Abstract
Background
While patients with interstitial lung disease may be particularly susceptible to ventilator-induced lung injury, ventilator strategies have not been studied in this group of patients.
Purpose
To describe the clinical course and outcome of patients with interstitial lung disease and acute respiratory failure in relation to ventilatory parameters.
Methods
We retrospectively identified a cohort of ventilated patients with interstitial lung disease admitted to five ICUs at a single institution. We analyzed demographic data, pulmonary function tests, severity of illness, and initial 24 hours of continuous ventilator parameters. Primary outcomes were survival to hospital discharge and one-year survival.
Main results
Of 94 patients with interstitial lung disease, 44(47%) survived to hospital discharge and 39(41%) were alive at one-year. Non-survivors were less likely to be postoperative, had higher severity of illness and were ventilated at higher airway pressures and lower tidal volumes. Step changes in positive end-expiratory pressure >10 cmH2O were attempted in 20 patients and resulted in an increase in plateau pressure (median difference +16; IQR, 9 to 24 cm H2O) and a decrease in respiratory system compliance (median difference -0.28: IQR, -0.43 to -0.13 mL/kg/cm H2O). Cox model revealed high positive end-expiratory pressure (hazard ratio 4.72; 95% CI, 2.06, 11.15), APACHE III predicted mortality (hazard ratio 1.33; 95% CI, 1.18,1.50), age (hazard ratio 1.03; 95% CI, 1,1.05) and low PaO2/FiO2(hazard ratio 0.96;95% CI, 0.92,0.99) to be independent determinants of survival.
Conclusion
Both severity of illness and high PEEP predict the outcome of interstitial lung disease patients receiving mechanical ventilation.
Keywords: Interstitial lung disease, intensive care unit, respiration, artificial
INTRODUCTION
Progression and exacerbation of chronic interstitial lung disease (ILD) generally denotes a very poor outcome once mechanical ventilation has been instituted.1 Saydain and colleagues1 reported a 68% hospital mortality rate in patients with idiopathic pulmonary fibrosis who developed respiratory failure requiring mechanical ventilation. Mechanical ventilation is an indispensable tool which supports critically ill patients with acute respiratory failure. However, it is also recognized that mechanical ventilation can initiate and exacerbate lung injury and contribute to patient morbidity and mortality, a condition recognized as ventilator-induced lung injury (VILI).2–5 Both over distension injury (“volutrauma”) and recruitment-derecruitment (atelectotrauma) determine the development of VILI. 5,6 Prevention of VILI with low tidal volume ventilation with or without high positive-end expiratory pressure has become a standard of care for patients with acute lung injury (ALI)and acute respiratory distress syndrome (ARDS) after several trials demonstrated improved outcome associated with this strategy 3,7,8. While patients with ILD may be susceptible to VILI, they were specifically excluded from these studies. Similar to ALI/ARDS, patients with ILD have a reduced volume of aerated lung (“baby lung”) and are at risk for over distension with conventional ventilator settings.5–9 However, in contrast to ALI these patients ought to be less susceptible to atelecto-trauma because their lungs are not edematous an “open lung” approach with the use of recruitment maneuvers and high positive end-expiratory pressure (PEEP), is of little benefit and may worsen VILI by causing over distension.
The influence of specific ventilator settings including tidal volumes and PEEP on the outcome of mechanically ventilated patients with ILD has not been previously studied. We collected data on ventilator settings, clinical course, and outcome of patients with ILD and respiratory failure requiring mechanical ventilation to describe and determine if specific ventilator settings as well as various clinical parameters influence outcome.
