunomodulatory properties in vivo.12–14 These observations have led some investigators to suggest that MSCs may aid tissue regeneration in more ways than previously believed.15–17 By introducing co-culture systems, scientists may be able to enhance the regeneration of tissues by exposing two or more cell populations to each other's regulatory molecules naturally and simultaneously, or at a sequence dictated by the cells themselves. All of these studies lead to the conclusion that MSCs can serve both as a cell source for tissue regeneration and/or as a source of regulatory cues for the differentiation of other cell types.
More recent studies have identified the presence of MSC-like cells in several tissues, including ligament and tendons.15–19 In the body, MSCs appear to function as support cells that can differentiate into specific cell types to regenerate injured tissues, and can promote regeneration by providing regulatory cues to native tissue cells that promote angiogenesis, stimulate progenitor cells from within the injured tissue, and reduce apoptosis and scar formation.15
This study was based on the premise that ACLcs and MSC co-cultures can initiate a regenerative response that could stimulate ACL tissue to enhance its own repair. Scientists in the field of ligament tissue engineering use the expression of Collagen-I, Collagen-III, and Tenascin-C as a tool to evaluate the degree of ligament regeneration.6–9,20,21 Microstructurally, close to 94% of ACL tissue is composed of fascicles of collagen fibers, with the remaining 6% comprised of cells and additional extracellular matrix (ECM) components, including Tenascin-C.22,23 Moreover, ∼90% of collagen in ACL is type I, with Collagen-III comprising the remaining 10%.20,22,24 Our objective was to determine the effects of direct co-culture of a variety of ratios of ACLcs and marrow-derived MSCs on the overall expression of ligament markers in vitro as a way to elucidate the optimal cell ratio for future ligament tissue-engineering studies. We hypothesized that a co-culture of ACLcs and MSCs would potentially enhance the expression of ligament markers Collagen-I, Collagen-III, and Tenascin-C when compared to ACLcs or MSCs cultured independently.
Materials and Methods
Tissue harvest and cell isolation
ACL tissue and bone marrow were harvested from 4–8-month-old Yorkshire pig legs purchased from a local butcher house. First, the ACL was isolated by an improvement on the protocol first developed by Nagineni and adapted by others.4,25–27 ACL tissue was aseptically dissected and washed in sterile Hank's Buffered Salt Solution, Calcium and Magnesium free (HBSS—; Invitrogen) and then placed in a Petri dish (BD Falcon) with fresh HBSS—. Two No. 21 blade scalpels were used to gently scrape off remaining synovial tissue from the surface of the ligament. The tissue was transferred to a second dish with fresh HBSS— and scraping was repeated gently. Subsequently, the tissue was transferred to a new dish with 10 mL of Dulbecco's modified Eagle's medium (DMEM; Gibco) with 15% fetal bovine serum (FBS; Gibco), 1% l-glutamine (Gibco), and 1% antibiotic mixture (Gibco) (ACL DMEM). The tissue was minced with scissors for ∼10 min until all pieces were <1-mm-long axis length, and then digested in a mixture of 2.4 U/mL Dispase-II (Roche) and 10 mg/mL Collagenase D (Roche) solution for 60 min in a 37°C shaker. Next, the resulting cell solution was filtered through a 100-μm strainer (BD Falcon) and centrifuged at 260 g for 6 min. The ACLcs obtained were resuspended in ACL DMEM and seeded at a density of 27×103 cells/cm2 in T-75 tissue culture flasks (BD Falcon).
Secondly, bone marrow (BM) was aspirated from the distal femoral end of the same pig leg as previously described.28 Briefly, 3–4 mL of BM were aspirated with a 16-gauge needle attached to a syringe with 0.5 mL sodium heparin (APP Pharmaceuticals) and seeded in T-75 flasks at a 1 mL BM-to-9 mL medium ratio with DMEM with 10% FBS and 1% antibiotic mixture (BM DMEM). Bone marrow-derived MSCs were isolated by their ability to attach to tissue culture plastic.28
All cells were grown to passages 2 or 3. Viability was determined by the trypan-blue (Sigma) exclusion method. MSCs were not allowed to reach more than 60%–70% confluency before passaging, whereas ACLcs were allowed to reach 100% confluency. The culture medium was changed twice a week for all cell types.
Osteogenesis
MSCs were seeded in 24-well plates (BD Falcon) at a density of 4.2×103 cells/cm2 and cultured in a BM DMEM until 50%–70% confluency was reached. The medium was then replaced with an osteogenic differentiation medium composed of α-MEM Basal Medium (Gibco) supplemented with dexamethasone, ascorbate, and β-glycerophosphate (Osteogenic Supplement; R&D Systems). The medium was changed every 3 days for 2 weeks. Cultures were then washed carefully with HBSS—, fixed with 10% formalin (Sigma) for 45 min, and rinsed with distilled water. Alizarin Red S staining solution was freshly made with 2g Alizarin Red S (EMD Chemicals) in 100 mL distilled water, adjusting pH to 4.1–4.3 with 0.1% NH4OH. Cell monolayers were then covered with the staining solution and incubated at room temperature in the dark for 45 min. Wells were then rinsed with distilled water and imaged using an Olympus IX70 microscope equipped with a SPOT digital camera and image processing software (Diagnostic Instruments).
