Abstract
The Escherichia coli DegP has been reported to function both as molecular chaperone and protease for the quality control of outer membrane protein biogenesis. Activation of the inactive DegP hexamers was believed to occur via their disassembly into trimeric units and subsequent reassembly into larger oligomers (12-mers and 24-mers). Here, we analyzed the thermal stability and the unfolding dynamics of the different secondary structure components of the DegP hexamers using Fourier transform infrared spectroscopy and temperature-jump nanosecond time-resolved IR difference absorbance spectroscopy. We found that the interfacial secondary structure components possess a degreed thermal stability, with the disassembly of the DegP hexamers follows a “proteinquake” manner, such that the fully exposed parts of the interfacial β-sheets serving as the temperature sensor and epicenter to drive the sequential unfolding/disassembly process that finishes within about 134 ns at room temperature.
The aggregation of misfolded proteins represents a severe danger for cell viability. To minimize such a hazard, effective quality-control systems, largely involving molecular chaperones and proteases, have evolved to suppress such aggregation and/or to refold or degrade the misfolded proteins1. The Escherichia coli (E. coli) DegP (also designated as HtrA), whose homologs are widely found in all forms of life, is a unique protein that has been reported to possess both chaperone and protease activities2. The DegP protein, present in the periplasmic space of E. coli cells, was found to be essential for bacterial survival at high temperatures3,4. It has been shown that DegP exists as inactive hexamers, which are transformed into the active and cage-like 12- or 24-mers upon substrate binding5,6. Therefore DegP provides an excellent example for understanding how an inactive protein becomes activated by readjusting its oligomeric quaternary structures.
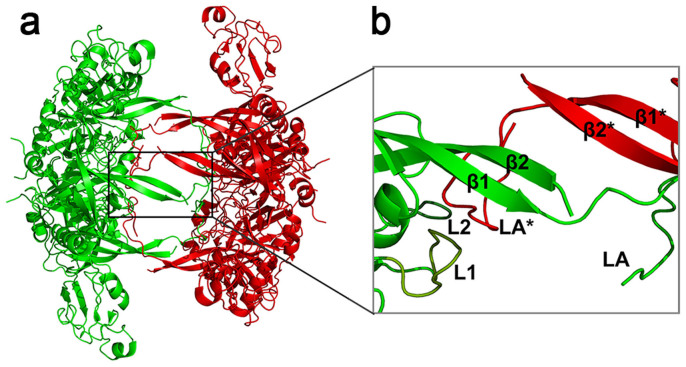
Crystal structure determination revealed that the DegP hexamer is formed by a staggered association of two trimeric units in a face-to-face manner7. The primary contacts between the two trimeric units consist of six (one from each of the six subunits) two-stranded β-sheets, as illustrated in Fig. 1a. In addition, at the interface of the two trimeric units, the six LA loops (each LA loop connecting two β strands designated as β1 and β2 from the six two-stranded β-sheets) protrude into their opposing subunits and intimately interact with loops L1 and L2 in active sites of the latter, forming six loop triads, LA*-L1-L2 (Fig. 1b; the asterisk denotes its contribution from an opposing subunit) that completely prevent the unfolded substrate proteins from entering the active sites, deactivating the protease function of the DegP hexamers6,7. It has been reported that upon binding unfolded substrate proteins, the DegP hexamers are converted into the larger oligomeric forms of 12 or 24 subunits (i.e., the 12- and 24-mers). Structural analyses of these larger oligomers have revealed that the inhibitory LA loops are liberated from the active sites, enabling DegP to adopt a functional state6,8. It follows that the conversion from hexamers to 12- and 24-mers is a crucial step for DegP to be activated9. Such conversion has been implicated to occur via oligomeric disassembly and reassembly, with the trimeric units being produced as the intermediate5, during which the six LA*-L1-L2 loop triads would have to be disrupted.
Figure 1. Cartoon representation of the crystal structure of the DegP hexamer (PDB ID:1ky9) emphasizing the interfacial linkers between the two trimeric units.
Shown in the enlarged view is the anti-parallel β-sheets and the loop triad LA*-L1-L2 (LA* comes from a monomer of the opposing trimeric unit). The crystal structure was drawn using Pymol program.
For the activation of DegP hexamers, outstanding unresolved issues include the following. First, what factor, the presence of unfolded substrate proteins (as proposed in ref. 9), the heat shock temperature (as implicated in ref. 2) or others, triggers the activation process of DegP? With equal importance, how the interactions between the trimeric units are disrupted in the DegP hexamers for the disassembly to occur?
We tried to resolve these issues via an approach of both thermodynamics and dynamics. For these we first performed thermal titration studies using Fourier transform infrared spectroscopy (FTIR) to reveal the varied thermal stability of the different secondary structure components in the DegP hexamer. We then applied temperature-jump time-resolved IR difference spectroscopy to probe the unfolding dynamics of these secondary structure components. Consistently, our thermodynamic and dynamic data unveil a mechanism for the inactive DegP hexamers to disassemble, such that the disruption of the interfacial secondary structure components, having degreed thermal stability, occurs in a proteinquake mode, which eventually leads to the dissociation of the two trimeric units. Remarkably, this occurs even at the room temperature (25°C) and within only about 134 nanoseconds. It is conceivable that similar proteinquake mechanism might be utilized by other oligomeric proteins (e.g., small heat shock proteins) that modulate their activities by changing their oligomeric forms10.