METHODS
Additional methods are provided in online data supplement. The study included patients with ILD admitted to the medical and surgical intensive care units (ICU) (72 beds) at Mayo Clinic, Rochester, MN, between February 1, 2002 and July 31, 2006. Mayo Foundation Institutional Review Board approved the study. Patients were identified from the institutional electronic International Classification of Diseases-9 database, Acute Physiology and Chronic Health Evaluation (APACHE) III database, and the diagnoses listed in the electronic medical records. The patients were included in the study if they were admitted to the ICU, required mechanical ventilation for more than 24 hours, and had prior diagnosis of 1) idiopathic pulmonary fibrosis (IPF) 10 2) Non -IPF-idiopathic interstitial pneumonias or 3) Other forms of chronic interstitial lung diseases. In addition, surgical patients had to have postoperative respiratory failure defined as the need for continuation of mechanical ventilation for greater than 48 hours after surgery11 or reinstitution of mechanical ventilation within the first 7 days after surgery.12 All patients had to have high-resolution computed tomography findings, documented clinical features and pulmonary function tests (PFT) that included evidence of restriction and/or decreased diffusing capacity for carbon monoxide.
Hospital and one-year survival were assessed as primary end points. Secondary outcomes included ICU and hospital lengths of stay. To define the impact of the underlying disease on outcome, we compared patients with IPF to those with other forms of ILD. Severity of illness on ICU admission was calculated from prospectively collected data in the institutional APACHE III database. Thoracic imaging, PFTs, lung histology and pertinent co-morbidities were collected from the electronic medical records. Continuous (q 15 minutes) mechanical ventilation settings were obtained during the first 24 hours from the clinical information system (PICIS Chart Plus) and included: tidal volume (Vt) (adjusted according to lung size based on the predicted body weight), respiratory rate, minute ventilation, mean airway pressure, peak airway pressure, plateau airway pressure, PEEP, FiO2 and oxygen saturation measured by pulse oximetry. To determine the response to step changes in PEEP, we compared average peak and plateau airway pressures, respiratory system compliance, expired tidal volume, and oxygen saturation before and after the increase in PEEP to >10 cm H2O.
Statistical Analysis
Wilcoxon and Student’s t-test for continuous variables and chi-square and Fisher’s exact tests were used for univariate comparisons as appropriate. The Kaplan-Meier estimate of survival curve was used for survival data with statistical evaluation through Mantel-Cox log-rank statistics. One-year survival time was determined from the time of ICU admission (starting point). Death certificates were available for all patients who died. Multivariate logistic models were created to determine independent risk factors for hospital mortality. Variables were selected into the multivariate analysis if they were measured on more than 80% of patients, were clinically plausible and showed a statistically significant and/or strong association in univariate analysis (p <0.1 and/or odds ratio > 2.0) taking into consideration co-linearity. The final model was chosen based on the highest area under the receiver operator curve after backward elimination of non-significant variables. Cox-proportional hazards model determined if specific ventilator settings and clinical characteristics were independently associated with one-year survival. JMP statistical software (JMP, version 6; SAS Institute Inc., Cary, NC) was used for data analyses.
RESULTS
We identified 104 patients with ILD admitted to the ICU during the study period. Ten patients were excluded; 6 did not authorize the use of their medical records for research, 3 patients were mechanically ventilated before surgery and complete data was lacking in one patient. The remaining 94 patients fulfilled the inclusion criteria and were included in the analysis.
The median age of patients was 70 years and the median interval between diagnosis of ILD and ICU admission was 6.3 months. Thirty of the patients (32%) had IPF, thirty-three (35%) had non-IPF idiopathic interstitial pneumonia and thirty-one (33%) had other forms of ILD. Histological documentation was present in 17 (57%) of patients with IPF, in 23 (70%) of patients with non-IPF idiopathic interstitial pneumonia, and in 15 (48%) patients with known causes other forms of ILD. The causes of interstitial lung diseases are outlined in Table 1.
Table 1.