Adipogenesis
MSCs were seeded in 24-well plates (BD Falcon) at a density of 2×104 cells/cm2 and cultured in a BM DMEM until 100% confluency was reached. The medium was then replaced with an adipogenic differentiation medium composed of α-MEM Basal Medium (Gibco) supplemented with hydrocortisone, isobutylmethylxanthine, and indomethacin in 95% ethanol (Adipogenic Supplement; R&D Systems). The medium was changed every 3 days for 2 weeks. Cultures were then washed carefully with HBSS—, fixed with 10% formalin (Sigma) for 45 min, and rinsed with distilled water. Wells were then covered with 60% isopropanol for 5 min and then stained with 0.3% Oil Red O staining solution for 15 min at room temperature. Wells were then rinsed with distilled water and imaged using an Olympus IX70 microscope equipped with a SPOT digital camera and image processing software (Diagnostic Instruments).
Chondrogenesis
About 250,000 MSCs were transferred to a 15-mL conical tube and centrifuged at 200 g for 5 min at room temperature. The medium was discarded, and cells were then resuspended in 0.5 mL of chondrogenic differentiation medium and centrifuged at 200 g for 5 min without discarding the medium. The chondrogenic differentiation medium was composed of a DMEM/F-12 basal medium (Gibco) supplemented with 1% ITS supplement (R&D Systems) and dexamethasone, ascorbate–phosphate, proline, pyruvate, and TFG-β3 (Chondrogenic Supplement; R&D Systems). Pellets were then incubated at 37°C and 5% CO2 for 2 weeks. The medium was changed every 3 days for 2 weeks. Pellets were then washed carefully with PBS, fixed with 10% formalin (Sigma) for 45 min, and 7-μm frozen sections were obtained for staining. Slides were stained with Hematoxylin I solution (Richard-Allan Scientific) for 10 min, rinsed in tap water for 5 min, and then incubated in 0.001% Fast Green (Fisher) solution for 5 min. Then, 1% acetic acid (Fisher) was added for 15 s, followed by 0.1% Safranin O solution (Fisher) for 5 min. Slides were rinsed and mounted for imaging using an Olympus IX70 microscope equipped with a SPOT digital camera and image processing software (Diagnostic Instruments).
Co-culture assay
Direct-contact co-culture between ACLcs and MSCs was performed using tissue culture-treated multiwell plates (BD Falcon). Five groups, 100% ACLcs/0% MSCs (n=4), 75% ACLcs/25% MSCs (n=3), 50% ACLcs/50% MSCs (n=4), 25% ACLcs/75% MSCs (n=3), and 0% ACLcs/100% MSCs (n=4), were examined in multiwell plates seeded at 2500 total cells/cm2 for 14 and 28 days. A control with 50% ACLcs/0% MSCs (total of 1250 ACLcs/cm2, n=3) was also examined. The ACL DMEM was used for all co-culture experiments and was changed twice per week.
Quantitative reverse transcription–polymerase chain reaction
Expression of ligament markers Collagen-I, Collagen-III, and Tenascin-C was quantified by real-time PCR. Total RNA was isolated from cells freshly harvested from ACL tissue (n=3) and from each well at 14 and 28 days of co-culture using an RNeasy Plus Midi kit (Qiagen) in accordance with the manufacturer's protocol. Total RNA (1 μg per sample) was then reverse-transcribed (RT) in a 20-μL total reaction volume using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer's specifications. The 20-μL samples were loaded onto a Mastercycler (Eppendorf) with the following program: 25°C 10 min→37°C; 120 min→85°C 5 min. Next, 2 μL of a 1:10 dilution of the RT reaction product was subjected to quantitative PCR (qPCR) using an iQ SYBR Green Supermix kit in 20-μL reactions (Biorad). A C100 Thermal Cycler (Biorad) was used with the following program: 95°C 3 min, 1 cycle; 95°C 10 s→64°C 30 s, repeat for 40 cycles total; 95°C 10 s→65°C 5 s, 1 cycle. 18s rRNA was used as the housekeeping gene for normalization. Preliminary mRNA studies showed that 18s rRNA levels remained stable over time and across ACLcs, MSCs, and ACLcs/MSC co-cultures (data not shown). Custom forward and reverse gene-specific primers for Collagen-I, Collagen-III, Tenascin-C, and 18s rRNA are shown in Table 1. Pig liver tissue was used as a negative control. Dissociation and amplification curve analysis was performed with Biorad CFX Manager software. Gene expression was obtained using the Pfaffl method to account for individual reaction efficiencies using freshly harvested native ACLcs as the calibrator sample.29 For freshly harvested native ACLcs expression analysis, the pig liver was used as the calibrator sample.
Table 1.
Custom Pig Primer Sequences for Quantitative Reverse Transcription–Polymerase Chain Reaction
| Target | Forward sequence | Reverse sequence |
|---|---|---|
| Collagen-I | 5′-CCTGGCTCTAGAGGTGAACG-3′ | 5′-AGGATTACCCACAGCACCAG-3′ |
| Collagen-III | 5′-TTGGCCCTGTTTGCTTTTTA-3′ | 5′-TGGTTGACAAGATGAGAACAAAA-3′ |
| Tenascin-C | 5′-TTAAGTACGCGCCCATCTCT-3′ | 5′-CCTTCACAGCAGACACTCCA-3′ |
| 18s rRNA | 5′-TCGCGGAAGGATTTAAAGTG-3′ | 5′-AAACGGCTACCACATCCAAG-3′ |
Collagen-I, Collagen type I; Collagen-III, Collagen type III.