Results
Temperature-dependent FTIR spectroscopic characterization of the secondary structure components of the DegP hexamer
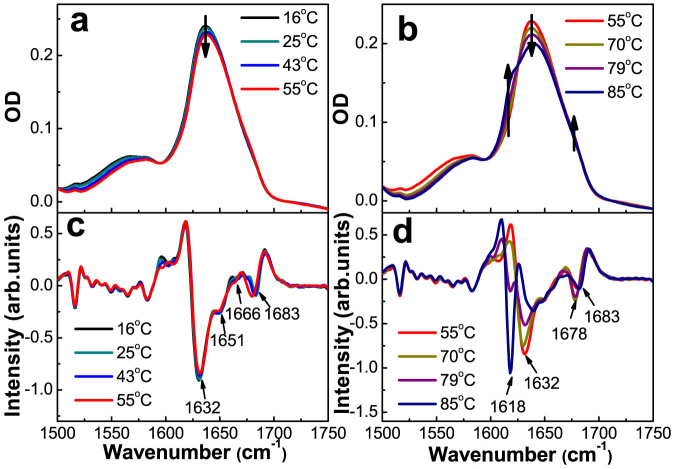
To unveil the mechanism for the inactive DegP hexamer to become active, in particular for its disassembly, we first examined the thermal stability of each of its secondary structure components, especially those participating in the interaction between the trimeric units. For this, we first conducted IR spectroscopic analysis, such that the temperature-dependent FTIR spectra with the DegPS210A protein sample dissolved in D2O, were recorded at 1500 cm−1–1750 cm−1, including the amide I′ (the prime denotes the frequencies of deuterated amide groups) band, i.e., between 1600 cm−1–1700 cm−1, a region at which each different secondary structure component would have absorption at a characteristic wavenumber. As shown in Figures 2a and 2b, the absorbance in the 1500 cm−1–1600 cm−1 region for the amide II band is low, indicating that the H2O content in the protein sample was insignificant11. The FTIR spectra of the inactive DegPS210A protein were collected in 3°C steps starting from 10°C and up to 85°C, representative ones recorded at the indicated temperatures are displayed in Figures 2a and 2b, and their corresponding second derivatives are presented in Figures 2c and 2d. For clarity, we presented the FTIR spectra as two groups, with those that only change slightly, i.e., between 10°C–55°C, displayed in Fig. 2a, while those that change significantly, i.e., between 55°C–85°C, displayed in Fig. 2b (the corresponding second derivatives displayed respectively in Figures 2c and 2d). For the second derivative spectra in the lower temperature group, the broadband centered at 1632 cm−1 (Fig. 2c) can be assigned to be produced from β-sheets in general, while the other peaks can be assigned to be produced from loops/helices (1651 cm−1), turns (1666 cm−1), and the high frequency vibration of anti-parallel β-sheets (1678 cm−1 and 1683 cm−1). Our secondary structure assignments of the major peaks are largely consistent to those reported earlier11,12. The simultaneous appearance of absorption at 1618 cm−1 and 1683 cm−1 at temperatures higher than 70°C (Fig. 2d) has been assigned to be produced from the intermolecular anti-parallel β-sheets present in the aggregate formed by denatured proteins13,14. Another feature for the spectra in the higher temperature group is the absorption peak at 1678 cm−1, which appears at 61°C but disappears at above 79°C, apparently being produced from a certain β-sheet structure intermediate formed prior to protein aggregation. It should be pointed out that the temperature-induced spectral changes recorded by us for the DegPS210A protein at the high temperature range are largely consistent with what were reported by Skorko-Glonek et al, who also observed similar unfolding-related temperature-induced spectral changes from 60°C to 70°C and the aggregation-related changes above 70°C11.
Figure 2. Overall characterization of the secondary structure components in the DegP hexamer via temperature-dependent FTIR absorption spectroscopy.
(a)(b) Absorption spectra of DegPS210A at 12.5 mg/ml, in D2O (pD 7.4). (c)(d) The second derivative spectra corresponding to (a) and (b). Arrows in the absorption spectra denote the direction of the absorbance change with increasing temperature.
We then focused on the broadband centered at 1632 cm−1 which we assigned to be derived from β-sheet structures. To find out the particular types of β-sheets that contribute to this band, we plotted the FTIR difference spectra of DegPS210A, as shown in Fig. 3a, by subtracting the spectrum recorded at a given higher temperature by that recorded at 16°C. We also plotted temperature-dependent changes for the wavenumber of the bleaching peaks, as displayed in Fig. 3b, using the information extracted from the FTIR difference spectra shown in Fig. 3a. The data displayed in Fig. 3b demonstrate that at least three different types of β-sheet structures can be clearly identified as indicated by the fact that the wavenumber curve has three different slopes in three typical temperature ranges, with one absorbing at 1623 cm−1–1626 cm−1 at temperatures lower than 55°C, and being blue shifted from 1623 cm−1 to 1626 cm−1 upon temperature rising. Upon raising the temperature from 55°C to 70°C, the bleaching peak wavenumber shifts drastically from 1626 cm−1 to 1634 cm−1 (Fig. 3b). Given that for β-sheet structures, the more solvent-exposed the more red shifted for its amide I′ absorption peak15,16, the β-sheet structures absorbing at 1623 cm−1 –1626 cm−1 would be more exposed to the solvent than those absorbing at 1626 cm−1–1634 cm−1. It follows that the former can be assigned to those fully exposed parts of the β-sheets located at the interface of the trimeric units, while the latter to those partially exposed parts of the interfacial β-sheets. When the temperature elevation exceeds 70°C, the bleaching peak remains at 1634 cm−1 and no longer shifts, thus to be assigned as the absorption of buried β-sheets.
Figure 3. Detailed assignments of the interfacial secondary structure components in the DegP hexamer via the comparison between three mutants.