Types of interstitial lung diseases in 94 patients
| Type of Interstitial Lung Disease | Number of patients and percentage No. (%) |
|---|---|
|
| |
| Idiopathic pulmonary fibrosis | 30 (32) |
|
| |
| Non-IPF-idiopathic interstitial pneumonia | 33 (35) |
| Cryptogenic organizing pneumonia | 22 (23) |
| Acute interstitial pneumonia | 4 (4) |
| Fibrotic nonspecific interstitial pneumonia | 4 (4) |
| RB-Interstitial lung disease | 1 (1) |
| Lymphocytic interstitial pneumonia | 1 (1) |
| Desquamative interstitial pneumonia | 1 (1) |
|
| |
| Other forms of chronic interstitial lung diseases | 31 (33) |
| Connective tissue disease | 11 (12) |
| Hypersensitivity pneumonitis | 6 (6) |
| Pneumoconiosis | 4 (4) |
| Drug-induced interstitial lung disease | 4 (4) |
| Sarcoidosis (stage 4) | 3 (3) |
| Chronic eosinophilic pneumonia | 2 (2) |
| Langerhans cells histiocytosis | 1 (1) |
IPF = idiopathic pulmonary fibrosis
RB = respiratory bronchiolitis
There were 53 medical and 41 postsurgical (cardiac 10, noncardiac-thoracic 6, abdominal 11, vascular 2, orthopedic 2, neuro 1, miscellaneous 9) ICU admissions. The most common causes of respiratory failure were cardiac diseases (11%) and infections (8.5%) but was not identifiable in 76.5% of patients. (Table 1, online data supplement).
Overall, 47% of patients survived to hospital discharge and 41% were alive at one year. The median survival was 75 days with IPF patients having non-significantly shorter survival (Figures 1 and 2, online data supplement). Causes of in-hospital death were: respiratory failure in 82% of patients, multi-organ failure in 6% of patients, sepsis in 6% patients, and other causes in 4%.
There were no significant differences in age, sex, the median duration of illness from the time of ILD diagnosis to ICU admission, pre-ICU PFT results, and use of high-dose steroids when comparing patients who did and did not survive (Table 2). Compared to survivors, nonsurvivors were less likely to be postoperative, had higher APACHE III score, had a higher ICU and hospital predicted mortality, and longer ICU stay (Table 2). Pre-ICU echocardiography revealed similar ejection fraction but systolic pulmonary artery pressure tended to be higher in non-survivors (Table 2).
Table 2.
Characteristics of ILD patients requiring mechanical ventilation who did and did not survive to hospital discharge•
| Variables | Survivors (n= 44) | Non-survivors (n=50) | P value |
|---|---|---|---|
|
| |||
| Sex, no. (%) | |||
| Male | 21 (48%) | 29 (58%) | 0.319 |
| Female | 23 (52%) | 21 (42%) | |
|
| |||
| Age, year | |||
| Median (range) | 71 (57 to 78) | 74 (67 to 79) | 0.122 |
|
| |||
| Duration from diagnosis to ICU admission, months | 18 (0.31 to 49) | 2 (0.10 to 40) | 0.248 |
|
| |||
| IPF | 12 (27%) | 18 (36%) | 0.507 |
| Non-IPF | 31 (70%) | 32 (64%) | |
|
| |||
| PFTs (% predicted) | |||
| FEV1 | 60 (46 to 73) | 62 (44 to 75) | 0.991 |
| FVC | 62 (53 to 76) | 62 (47 to 74) | 0.551 |
| TLC | 73 (61 to 84) | 67 (52 to 84) | 0.572 |
| DLCO | 47 (38 to 66) | 41 (33 to 55) | 0.113 |
|
| |||
| Post-operative respiratory failure | 29 (71%) | 12 (29%) | <0.001 |
|
| |||
| Systolic pulmonary artery pressure, mmHg | 46 (33 to 59) | 53 (36 to 66) | 0.062 |
|
| |||
| Ejection fraction, % | 60 (45 to 60) | 60 (52 to 65) | 0.918 |
|
| |||
| ICU high dose steroids | |||
| 13 (29%) | 16 (32%) | 0.797 NS |
|
| IPF | 4 (31%) | 5 (31%) | |
| Non-IPF | 9 (69%) | 11(69%) | |
|
| |||
| PaCO2, mmHg | 39 (37 to 44) | 41 (36 to 47) | 0.289 |
| PaO2/FIO2 ratio | 252 (158 to 388) | 117 (71 to 182) | <0.001 |