Immunofluorescence
Wells were fixed with 4% paraformaldehyde (Wako) for 20 min at room temperature, washed with PBS, then permeabilized, and blocked with 0.3% TritonX-100 (Sigma), 1% BSA (Fisher), and 10% normal goat serum (Jackson ImmunoResearch) for 45 min at room temperature. Mouse primary antibodies for Collagen-I (1:250, Cat. no. C2456; Sigma), Collagen-III (1:200, Cat. no. ab6310; Abcam), and Tenascin-C (1:250, Cat. no. ab88280; Abcam) were added to respective wells and incubated overnight at 4°C. Goat anti-mouse Cy3 secondary antibody (1:500, Cat. no. 115-165-062; Jackson ImmunoResearch) was added for 60 min at room temperature. DAPI (1:500; Sigma) counterstain was added for 10 min at room temperature. Images of each well were then obtained with an Olympus IX70 fluorescent microscope equipped with an Olympus UM4-100 7A Cy3-710 fluorescent light filter, and a SPOT digital camera and image processing software (Diagnostic Instruments). Orange coloring in all images represents Cy3 staining.
Data analysis
qPCR was performed in triplicate per group per pig. Collagen I/Collagen III and Collagen I/Tenascin-C ratios within each sample were calculated using relative expression (2CT), since their primer efficiencies were all above 97%. No control genes were considered in this case, as the calculation of a direct ratio does not need normalization. Statistical significance was calculated using a Student's t-test. p-values<0.05 were considered significant.
Results
Optimized primary ACL harvest
We find it important to note the difficulties we encountered in our initial attempts at harvesting ACLcs using previously published methodologies, and the subsequent improvements we made to these protocols.4,25–27 First, we found that mincing the ACL tissue with scissors in ACL DMEM resulted in an increased number of viable cells recovered as compared to the published method of doing so in HBSS— (data not shown). In addition, using only collagenase was not sufficient for digestion if done for 2 h or less, and if done for more than 2 h, it resulted in cell suspensions with low viability (<60%). The combination of collagenase and dispase for 1 h, as previously described by our laboratory for muscle tissue,30 yielded a high quantity of healthy fibroblasts (an average of 4×106±1.5×106 cells per 1 pig ACL with 90%+ viability) in a significantly smaller amount of time. Thus, we have established an improved protocol for primary ACLcs isolation, where the midsubstance of a ligament is cleaned of nonligamentous tissue, minced in ACL DMEM, and digested in a solution of 0.3% Dispase-II and 1% Collagenase D for 60 min.