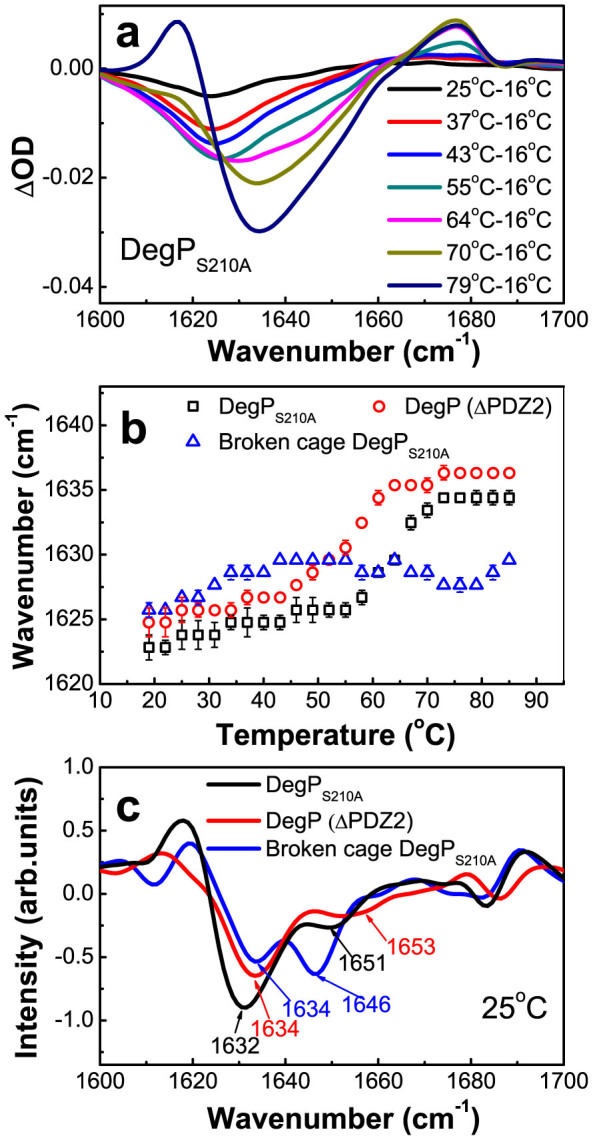
(a) Absorption difference spectra of DegPS210A between the spectrum acquired at the indicated temperature and that of 16°C. (b) The plot of the bleaching peak wavenumber against the temperature for DegPS210A, DegP (ΔPDZ2), and broken cage DegPS210A (Δ39–78). (c) The second derivative spectra of DegPS210A, DegP (ΔPDZ2) and broken cage DegPS210A (Δ39–78) at 25°C.
To validate these assignments, we repeated the above experiment on two previous reported trimeric mutants, the DegP (ΔPDZ2), having the PDZ2 domain (residues 356–446) deleted, and the broken cage DegPS210A, having residues 39 to 78 within the LA loop removed17. The DegP (ΔPDZ2) mutant protein was demonstrated to exist mainly as trimers, while the broken cage DegPS210A mutant existing mostly as trimers and monomers17. It follows that the interfacial β-sheets and LA loops present in the hexameric forms would no longer exist in these two mutant forms of DegP.
The FTIR absorption spectra of these two forms of DegP recorded at 25°C are shown in Supplementary Fig. S1 and their corresponding second derivative spectra are displayed in Fig. 3c. Remarkably, instead of detecting a significant peak centered at 1632 cm−1 for the hexameric DegP, we only detected a peak that is much weaker and centered at 1634 cm−1 for the DegP (ΔPDZ2) and broken cage DegPS210A mutants (Fig. 3c). Conceivably, those disappeared absorption, located at the red side in a spectral range of 1623 cm−1–1634 cm−1, were most likely contributed by the solvent-exposed interfacial β-sheets in the hexamers. Similar plotting of temperature-dependent changes for the wavenumber of the bleaching peaks for the trimeric mutants, also displayed in Fig. 3b, reveals a blue-shift of 2–4 cm−1 at temperatures lower than 55°C in comparison with that of the DegP hexamer. Taken together, these observations strongly validate our assignment of the IR absorption band at 1623 cm−1–1626 cm−1 to be derived from those fully exposed parts of the β-sheets located at the interface of the trimeric units of the hexamer.
The secondary structure components of the DegP hexamers possess a degreed thermal stability
We next studied the thermal stability of these secondary structure components identified above (Fig. 2 and 3). To avoid the potential overlapping between the absorption spectra of the fully and partially exposed interfacial β-sheets (absorbing at 1623 cm−1–1626 cm−1 and 1626 cm−1–1634 cm−1 respectively), we decided to perform Singular Value Decomposition (SVD) analysis, being a powerful mathematical tool commonly used for multicomponent spectral resolution18,19, on the temperature-dependent FTIR spectra between 1610 cm−1 and 1632 cm−1 that we acquired in the low temperature region between 10°C–55°C (for details, see Supplementary Note and Fig. S2). The plotting of intensity against wavenumber, as shown in Fig. 4a, clearly resolves two absorption peaks centered at 1623 cm−1 and 1629 cm−1, which can be respectively assigned to those fully exposed and those partially exposed parts of the interfacial β-sheets. Meanwhile, the SVD-resolved population thermal titration curve for the secondary structural component absorbing at 1623 cm−1, as shown in Fig. 4b, can be fitted by a two-state unfolding model, with the transition midpoint temperature (Tm) being at 23.6 ± 2.3°C. It should be pointed out that a highly similar Tm value of 23.9 ± 0.8°C was also obtained by analyzing the absorbance thermal titration curve at 1623 cm−1 (see Supplementary Note and Fig. S3). This relatively low Tm value strongly indicates that the fully exposed interfacial β-sheet structure is actually quite unstable, with its thermal-induced disruption starting at least from such a low temperature as 10°C. A similar SVD-resolved population thermal titration curve at 1629 cm−1, also presented in Fig. 4b, failed to be fitted by a two-state unfolding process, suggesting that this absorption band is contributed from β-sheets of varied thermal stability. Nevertheless, the data presented in Fig. 4b clearly indicate that these partially exposed β-sheet structures absorbing at 1629 cm−1 are more stable than the fully exposed β-sheets absorbing at 1623 cm−1.
Figure 4. The thermal stability analysis of the interfacial secondary structure components in the DegP hexamer.