| NIPPV before intubation | 5 (11%) | 16 (32%) | 0.014 |
| Vt mL/kg PBW | 8 (7 to 10) | 7 (6 to 9) | 0.023 |
| Set respiratory rate | 11 (8 to 13) | 18 (10 to 23) | <0.001 |
| PEEP, cmH2O | 5 (5 to 6) | 9.5 (5 to 10) | 0.001 |
| FIO2 | 0.4 (0.4 to 0.6) | 0.6 (0.5 to 0.8) | <0.001 |
| Mean airway pressure, cmH2O | 10 (8 to 12) | 14 (10 to 18) | 0.001 |
| Peak airway pressure, cmH2O | 24 (22 to 29) | 30 (25 to 33) | 0.001 |
| Plateau pressure, cmH2O, n=45 | 21 (16 to 25) | 33(25 to 39) | 0.001 |
|
| |||
| APACHE III score | 57 (47 to 69) | 78 (61 to 96) | <0.001 |
| ICU predicted mortality | 6 (2 to 12) | 18 (7 to 36) | <0.001 |
| Hospital predicted mortality | 9 (5 to 21) | 37 (16 to 59) | 0.001 |
|
| |||
| ICU length of stay, days | 2 (1 to 8) | 5 (2 to 10) | 0.016 |
| Hospital length of stay, days | 10 (6 to 16) | 11 (7 to 21) | 0.543 |
Data are represented as median (interquartile range) and number (percentage). IPF, idiopathic pulmonary fibrosis; ICU, intensive care unit; TLC, total lung capacity; DLCO, diffuse capacity of lungs for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PaO2, partial pressure of oxygen, FIO2, fraction of inspired oxygen; SaO2, arterial oxygen saturation; PaCO2, partial pressure of carbon dioxide; PEEP, positive end expiratory pressure; NIPPV, noninvasive positive pressure ventilation; Vt, tidal volume; PBW, predicted body weight; PFT, pulmonary function test; ARDS, acute respiratory distress syndrome; APACHE, Acute Physiology and Chronic Health Evaluation.
Twenty-one patients received a trial of noninvasive ventilation before intubation. The use of noninvasive ventilation was more common among nonsurvivors (32% vs 11%, p=0.01). Compared to those who did survive, nonsurvivors received lower tidal volume, had lower PaO2/FiO2 ratio, had higher pea k, plateau and mean airway pressures and higher PEEP (Table 2, Figure 3). High PEEP (>10 cm H2O) was attempted in 20 patients, but did not result in improved oxygenation (Table 3). Step increase in PEEP resulted in worsening respiratory system compliance and increased airway pressures suggestive of overdistension (Table 3).
Table 3.
Difference in ventilator variables before and after PEEP titration in 20 patients receiving PEEP >10 cm H2O in the first 24 hours of mechanical ventilation
| Variables | Mean change and 95% CI | P value |
|---|---|---|
| Δ PEEP, cmH2O | 6 (4 to 8) | <0.001 |
| Δ peak airway pressure, cmH2O | 16 (10 to 21) | <0.001 |
| Δ plateau pressure, cmH2O | 16 (9 to 24) | <0.001 |
| Δ respiratory system compliance, mL/kg/PBW/cmH2O | −0.28 (−0.43 to −0.13) | 0.001 |
| Δ Vt, mL/kg PBW | −0.4 (−0.9 to 0.23) | 0.211 |
| Δ SaO2, % | −1.7 (−8 to 4) | 0.571 |
PEEP, positive end expiratory pressure Vt, tidal volume; PBW, predicted body weight SaO2, arterial oxygen saturation
Multivariate analysis of these risk factors, using Cox proportional hazard model revealed high PEEP, APACHE III predicted hospital mortality, age and hypoxemia to be independent determinants of one-year survival (Table 4). In a multivariate logistic regression analysis, hospital mortality was predicted by severe hypoxemia (lower PaO2/FiO2), higher PEEP during the first 24 hours of mechanical ventilation, older age, and higher severity of illness on the day of ICU admission (Table 4). Post-hoc subgroup analysis, demonstrated a similar proportional increased in the risk of death on those exposed to PEEP >10 cm H2Oin both medical and post -surgical patients (see electronic supplementary material-Table 2 and 3).