Cell characterization
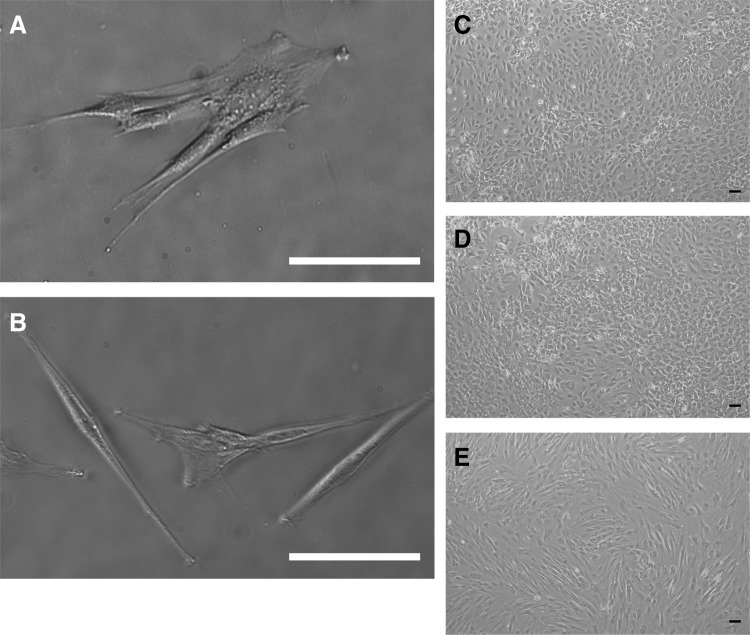
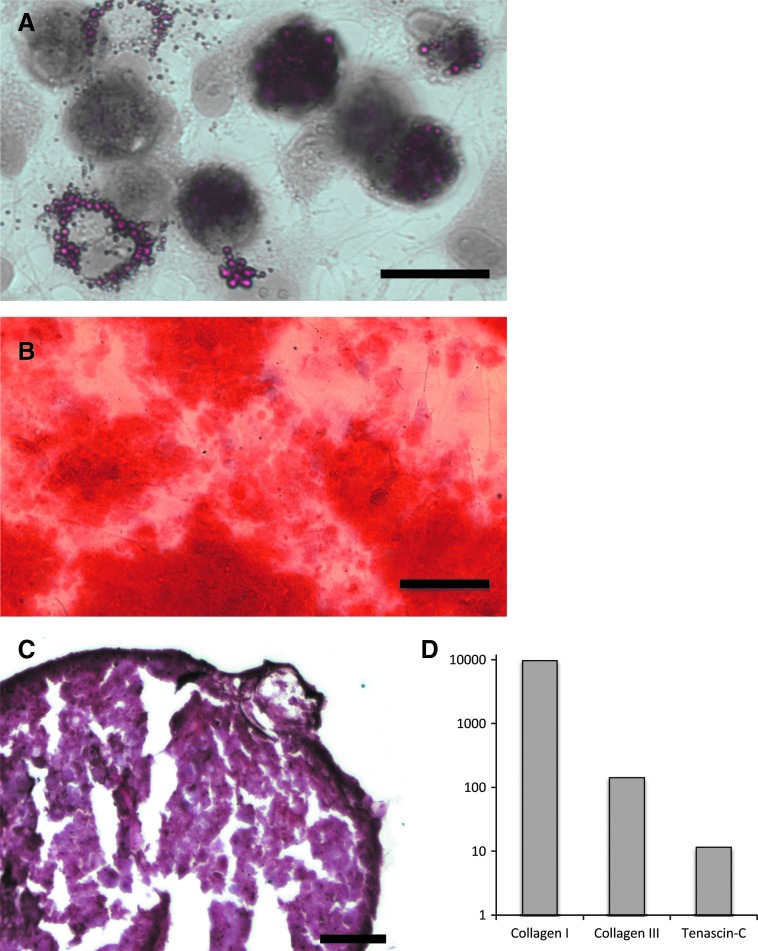
The morphology of the ACLcs isolated and used throughout the study is fibroblastic in nature, as is typical of ACLcs (Fig. 1A, C). In addition, cells freshly isolated from ACL tissue expressed ligament makers Collagen-I, Collagen-III, and Tenascin-C, further characterizing them as ACL fibroblasts (Fig. 2D). MSCs isolated from bone marrow also showed the expected fibroblastic morphology (Fig. 1B, E), as well as the ability to differentiate into osteogenic, adipogenic, and chondrogenic lineages (Fig. 2A–C).
FIG. 1.
In vitro morphological characterization. (A) MSCs isolated from Pig bone marrow show fibroblastic morphology. (B) ACL fibroblasts isolated from Pig ligament. Day-14 unstained cultures of 100% ACLcs (C), 50% ACLcs 50% MSCs, (D) and 100% MSCs (E). Scale bar=100 μm. ACL, anterior cruciate ligament; ACLcs, ACL cells; MSCs, mesenchymal stem cells.
FIG. 2.
Fibroblast characterization. Pig MSCs differentiability to (A) adipogenic (scale bar=25 μm), (B) osteogenic (scale bar=100 μm), and (C) chondrogenic (scale bar=100 μm) lineages. (D) Log10 of the fold expression of ligament markers from freshly harvested ACL fibroblasts calibrated with Pig hepatocytes. Color images available online at www.liebertpub.com/tea
Quantitative effect of co-culture on ligament marker expression
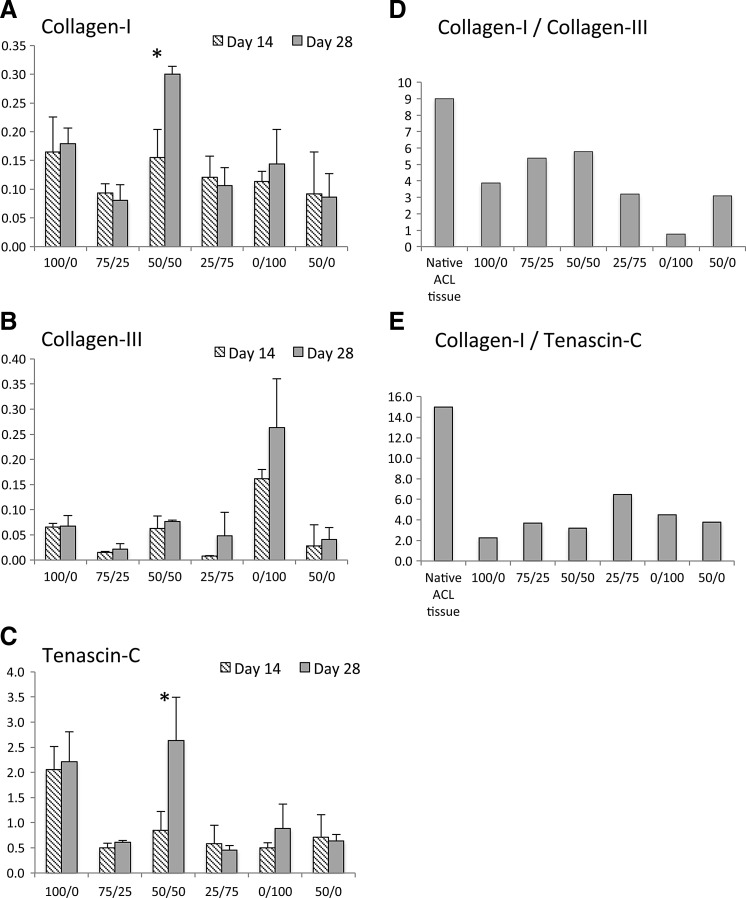
Results show that at day 14, the expression of Collagen-I was statistically similar across cultures (p>0.05, Fig. 3A). At day 28, the 50% ACLcs/50% MSCs sample was the only one to show a significant increase in Collagen-I expression (p≤0.02), and the level of Collagen-I expression was significantly higher than any other culture group (p≤0.01). Collagen-III expression was significantly higher in the MSCs alone throughout the experiment (p≤0.03), and expression remained relatively constant across groups from 14 to 28 days (Fig. 3B). At 14 days, Tenascin-C expression was significantly higher (p≤0.02) only in ACLcs-alone compared to all other samples (Fig. 3C). By day 28, the 50% ACLcs/50% MSC sample was the only group that showed a statistically significant increase in Tenascin-C expression (p≤0.03). Moreover, at 28 days, ACLcs-alone and 50% ACLcs/50% MSCs samples had significantly higher Tenascin-C expression compared to all other samples (p≤0.02), and the difference in their expression at this time point was not statistically