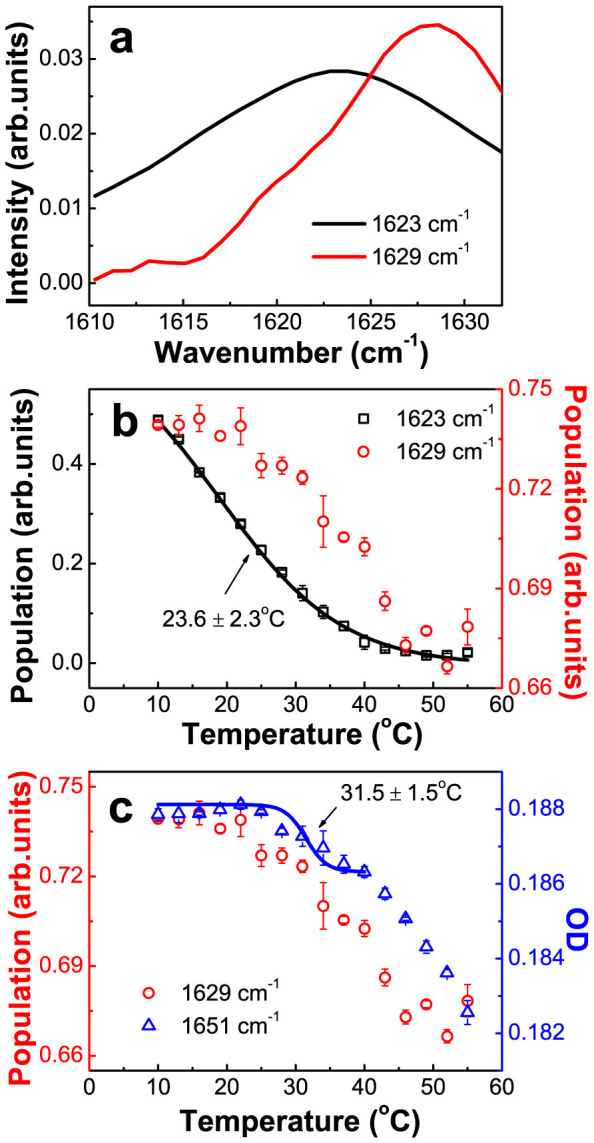
(a) The SVD-resolved two species-associated spectra centered at 1623 cm−1 and 1629 cm−1. (b) The population thermal titration curves of the structure components (1623 cm−1, 1629 cm−1) retrieved by SVD analysis. (c) The absorbance thermal titration curve at 1651 cm−1 and the population thermal titration curve of the structure component at 1629 cm−1.
We subsequently analyzed the thermal stability of the loop/helical structures that absorb at 1651 cm−1 also in the low temperature region (between 10°C–55°C). The crystal structure of DegP indicates that most of the α-helices are located far away from the trimer-trimer interface in the hexamer, and are thus expected to be more stable than the loops that are largely located closer to the trimeric interface7. In view of this, we assumed that the thermal-induced IR absorbance change at the above low temperatures largely reflects the structural change of the loops, although the absorption spectra from α-helices and loops in proteins may overlap in this spectral region20. The thermal titration curve for IR absorbance at 1651 cm−1, displayed in Fig. 4c, shows that the absorption begins to decrease at 25°C and reaches a plateau at 40°C, giving a Tm value of 31.5 ± 1.5°C, reflecting the unfolding process of a certain kind of loop structures. Upon further increase of the temperature, no plateau appears till 55°C, indicating a continuously opening of the loop structures. It has been previously reported that the LA loop undergoes a significant thermal-induced opening at a temperature of 25°C–30°C, the L2 loop at a temperature exceeding 35°C, and the L1 loop at a temperature higher than 40°C12. In view of these, the observed Tm value of 31.5 ± 1.5°C should be assigned to the unfolding process of the LA loop. It should be pointed out that the loops absorbing at 1651 cm−1 are apparently more stable than the partially exposed β-sheet structures absorbing at 1629 cm−1, as indicated by our plotted SVD-resolved population thermal titration curve presented in Fig. 4c.
The assignment of the peak at 1651 cm−1 to the LA loop is again validated by our FTIR spectroscopy analysis of the broken cage DegPS210A, for which the residues from positions 39 to 78 within the LA loop were removed and thus existing predominantly as trimers and monomers17. As indicated by the second derivative spectrum shown in Fig. 3c, for the broken cage DegPS210A, the absorption at 1651 cm−1 was no longer detectable, instead, a new peak centered at 1646 cm−1 was recorded, most likely due to the formation of random coil structures resulted from the deletion of the LA loop. Taken together, these observations confirm that the absorption peak centered at 1651 cm−1 is indeed derived from the LA loop in the DegP hexamer.
Last but not least, we analyzed the thermal stability of the anti-parallel β-sheet structures that absorb at 1683 cm−1 and are located within the trimeric units. In the second derivative spectra (Fig. 2d), we noticed that when the temperature exceeds 55°C, the peak at 1683 cm−1 is first converted to one at 1678 cm−1 and then back to 1683 cm−1 upon further temperature elevation. To gain detail information on the structure species attributing to these two absorption peaks, we carried out SVD analysis on the temperature-dependent FTIR spectra between 1670 cm−1 and 1690 cm−1 (for details, see Supplementary Fig. S4). The results reveal that two absorption bands with a peak centered at 1678 cm−1 and 1683 cm−1 respectively are resolved clearly (Fig. 5a). The SVD-resolved population thermal titration curves for the two different structure species that absorb at 1683 cm−1 and 1678 cm−1 respectively, as shown in Fig. 5b, show that the disruption of the anti-parallel β-sheet structures that absorb at 1683 cm−1 starts at 40°C by first converting to an intermediate form that absorbs at 1678 cm−1. This red shift in peak position (from 1683 cm−1 to 1678 cm−1) suggests that these anti-parallel β-sheets somehow become loosened. The disruption apparently completes at around 70°C, with the fitted Tm value being at 56.7 ± 0.4°C. Our conclusion that the structure species absorbing at 1678 cm−1 is derived from that initially absorbing at 1683 cm−1 is strongly supported by the fact that the population decrease of the latter is accompanied by an increase of the former in an inverse and proportional manner upon temperature elevation from 40°C to 70°C.