Table 4.
Risk factors associated with hospital mortality and one-year survival: multivariate logistic regression analysis and cox proportional hazard model (AUC 0.89)
| Hospital Mortality | 12 months Survival | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variables | Odds Ratio | 95% CI | P | Hazard ratio | 95% CI | P |
| PaO2/FIO2 (for each 10 units increase) | 0.93 | 0.86–0.99 | 0.039 | 0.96 | 0.92–0.99 | 0.010 |
|
| ||||||
| Post-operative | 0.23 | 0.05–0.95 | 0.048 | 0.55 | 0.28–1.01 | 0.055 |
|
| ||||||
| PEEP first 24h, cm H2O | ||||||
| 0–5 | 1 | |||||
| 6–10 | 1.52 | 0.37–6.39 | 0.559 | 1.53 | 0.71–3.19 | 0.259 |
| >10 | 17.26 | 1.98–412.89 | 0.001 | 4.72 | 2.1–11.15 | <0.001 |
|
| ||||||
| Patient Age, years | 1.07 | 1.01–1.014 | 0.020 | 1.03 | 1–1.05 | 0.023 |
|
| ||||||
| Predicted death (for each 10% increase) | 1.47 | 1.12–2.05 | 0.012 | 1.33 | 1.18–1.5 | <0.001 |
PaO2, partial pressure of oxygen; FIO2, fraction of inspired oxygen; PEEP, positive end expiratory pressure. AUC Area under receiver operator curve
DISCUSSION
This observational cohort study describes the clinical course, mechanical ventilation practice, and outcome of 94 ILD patients mechanically ventilated for acute respiratory failure at a teaching tertiary-referral institution. Both hospital and one-year mortality were high, particularly in medical patients. Older age, higher severity of illness at the time of ICU admission, severe hypoxemia, and high PEEP during the first 24 hours of mechanical ventilation were independent predictors for both hospital and one -year survival. High PEEP settings failed to improve oxygenation and were associated with overdistension (unsafe inflation pressures and decreased respiratory system compliance) and worse outcome.
No prior study has described the long-term survival of patients with both IPF and non-IPF forms of ILD requiring mechanical ventilation in the ICU. Similar to those with IPF, patients with chronic non-IPF ILD frequently experience acute exacerbation without an identifiable cause. 13,14 No specific cause was found for the acute respiratory decompensation during the nonsurgical admission in 80% (16/20) of patients with idiopathic interstitial pneumonias and in 65% (11/17) of patients with other forms of ILDs. Similar to patients with IPF, most patients with non-IPF ILD died of respiratory failure due to progression of lung disease. These findings suggest that an accelerated phase of the disease is relatively common in the clinical course of these patients. If so, optimal management of patients with non-IPF ILD would require better understanding of the true incidence, pathogenic mechanisms, and clinical effects of these acute episodes.