significant (p=0.4). Collagen-I-to-Collagen III and Collagen-I-to-Tenascin-C expression ratios for all samples were compared to published ratios for native ACL ligament (Figs. 3D, E). In native ACL tissue, the Collagen-I-to-Collagen-III ratio is ∼9:1, whereas the Collagen-I-to-Tenascin-C ratio is greater than 15.7:1.20,22,24,31 The highest ratio of Collagen-I to Collagen-III was observed in the 50% co-culture group with a value of 5.8, whereas the lowest ratio was seen in the MSCs-alone group at 0.8. The 75% ACLcs/25% MSCs had the second highest Collagen-I-to-Collagen-III ratio at 5.4; however, the overall expression of both markers in this group was markedly lower than the 50% co-culture group throughout the experiment. For Collagen-I-to-Tenascin-C ratios, the highest value was seen in the 25% ACLcs/75% MSCs group at 6.5, whereas the lowest value was seen in the ACLcs-alone group at 2.2, with the 50% co-culture group a close second at 3.2. Primer efficiencies for qPCR gene expression analysis were 90.4% for 18s rRNA, 97.5% for Collagen-I, 97.6% for Collagen-III, and 98.7% for Tenascin-C.
FIG. 3.
Fold expression of (A) Collagen-I, (B) Collagen-III, and (C) Tenascin-C at 14 and 28 days. (D) Collagen-I-to-Collagen-III ratios for native ACL tissue and all co-culture conditions. (E) Collagen-I-to-Tenascin-C ratios for native ACL tissue and all co-culture conditions. X-axis labels indicate the %ACLcs/%MSCs ratio during co-culture. mRNA expression was detected using quantitative PCR and normalized to 18s rRNA. For 100/0, 50/50, and 0/100, n=4, and all others n=3. * indicates statistically significant increase with p<0.05. Means±SD shown. Collagen-I, Collagen type I; Collagen-III, Collagen type III.
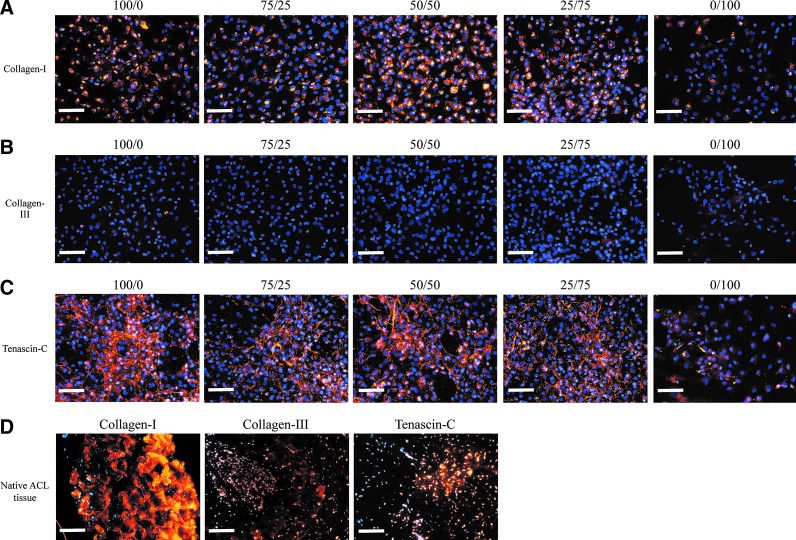
ECM ligament marker expression
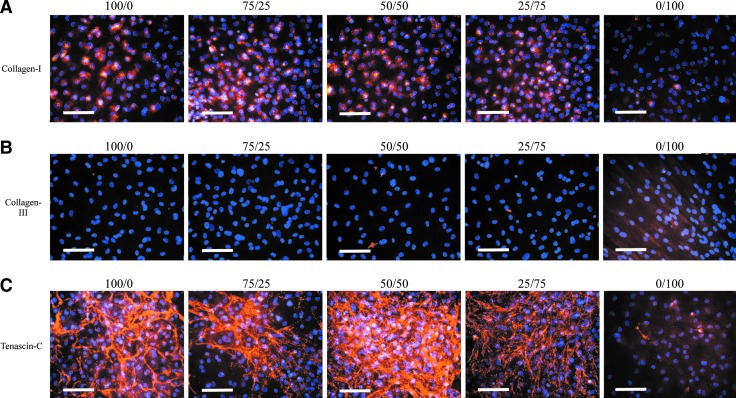
At 14 days, Collagen-I staining was similar across all samples (Fig. 4A); by day 28, Collagen-I staining intensity was clearly increased in all samples, except in MSC alone (Fig. 5A). At 14 days, Collagen-III staining was negligible in all samples (Fig. 4B); by day 28, only the MSCs-alone sample showed increased Collagen-III staining intensity (Fig. 5B). At 14 and 28 days, Tenascin-C staining was more intense in ACLcs-alone and 50% ACLcs/50% MSCs samples, whereas MSCs alone showed minimal Tenascin-C staining at both time points (Figs. 4C and 5C). Unstained day-14 cultures for ACLcs alone, 50% ACLcs/50% MSCs, and MSCs alone are shown in Figure 1C–E. Native ACL tissue staining for the three markers is shown in Figure 4D.