Figure 5. The thermal stability analysis of the secondary structure components within the interior of the trimeric units in the DegP hexamer.
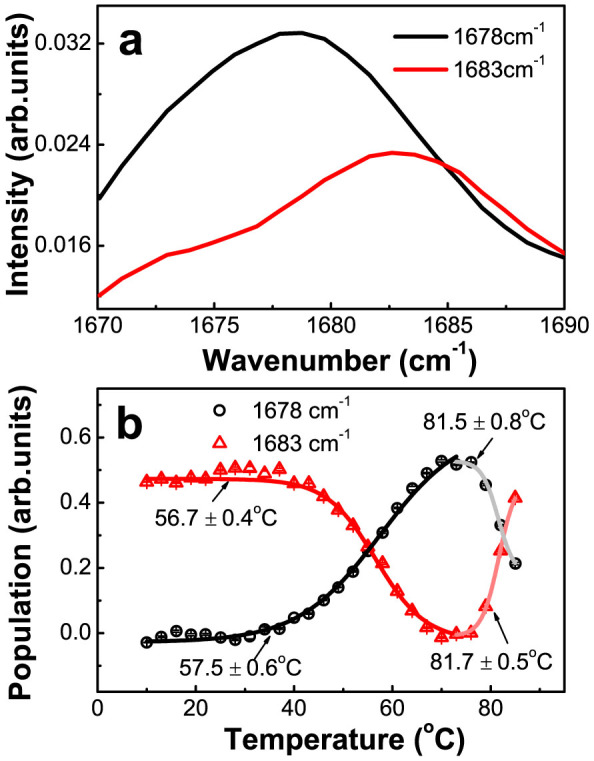
(a) The SVD-resolved two species-associated spectra centered at 1678 cm−1 and 1683 cm−1. (b) The population thermal titration curves of the structure components (1678 cm−1, 1683 cm−1) retrieved by SVD analysis.
The results of the thermal stability analysis described above clearly suggest that the different secondary structure components of the DegP hexamer possess a degreed thermal stability. In particular, their relative stability increases in the following order, the fully exposed parts of the interfacial β-sheets (Tm = 23.6 ± 2.3°C), the partially exposed parts of the interfacial β-sheets, the LA loops (Tm = 31.5 ± 1.5°C), the buried anti-parallel β-sheet structures within the interior of the trimeric unit (Tm = 56.7 ± 0.4°C).
The unfolding dynamics of the secondary structure components of the DegP hexamers as revealed by temperature-jump time-resolved IR difference absorbance spectroscopy
To find out how these secondary structure components that possess a degreed thermal stability are involved in the disassembly process of the DegP hexamers, we next examined the dynamics for their thermal-induced disruption. For this, we investigated the thermal-induced unfolding dynamics of the DegP hexamers as initiated by temperature-jump from 16°C to 25°C, as followed by using time-resolved IR absorbance difference spectroscopy. The reason to choose this temperature jump range is based on the following observations: (i) It has been reported that the larger oligomeric forms (12- and 24-mers) can be formed from the DegP hexamers even at the room temperatures21, suggesting that the DegP hexamers are able to dissociate into their trimeric units at such low temperatures; (ii) our thermal titration results described above indicate that the disruption of the secondary structure components involved in the interaction between the trimeric units of the DegP hexamer apparently starts at least from a temperature as low as 10°C (Fig. 4b).
The transient IR difference spectrum, as recorded two microseconds after the temperature-jump, is presented in Fig. 6. For comparison, the steady-state FTIR difference spectrum between the spectrum recorded at 25°C and that at 16°C (as shown in Fig. 3a) is also displayed here. The reliability of such transient IR spectrum is indicated by its overall correlation with the steady-state difference spectrum described above (Fig. 3a). This transient spectrum reveals the disappearance of three secondary structure components, as indicated by the recording of the bleaching peaks centered at 1622 cm−1, 1629 cm−1 and 1650 cm−1, which correlate respectively to the disruption of the fully exposed parts of the interfacial β-sheets, the partially exposed parts of the interfacial β-sheets and the LA loops. It meanwhile reveals the appearance of two major absorption peaks centered at 1646 cm−1 and 1663 cm−1, which correlate respectively to the formation of random coil and β-turn structures.
Figure 6. The transient IR absorbance difference spectrum of DegPS210A recorded at 2 μs after the temperature-jump from 16°C to 25°C.
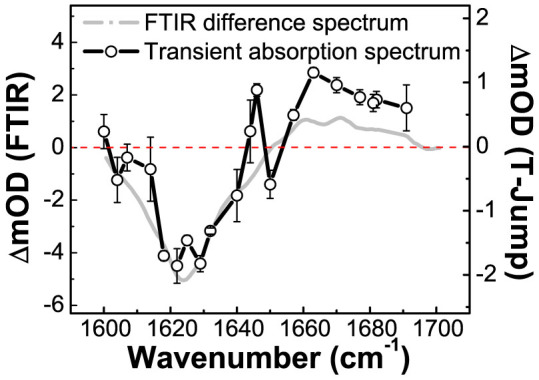
The steady-state FTIR absorbance difference spectrum between 25°C and 16°C is also included for comparison.