The in-hospital mortality rate of nonsurgical IPF patients admitted for acute respiratory failure from this study (11/16 patients, 69%) is similar to that previously reported by Saydain et al.1 from our institution (23/38, 60%). In our study, all 5 patients with IPF that survived to hospital discharge were alive at one-year. Two randomized studies provide information regarding mortality of acute exacerbation of IPF. Of 107 patients followed for 9-months in a double-blind, randomized, placebo-controlled trial of pirfenidone in the treatment of IPF,15 acute exacerbation of IPF was manifested in 14% of the placebo group (5/35) and in none of the pirfenidone group. One of the five patients died after the onset of acute exacerbation in the placebo group (mortality rate 20%), whereas no deaths occurred in the pirfenidone group during the 9-month study period. Of 56 patients followed for approximately 3 years in a randomized controlled trial of anticoagulant therapy in IPF,16 32 (57%) were hospitalized for acute exacerbation and 53% of those patient died. Other retrospective case series have reported a higher mortality rate compared to our results among patients admitted to the ICU with acute exacerbation of IPF.1,17–20 Our data support the results recently reported by Churg et al. 21 In their report, 10 of 12 patients (9 with usual interstitial pneumonia, 2 with nonspecific interstitial pneumonia, and 1 with chronic hypersensitivity pneumonitis)survived the acute exacerbation and were discharged with survival times of 1 to 11 months. Pulmonary function tests in our cohort revealed relatively mild to moderate impairment prior to presentation. Not surprisingly, our data suggest that the test obtained during clinical stability does not correlate with the severity of the acute event.
The literature suggests that initiation of invasive mechanical ventilation in medical patients with IPF is questionable.1,17,20,23 Patients with IPF have profound alterations in mechanical lung properties9 and may be potentially susceptible to VILI. In our study, low tidal volumes were used inconsistently and were not associated with improved outcome. Non-survivors had more severe baseline hypoxemia and were ventilated at higher airway pressures. An important finding in our multivariate analysis is identification of high PEEP being independently associated with lower short and long term survival. Gattinoni et al24 showed that use of higher PEEP in patients with ARDS who had a low percentage of recruitable lung (i.e., lung tissue in which aeration can be restored) provides little benefit and may actually be harmful, since it will serve only to overinflate lung regions that are already open.24 Patients with chronic ILD may have little or no recruitable lung and may be particularly prone to overdistension injury.9 Despite the fact that step change in PEEP caused a disproportional increase in peak and plateau airway pressures and a decrease in respiratory system compliance (suggesting lung overdistension, (Table 3), the majority of our patients were continued on high PEEP settings, and 18 out of 20 died before hospital discharge. While the plateau airway pressure may be a better determinant of lung overdistension than PEEP, this measurement was not obtained in 40% of our patients precluding its use in multivariate analysis. Peak airway pressure on the other hand reflects both the resistance and compliance of the respiratory system, and is a more variable determinant of lung overdistension. Adjusted for baseline patient characteristics, PEEP was more strongly associated with outcome than peak airway pressure in our study. Neither plateau nor peak airway pressure distinguishes between lung and chest wall elastic properties and are therefore non-specific surrogate measures of alveolar overdistension.
Non-postoperative ILD patients had a greater risk of death compared to ILD patients ventilated for postoperative respiratory failure. The difference did not, however, reach statistical significance in the multivariate analysis. This finding may reflect the elective nature of the surgical procedures as defined in our inclusion criteria. Chiyo et al25 investigated the postoperative morbidity and mortality of patients with lung cancer and ILD. The 30-day mortality was only 2.8%. Similarly, Martinod et al. 26 observed no postoperative deaths in 27 patients with ILD undergoing lung cancer resection. However, methodology, patient selection, and type of surgery differ significantly in these two studies compared to ours. Whether ILD is an independent risk factor for postoperative pulmonary complications remains to be established.