FIG. 4.
Ligament marker expression for (A) Collagen-I, (B) Collagen-III, and (C) Tenascin-C at 14 days. Column labels indicate the %ACLcs/%MSCs ratio during co-culture. (D) Native Pig ACL stains. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
FIG. 5.
Ligament marker expression for (A) Collagen-I, (B) Collagen-III, and (C) Tenascin-C at 28 days. Column labels indicate the %ACLcs/%MSCs ratio during co-culture. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Discussion
A key component of the successful creation of a tissue-engineered ligament is the source of the cells from which regeneration is to occur. The goal of this study was to explore the effect of direct co-culture of several ACLcs/MSCs ratios on the overall expression of ligament markers. Recent evidence suggests that co-culture systems may lead to enhanced regenerative responses, especially when MSCs are involved.6–8,10,11,32 However, the majority, if not all, of the studies focus on the differentiation potential of MSCs toward a specific lineage, and disregard the plethora of additional benefits that MSCs can provide to a regenerative response.15–17 The ACL is a tissue that has limited regenerative potential, due in part to its restricted vascular supply and also to its inability to form a functional healing bridge between the injured or torn ends.33,34 Nevertheless, recent evidence suggests that the tissue has the inherent capacity to heal,19 with MSC-like progenitor cells readily available in the tissue, although this resource must potentially be stimulated to induce a regenerative response.
Our results show that a direct co-culture of ACLcs and MSCs leads to increased expression of ligament markers over 28 days. Collagen-I and Tenascin-C mRNA expression increased over time in 50% co-culture compared to ACLcs or MSCs cultured alone, suggesting that in vitro ECM formation of ACLcs may be enhanced in the presence of MSCs. Immunofluorescent staining supported our mRNA observations, with Collagen-I staining intensity becoming more widespread from 14 to 28 days, particularly in the ACLcs and 50% co-culture groups, and Tenascin-C staining intensity increasing in ACLcs-alone and most remarkably on 50% co-culture. Collagen-I is the marker that stains most prominently in native pig ACL tissue, and the marker whose mRNA expression most remarkably increased when 50% ACLcs/50% MSCs were co-cultured. This observation becomes more notable when considering that only half as many ACLcs were seeded for the co-culture as compared to the ACLcs-alone group. The control with ACLcs seeded in a well at the 50% density without MSCs did not show a Collagen-I expression response comparable to the 50% co-culture. In addition, Tenascin-C mRNA expression significantly increased over 28 days in 50% co-culture, a response not observed in any of the other groups. With the understanding that ECM deposition does not always follow patterns of mRNA expression, our gene expression and immunofluorescence data are complementary and suggest that MSCs in 1:1 co-culture with ACLcs may enhance the expression of Collagen-I and Tenascin-C.
An unanticipated result from our study was the finding that the MSCs-alone culture exhibited a significantly higher expression of Collagen-III throughout the experiment. Moreover, our finding that Collagen-III expression in ACLcs-containing cultures remained relatively constant over time is consistent with previous reports.35 Collagen-III is a fibrillar component of the ECM of ACL that is universally located throughout the ligament, but is most prominently found close to the attachment zones of the tissue.1,22 It comprises about 10% of all collagen content, and plays a key role during the early phases of ligament healing and during the ligamentization of tendon grafts undergoing remodeling after ACL reconstruction.22,36 Collagen-III is known for its ability to make strong intermolecular disulfide crosslinks in a newly formed connective tissue ECM, and has been detected in high quantities at sites of injury during the early phases of the healing response in bone, ligament, and tendon.31 These responses during connective tissue healing may explain why we see MSCs alone as the group with the highest expression of Collagen-III, since mesenchymal cells that differentiate into bone, cartilage, and ligament cells tend to be the first to migrate to the injury sites of these tissues and produce the early matrix of the reparative response.31 These observations about Collagen-III suggest that MSCs could have a positive effect in healing during ACL regeneration. Perhaps, a significant presence of MSCs in a construct aimed at ligament regeneration may have a greater potential to initiate ligament healing and establish a structurally sound framework for the early ECM, whereas a similar presence of ACLcs may be required for long-term deposition of the predominant and stronger Collagen-I. Taking all our conclusions into account, our results suggest that utilizing a 50% co-culture of ACLcs and MSCs in future ACL tissue-engineering studies may better maintain or enhance ligament ECM expression, and thus improve healing.