We then tried to elucidate the kinetics regarding the temperature-jump induced cooperative conversion of the secondary structure components by analyzing the temporal coincidence between the disappearance of the transient bleaching peaks and the appearance of the absorption peaks (shown in Fig. 6). The kinetic curves, displayed in Fig. 7a, indicate a clear correlation between the disappearing bleaching peak centered at 1622 cm−1, having a time constant of 40 ± 10 ns, and the appearing absorption peak centered at 1646 cm−1, having a time constant of 45 ± 10 ns. This demonstrates that the disruption of the fully exposed parts of the interfacial β-sheets that absorb at 1622 cm−1 leads to the formation of the random coil structures that absorb at 1646 cm−1. Similarly, the disappearing bleaching peak centered at 1629 cm−1, having a time constant of 65 ± 10 ns, correlates to the appearing absorption peak centered at 1663 cm−1, having a time constant of 75 ± 10 ns (Fig. 7b), indicating a correlation between the disruption of the partially exposed parts of the interfacial β-sheet structures that absorb at 1629 cm−1 and the formation of the turn structures that absorb at 1663 cm−1. In contrast, the disappearing bleaching peak centered at 1650 cm−1, having a time constant of 134 ± 6 ns (Fig. 7c), finds no counterpart among the appearing transient absorption peaks, suggesting that the LA loops that absorb at 1650 cm−1 detach from the opposing trimeric units, which in turn leads to the falling apart of the two interacting trimeric units in the DegP hexamer.
Figure 7. Unfolding and folding kinetic curves for the interfacial secondary structure components of the DegP hexamer acquired by time-resolved IR absorbance difference spectroscopy at the indicated wavenumbers, after a temperature-jump from 16°C to 25°C.
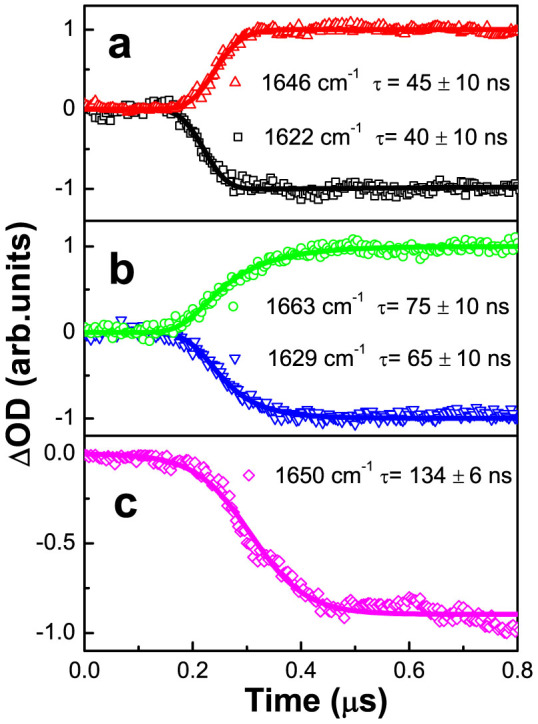
(a) 1622 cm−1 and 1646 cm−1, (b) 1629 cm−1 and 1663 cm−1 and (c) 1650 cm−1.
Together, these observations suggest the following time course for the sequential opening of the secondary structure components and dissociation of the DegP hexamer. First, the fully exposed parts of the interfacial β-sheets unfold in about 40 ns, then the partially exposed parts of the interfacial β-sheets open in about 70 ns, and finally the LA loops detach from the opposing trimeric units in about 134 ns. In consistent with the results of the thermal stability analysis described above (Fig. 4), these dynamic data suggest an increase of the dynamic stability of the secondary structure components in the following order, the fully exposed parts of the interfacial β-sheets, the partially exposed parts of the interfacial β-sheets and the LA loops.
Discussion
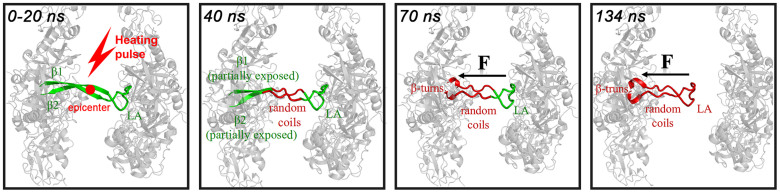
In an attempt to elucidate the structural mechanism for the inactive DegP hexamers to be converted to the active 12- and 24-mers, we hereby examined the process for the DegP hexamer to disassemble into its trimeric units in both thermodynamic and dynamic terms, by using high time-resolved spectroscopic methods to trace the temperature-jump induced structure conversions. Our most remarkable findings include the following. First, our qualitative and quantitative thermal titration analysis reveals that the different secondary structure components of the DegP hexamers possess a degreed thermal stability. Second, our temperature-jump nanosecond time-resolved IR difference spectroscopic results demonstrate also a degreed dynamic stability for the interfacial secondary structure components of the DegP hexamers. Together these revelations strongly suggest that the interfacial secondary structure components unfold in a sequential manner such that the disassembly of the DegP hexamers occurs via a “proteinquake” mechanism, as illustrated in Fig. 8. Here the term “proteinquake” is defined as such that a perturbation in one part of the protein molecule leads to a successive further perturbations nearby in a quake-like manner, eventually leading to a large conformation change22,23,24. For the DegP hexamer, in particular, the epicenter locates at the fully exposed parts of the interfacial β-sheets which unfold in about 40 ns at 25°C, the perturbation then propagates to the partially exposed parts of the interfacial β-sheets in about 70 ns, and subsequently to the LA loops in about 134 ns, eventually leading to the dissociation of the DegP hexamer to its trimeric units. Owing to the formation of the random coils from the fully exposed part and the formation of β-turns from the partially exposed part of the interfacial β-sheets, the backbone of which is made less extended. This creates a tension (denoted as F in Fig. 8) along this backbone, which meanwhile would detach the LA loop away from the opposing trimeric unit, eventually leading to the dissociation of the DegP hexamer into two trimers. The fact that the middle parts of the interfacial links are the most exposed and thus most fragile would make them the ideal sensor for the environmental changes, and thus being the ideal epicenter for the proteinquake, a small perturbation of which would initiate a domino effect that causes a cooperative disruption of the more stable neighboring secondary structures, leading to the effective disassembly of the DegP hexamers. It should be pointed out that the unfolding midpoint temperature for the LA loop, being at 31.5 ± 1.5°C, can be taken as that for the DegP hexamer to disassemble into its trimeric units. This midpoint temperature correlates well with the earlier reports, as achieved by size-exclusion chromatography analysis, that the DegP protein exists as hexamers at 25°C but completely dissociates into trimers at 42°C5,6, the midpoint temperature of which would thus be 33.5°C.