Our study has several potential shortcomings. First, the data were retrospectively collected, and we cannot exclude that, in some patients, the cause of acute respiratory failure was missed. Second, the study design does not allow estimation of the cause-and-effect relationship between the risk factors and outcome because unmeasured confounding factors may not have been accounted for. The specific reason for each titration of PEEP >10 cm H2O on the 20 identified patients from the cohort was not collected. For example, higher PEEP may be a marker for lung disease and hypoxemia and not causative of lung injury and consequently increased mortality. However, higher PEEP was associated with changes in lung mechanics suggestive of overdistension and the effect of PEEP persisted after the adjustment for initial severity of illness and hypoxemia. Finally, our results represent the experience of only one medical center.
In conclusion, the mortality of mechanically ventilated patients with ILD is high. Both underlying severity of illness and high PEEP appear to determine the outcome of mechanically ventilated ILD patients. Prospective controlled studies therefore should be performed to determine optimal ventilatory support of these patients.
Supplementary Material
Figure 1.
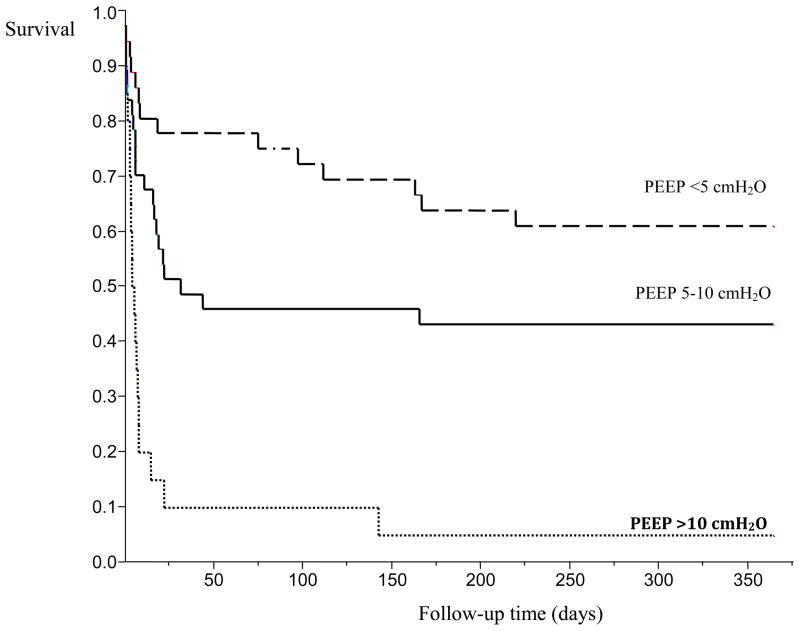
One-year survival for levels of PEEP utilized within the first 24 hours of mechanical ventilation after ICU admission. PEEP <=5 cmH2O, median time survival >350 days. PEEP 5–10 cmH2O, median time survival 32 days. PEEP >10 cmH2O, median time survival 5.8 days (95% CI, 3.5 to 8). Log-Rank, p=<0.001.
Footnotes
Publication Disclosure: An abstract of this study was presented at the 73rd Annual International Meeting of the American College of Chest Physician, Salk Lake City, Utah, 2006. Chest Foundation Young Investigator Award.
Financial Disclosure: The above authors have no financial involvement in the subject matter discussed in the manuscript.
Contributor Information
Evans R. Fernández-Pérez, Instructor in Medicine, Fellow, Pulmonary and Critical Care Medicine, Mayo Clinic College of Medicine Rochester, MN
Murat Yilmaz, Research fellow, Critical Care Medicine, Mayo Clinic College of Medicine, Rochester, MN
Hussam Jenad, Fellow, Hospital Medicine, Mayo Clinic College of Medicine, Rochester, MN
Craig E. Daniels, Assistant Professor of Medicine, Pulmonary and Critical Care Medicine, Mayo Clinic College of Medicine, Rochester, MN
Jay H. Ryu, Professor of Medicine, Pulmonary and Critical Care Medicine, Mayo Clinic College of Medicine, Rochester, MN.
Rolf D Hubmayr, Professor of Medicine, Walter and Leonore Annenberg Professor in Cardiology and Critical Care, Pulmonary and Critical Care Medicine, Mayo Clinic College of Medicine, Rochester, MN.