Native tissue studies have determined that about 90% of all collagen in ACL is type I, whereas the remaining 10% is type III, yielding a 9:1 ratio of Collagen-I to Collagen-III.20,22,24 This fact strengthens our conclusion that the 50% co-culture sample may be the best cell combination in future tissue-engineering studies of the ACL, since it exhibited the highest Collagen-I-to-Collagen-III ratio of all samples at 5.8. Previous ligament-healing studies have noted that, even after long-term follow-up, the ligament scar found at a site of injury exhibited close to a 30% decrease in normal collagen content and a significant increase in the relative amount of Collagen-III content.31 Thus, a ratio lower than the native level of 9 should not be unexpected in healing ligaments.
Tenascin-C is a glycoprotein found in tissues that experience high tensile and compressive stresses, such as ligament and tendons, and it is involved in the binding of cell surface receptors with the ECM.23 Even though its native levels in ACL tissue have not been determined to our knowledge, it is part of the 6% of ligament components that are not a form of collagen (94%),22 making the true Collagen-I-to-Tenascin-C ratio in native ligament significantly higher than 94:6. For this reason, our analysis estimated this native ratio to be at least 15. After tissue injury and during wound healing, Tenascin-C levels have been shown to be transiently overexpressed.37,38 Because of this, high levels of the marker are not expected in native ligament tissue, but are expected during a reparative response. Thus, the Collagen-I-to-Tenascin-C ratio would be expected to be large in native ligament and low in instances of ligament repair. Our mRNA analysis showed that the lowest ratios of Collagen-I to Tenascin-C were observed in the ACLcs and 50% co-culture groups, 2.2 and 3.2, respectively. This result would imply that Tenascin-C is being overexpressed, which is expected in cells isolated from ACLcs that may detect a state of injury and are mounting a healing response. This observation also strengthens our support for the use of 50% co-cultures of ACLcs and MSCs for tissue engineering ACL attempts.
A co-culture of ACLcs and MSCs may lead to an enhanced regenerative response in vivo by providing not only a cell source from which cell differentiation can replenish an implanted scaffold but also by the production of chemokines and cytokines that promote angiogenesis within the scaffolds, prevent scar formation, and awaken/recruit tissue-intrinsic progenitor cells (ACL-derived MSCs) to the scaffold to increase the regeneration. Furthermore, MSC anti-inflammatory and immunomodulatory properties can provide a beneficial effect for biomaterial-based implants.39,40 Many of the problems caused by biodegradable scaffolds implanted in the body relate to the triggering of an immune response against the scaffold. MSCs could prevent the immune response and decrease related inflammation at the site by their anti-inflammatory effects, which in turn would protect the implant and enhance the repair. Ongoing studies in our laboratory are examining this effect in a 3D scaffold culture, as well as attempting to elucidate the fate of MSCs in direct co-culture. An in vivo ACL regeneration model is also ongoing, where the immunomodulatory and anti-inflammatory roles of MSCs are being more directly assessed.
Acknowledgments
The authors would like to thank Dr. Chenhui Zou for his expertise and help with qPCR, and Dr. Toru Hosoda for his advice with statistical analyses. Funds from the Department of Anesthesiology, Perioperative and Pain Medicine at the Brigham and Women's Hospital were used for this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.van Eijk F. Saris D.B.F. Riesle J. Willems W.J. van Blitterswijk C.A. Verbout A.J. Dhert W.J.A. Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004;10:893. doi: 10.1089/1076327041348428. [DOI] [PubMed] [Google Scholar]
- 2.Laurencin C. Freeman J. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 3.Cimino F. Volk B.S. Setter D. Anterior cruciate ligament injury: diagnosis, management, and prevention. Am Fam Physician. 2010;82:917. [PubMed] [Google Scholar]
- 4.Cooper J. Bailey L. Carter J. Castiglioni C. Kofron M. Ko F. Laurencin C. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials. 2006;27:2747. doi: 10.1016/j.biomaterials.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Ge Z. Goh J.C.H. Lee E.H. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- 6.Luo Q. Song G. Song Y. Xu B. Qin J. Shi Y. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61:1. doi: 10.1007/s10616-009-9233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I.-C. Wang J.-H. Lee Y.-T. Young T.-H. The differentiation of mesenchymal stem cells by mechanical stress or/and co-culture system. Biochem Biophys Res Commun. 2007;352:147. doi: 10.1016/j.bbrc.2006.10.170. [DOI] [PubMed] [Google Scholar]
- 8.Fan H. Liu H. Toh S.L. Goh J.C.H. Enhanced differentiation of mesenchymal stem cells co-cultured with ligament fibroblasts on gelatin/silk fibroin hybrid scaffold. Biomaterials. 2008;29:1017. doi: 10.1016/j.biomaterials.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L. Tran N. Chen H.-Q. Kahn C.J.F. Marchal S. Groubatch F. Wang X. Time-related changes in expression of collagen types I and III and of tenascin-C in rat bone mesenchymal stem cells under co-culture with ligament fibroblasts or uniaxial stretching. Cell Tissue Res. 2008;332:101. doi: 10.1007/s00441-007-0564-6. [DOI] [PubMed] [Google Scholar]