Figure 8. Schematic illustration of the proteinquake mechanism for the thermal-induced dissociation of the DegP hexamer with four typical snapshots at the indicated time points.
Only one (green colored) of the six β1-LA-β2 structures at the interface of the trimeric units is displayed. The unfolded structures are red colored. The epicenter (denoted as a red circle) locates at the fully exposed part of the interfacial β-sheet. F denotes the force of the tension. The black arrows denote the direction of the motion of the LA loop.
The fact that the DegP hexamers start to dissociate into trimers even at room temperatures, strongly implicates that this protein can become fully active at the normal growth temperature of 37°C of the bacterial cells. In this regard, the essentiality of DegP for bacterial cell to grow at the heat shock temperatures should be attributed to the increased level of its substrate (i.e., unfolded proteins), instead of the activation of DegP proteins at such temperatures. It follows that the binding of the unfolded protein substrates would help to shift the equilibrium towards the dissociation side by binding to the trimeric units, which leads to the formation of the active and larger oligomers (12- and 24-mers). We envisage that this proteinquake mechanism for oligomeric dissociation might be utilized for other oligomeric proteins that modulate their activities through a similar disassembly and reassembly process.
Methods
Protein preparation
The proteolytically inactive and C-terminal His-tagged hexameric DegPS210A (in which the active site serine residue was replaced by an alanine) was utilized in this study to avoid the self-cleavage of the wild-type DegP. The broken cage DegPS210A and DegP (ΔPDZ2) mutants were derived from pET28a-DegPS210A-His6, which was constructed in our previous work25, by using the QuikChange site-directed mutagenesis method17.
To express DegPS210A, the broken cage DegPS210A, and the DegP (ΔPDZ2) mutants, as C-terminal His tagged proteins, the expression vector pET28a containing these mutants were transformed into E. coli BL21 (DE3) competent cells. The cells were cultured in LB medium at 37°C to an optical density at 600 nm (OD600) of 0.6, and protein expression was induced by addition of 0.5 mM IPTG (isopropyl-β-D-thiogalactopyranoside). The cells were further incubated for 6 hours at 37°C. After being harvested, washed and resuspended in buffer A (20 mM phosphate buffered saline containing 500 mM NaCl and 20 mM imidazole, pH 7.5), cells were lysed by sonication on ice. After centrifugation at 20,000 × g for 40 min, the supernatant was loaded onto a pre-equilibrated 5 ml Ni-NTA agarose column (GE Healthcare), washed sequentially with 30 ml buffer A and 30 mL buffer B (20 mM phosphate buffered saline containing 1 M NaCl and 40 mM imidazole, pH 7.5), before the bound proteins were eluted with 25 ml of buffer C (20 mM phosphate buffered saline containing 500 mM NaCl and 300 mM imidazole, pH 7.5). The eluted samples were then desalted by dialysis. In all the infrared spectroscopy experiments, the freeze-dried protein powder was dissolved in 99.9% D2O (pD 7.4) at a concentration of 12.5 mg/ml.
FTIR spectroscopy
Temperature-dependent FTIR spectra were collected on a spectrometer (VERTEX 70 V, Bruker Optics, DE). A two-compartment CaF2 sample cell with a 50-μm thick Teflon spacer was used for protein solution and the reference D2O, respectively. The measurements were performed in a home-built vacuum chamber with a temperature controlled at an accuracy of ±0.1°C by water circulation26. Each spectrum was recorded in the 1500 cm−1–1750 cm−1 region at a resolution of 4 cm−1 and 40 scans were signal-averaged. As the presence of salt in protein solution could cause complexity in amide I′ IR absorption27,28, all the infrared spectroscopy experiments were carried out in D2O.
Temperature-jump time-resolved IR difference absorbance spectra
The details of temperature-jump time-resolved IR absorbance difference spectrometer have been described previously20,26,29. Briefly, the fundamental output of a Nd:YAG laser (Lab 170, repetition rate: 10 Hz, pulse width: 8–12 ns; Spectra Physics, Mountain View, CA) generates the 1.9-μm temperature-jump pulse via Raman shifting in a cell that was filled with 750 psi H2 gas. The temperature-jump induced transient IR absorbance changes were probed by a CO mid-IR laser at a spectral spacing about 4 cm−1 (Dalian University of Technology, Dalian, CN) in conjunction with a MCT detector (Kolmar, Newburyport, MA) and a digital oscilloscope (Tektronix, TDS520D, Santa Clara, CA). We took the temperature-dependent FTIR spectra of D2O as an internal standard for temperature-jump calibration20,30,31. The temporal resolution of this system after the deconvolution of the instrumental response is about 30 ns20,26. Heating-and-cooling cycled FTIR spectra were collected to determine the highest accessible temperature at which the reversibility of the protein folding/unfolding can still be achieved20, which is about 40°C for DegPS210A in D2O solution.
Author Contributions
Y.W. conceived and designed the research. S.L., R.W., D.L., J.M. and H.L. conducted the experiments. S.L., Y.W., Z.C. and X.H. performed analyses. S.L., Y.W., Z.C. and R.W. wrote the manuscipt. S.L. prepared all the figures.
Supplementary Material
Supplementary Information
Acknowledgments
This work is supported by the Natural Science Foundation of China (grant 20925313 and 21090342 to Y.W.; grant 31170738 to Z.C.), National Basic Research Program of China (grant 2006CB910302 to Y.W.; grant 2012CB917300 to Z.C.) and Chinese Academy of Sciences Innovation Program (KJCX2-YW-W25).