Ognjen Gajic, Assistant Professor of Medicine, Pulmonary and Critical Care Medicine, Mayo Clinic College of Medicine, Rochester, MN.
References
- 1.Saydain G, Islam A, Afessa B, et al. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med. 2002;166:839–842. doi: 10.1164/rccm.2104038. [DOI] [PubMed] [Google Scholar]
- 2.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 3.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis. 1993;148:1194–1203. doi: 10.1164/ajrccm/148.5.1194. [DOI] [PubMed] [Google Scholar]
- 5.International consensus conferences in intensive care medicine. Ventilator-associated lung injury in ARDS. American Thoracic Society, European Society of Intensive Care Medicine, Societe de Reanimation Langue Francaise. Intensive Care Med. 1999;25:1444–1452. [PubMed] [Google Scholar]
- 6.Slutsky AS, Tremblay LN. Multiple system organfailure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 7.Villar J, Kacmarek RM, Perez-Mendez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 8.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax. 1999;54:390–395. doi: 10.1136/thx.54.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J. 2002;19:794–796. doi: 10.1183/09031936.02.00492002. [DOI] [PubMed] [Google Scholar]
- 11.Arozullah AM, Daley J, Henderson WG, et al. Multifactorial risk index for predicting postoperative respiratory failurein men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232:242–253. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. Jama. 2005;293:589–595. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 13.Parambil JG, Myers JL, Lindell RM, et al. Interstitial lung disease in primary Sjogren syndrome. Chest. 2006;130:1489–1495. doi: 10.1378/chest.130.5.1489. [DOI] [PubMed] [Google Scholar]
- 14.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127:2019–2027. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 15.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 16.Kubo H, Nakayama K, Yanai M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11:117–122. doi: 10.1155/2004/379723. [DOI] [PubMed] [Google Scholar]
- 18.Fumeaux T, Rothmeier C, Jolliet P. Outcome of mechanical ventilation for acute respiratory failure in patients with pulmonary fibrosis. Intensive Care Med. 2001;27:1868–1874. doi: 10.1007/s00134-001-1150-0. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini V, Cancellieri A, Chilosi M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22:821–826. doi: 10.1183/09031936.03.00022703. [DOI] [PubMed] [Google Scholar]
- 20.Blivet S, Philit F, Sab JM, et al. Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest. 2001;120:209–212. doi: 10.1378/chest.120.1.209. [DOI] [PubMed] [Google Scholar]
- 21.Churg A, Muller NL, Silva CI, et al. Acute Exacerbation (Acute Lung Injury of Unknown Cause) in UIP and Other Forms of Fibrotic Interstitial Pneumonias. Am J Surg Pathol. 2007;31:277–284. doi: 10.1097/01.pas.0000213341.70852.9d. [DOI] [PubMed] [Google Scholar]
- 22.Akira M, Hamada H, Sakatani M, et al. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 1997;168:79–83. doi: 10.2214/ajr.168.1.8976924. [DOI] [PubMed] [Google Scholar]
- 23.Stern JB, Mal H, Groussard O, et al. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120:213–219. doi: 10.1378/chest.120.1.213. [DOI] [PubMed] [Google Scholar]
- 24.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 25.Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg. 2003;126:1141–1146. doi: 10.1016/s0022-5223(03)00791-8. [DOI] [PubMed] [Google Scholar]
- 26.Martinod E, Azorin JF, Sadoun D, et al. Surgical resection of lung cancer in patients with underlying interstitial lung disease. Ann Thorac Surg. 2002;74:1004–1007. doi: 10.1016/s0003-4975(02)03848-1. [DOI] [PubMed] [Google Scholar]
- 27.How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J. 1998;19:990–1003. doi: 10.1053/euhj.1998.1057. [DOI] [PubMed] [Google Scholar]
- 28.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 29.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.