- 10.Ball S.G. Shuttleworth A.C. Kielty C.M. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36:714. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Schneider P.R.A. Buhrmann C. Mobasheri A. Matis U. Shakibaei M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res. 2011;29:1351. doi: 10.1002/jor.21400. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain G. Fox J. Ashton B. Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 13.Nauta A.J. Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 14.Newman R.E. Yoo D. LeRoux M.A. Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 15.Caplan A.I. New era of cell-based orthopedic therapies. Tissue Eng Part B Rev. 2009;15:195. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 18.Cheng M.-T. Yang H.-W. Chen T.-H. Lee O.K.-S. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009;42:448. doi: 10.1111/j.1365-2184.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinert A.F. Kunz M. Prager P. Barthel T. Jakob F. Nöth U. Murray M.M. Evans C.H. Porter R.M. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 2011;17:1375. doi: 10.1089/ten.tea.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan H. Liu H. Toh S.L. Goh J.C.H. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Yates E.W. Rupani A. Foley G.T. Khan W.S. Cartmell S. Anand S.J. Ligament tissue engineering and its potential role in anterior cruciate ligament reconstruction. Stem Cells Int. 2012 doi: 10.1155/2012/438125. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthon V.B. Barea C. Abrassart S. Fasel J.H. Fritschy D. Menetrey J. Anatomy of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc: Off J ESSKA. 2006;14:204. doi: 10.1007/s00167-005-0679-9. [DOI] [PubMed] [Google Scholar]
- 23.September A.V. Schwellnus M.P. Collins M. Tendon and ligament injuries: the genetic component. Br J Sports Med. 2007;41:241. doi: 10.1136/bjsm.2006.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amiel D. Frank C. Harwood F. Fronek J. Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1:257. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- 25.Nagineni C.N. Amiel D. Green M.H. Berchuck M. Akeson W.H. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10:465. doi: 10.1002/jor.1100100402. [DOI] [PubMed] [Google Scholar]
- 26.Ross S.M. Joshi R. Frank C.B. Establishment and comparison of fibroblast cell lines from the medial collateral and anterior cruciate ligaments of the rabbit. In Vitro Cell Dev Biol. 1990;26:579. doi: 10.1007/BF02624206. [DOI] [PubMed] [Google Scholar]
- 27.Liu H. Fan H. Toh S.L. Goh J.C.H. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008;29:1443. doi: 10.1016/j.biomaterials.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Kojima K. Ignotz R.A. Kushibiki T. Tinsley K.W. Tabata Y. Vacanti C.A. Tissue-engineered trachea from sheep marrow stromal cells with transforming growth factor beta2 released from biodegradable microspheres in a nude rat recipient. J Thorac Cardiovasc Surg. 2004;128:147. doi: 10.1016/j.jtcvs.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerman K.A. Penvose A. Yang Z. Allen P.D. Vacanti C.A. Adult muscle “stem” cells can be sustained in culture as free-floating myospheres. Exp Cell Res. 2010;316:1966. doi: 10.1016/j.yexcr.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S.H. Yang R.S. al-Shaikh R. Lane J.M. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;265 [PubMed] [Google Scholar]
- 32.Bian L. Zhai D.Y. Mauck R.L. Burdick J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray M.M. Current status and potential of primary ACL repair. Clin Sports Med. 2008;28:51. doi: 10.1016/j.csm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bray R.C. Leonard C.A. Salo P.T. Vascular physiology and long-term healing of partial ligament tears. J Orthop Res. 2002;20:984. doi: 10.1016/S0736-0266(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 35.Almarza A.J. Augustine S.M. Woo S.L.-Y. Changes in gene expression of matrix constituents with respect to passage of ligament and tendon fibroblasts. Ann Biomed Eng. 2008;36:1927. doi: 10.1007/s10439-008-9565-1. [DOI] [PubMed] [Google Scholar]
- 36.Cheng M.-T. Liu C.-L. Chen T.-H. Lee O.K. Comparison of potentials between stem cells isolated from human anterior cruciate ligament and bone marrow for ligament tissue engineering. Tissue Eng Part A. 2010;16:2237. doi: 10.1089/ten.TEA.2009.0664. [DOI] [PubMed] [Google Scholar]
- 37.Midwood K.S. Hussenet T. Langlois B. Orend G. Advances in tenascin-C biology. Cell Mol Life Sci. 2011;68:3175. doi: 10.1007/s00018-011-0783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geffrotin C. Garrido J.J. Tremet L. Vaiman M. Distinct tissue distribution in pigs of tenascin-X and tenascin-C transcripts. Eur J Biochem. 1995;231:83. doi: 10.1111/j.1432-1033.1995.tb20673.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoogduijn M.J. Popp F. Verbeek R. Masoodi M. Nicolaou A. Baan C. Dahlke M.H. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10:1496. doi: 10.1016/j.intimp.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Phinney D.G. Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]