References
- Wickner S., Maurizi M. R. & Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science 286, 1888–1893 (1999). [DOI] [PubMed] [Google Scholar]
- Spiess C., Beil A. & Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97, 339–347 (1999). [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K. & Beckwith J. Characterization of degp, a gene required for proteolysis in the cell-envelope and essential for growth of escherichia-coli at high-temperature. J. Bacteriol. 171, 2689–2696 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Zylicz M. & Georgopoulos C. The Htra (Degp) protein, essential for escherichia-coli survival at high-temperatures, is an endopeptidase. J. Bacteriol. 172, 1791–1797 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. et al. Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proc. Natl. Acad. Sci. USA 105, 11939–11944 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krojer T. et al. Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885–890 (2008). [DOI] [PubMed] [Google Scholar]
- Krojer T., Garrido-Franco M., Huber R., Ehrmann M. & Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature 416, 455–459 (2002). [DOI] [PubMed] [Google Scholar]
- Krojer T., Sawa J., Huber R. & Clausen T. HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat. Struct. Mol. Biol. 17, 844–852 (2010). [DOI] [PubMed] [Google Scholar]
- Ortega J., Iwanczyk J. & Jomaa A. Escherichia coli DegP: a structure-driven functional model. J. Bacteriol. 191, 4705–4713 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M., Franzmann T., Weinfurtner D. & Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 12, 842–846 (2005). [DOI] [PubMed] [Google Scholar]
- Skorko-Glonek J., Krzewski K., Lipinska B., Bertoli E. & Tanfani F. Comparison of the structure of wild-type HtrA heat shock protease and mutant HtrA proteins. J. Biol. Chem. 270, 11140–11146 (1995). [DOI] [PubMed] [Google Scholar]
- Sobiecka-Szkatula A. et al. Temperature-induced conformational changes within the regulatory loops L1-L2-LA of the HtrA heat-shock protease from Escherichia coli. Biochim. Biophys. Acta 1794, 1573–1582 (2009). [DOI] [PubMed] [Google Scholar]
- Ismail A. A., Mantsch H. H. & Wong P. T. Aggregation of chymotrypsinogen: portrait by infrared spectroscopy. Biochim. Biophys. Acta 1121, 183–188 (1992). [DOI] [PubMed] [Google Scholar]
- Sarroukh R., Goormaghtigh E., Ruysschaert J. M. & Raussens V. ATR-FTIR: A “rejuvenated” tool to investigate amyloid proteins. Biochim. Biophys. Acta 1828, 2328–2338 (2013). [DOI] [PubMed] [Google Scholar]
- Fang Y. & Dalgleish D. G. Conformation of beta-lactoglobulin studied by FTIR: Effect of pH, temperature, and adsorption to the oil-water interface. J. Colloid. Interf. Sci. 196, 292–298 (1997). [DOI] [PubMed] [Google Scholar]
- Jones K. C., Peng C. S. & Tokmakoff A. Folding of a heterogeneous beta-hairpin peptide from temperature-jump 2D IR spectroscopy. Proc. Natl. Acad. Sci. USA 110, 2828–2833 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomaa A. et al. The inner cavity of Escherichia coli DegP protein is not essential for molecular chaperone and proteolytic activity. J. Bacteriol. 189, 706–716 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. & Hamaguchi H. Femtosecond ultraviolet-visible absorption study of all-trans → 13-cis center dot 9-cis photoisomerization of retinal. J. Chem. Phys. 109, 1397–1408 (1998). [Google Scholar]
- Zhang L., Yang J., Wang L., Yang G. & Weng Y. Direct observation of interfacial charge recombination to the excited-triplet state in all-trans-retinoic acid sensitized TiO2 nanoparticles by femtosecond time-resolved difference absorption spectroscopy. J. Phys. Chem. B 107, 13688–13697 (2003). [Google Scholar]
- Ye M. et al. Infrared spectroscopic discrimination between the loop and alpha-helices and determination of the loop diffusion kinetics by temperature-jump time-resolved infrared spectroscopy for cytochrome c. Biophys. J. 93, 2756–2766 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. & Sauer R. T. Cage assembly of DegP protease is not required for substrate-dependent regulation of proteolytic activity or high-temperature cell survival. Proc. Natl. Acad. Sci. USA 109, 7263–7268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A. et al. Protein states and protein quakes. Proc. Natl. Acad. Sci. USA 82, 5000–5004 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A. et al. Formation of a new buried charge drives a large-amplitude protein quake in photoreceptor activation. Biochemistry 40, 1510–1517 (2001). [DOI] [PubMed] [Google Scholar]
- Zang C. et al. Ultrafast proteinquake dynamics in Cytochrome c. J. Am. Chem. Soc. 131, 2846–2852 (2009). [DOI] [PubMed] [Google Scholar]
- Wu S. et al. Interaction between bacterial outer membrane proteins and periplasmic quality control factors: a kinetic partitioning mechanism. Biochem. J. 438, 505–511 (2011). [DOI] [PubMed] [Google Scholar]
- Li H. et al. Thermal-induced dissociation and unfolding of homodimeric DsbC revealed by temperature-jump time-resolved infrared spectra. Biophys. J. 97, 2811–2819 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. W., Laberge M. & Vanderkooi J. M. Surface of cytochrome c: infrared spectroscopy of carboxyl groups. Biochemistry 36, 14724–14732 (1997). [DOI] [PubMed] [Google Scholar]
- Li H., Xu Y. & Weng Y. Infrared absorption intensity analysis as a new tool for investigation of salt effect on proteins. Chin. J. Chem. Phys. 22, 556–562 (2009). [Google Scholar]
- Zhang Q. et al. Nanosecond-time-resolved infrared spectroscopic study of fast relaxation kinetics of protein folding by means of laser-induced temperature-jump. Chin. Phys. 14, 2484–2490 (2005). [Google Scholar]
- Wang T. et al. Length dependent helix-coil transition kinetics of nine alanine-based peptides. J. Phys. Chem. B 108, 15301–15310 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. et al. Helix formation via conformation diffusion search. Proc. Natl. Acad. Sci. USA 99, 2788–2793 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information