Abstract
Video electroencephalography (EEG) plays an important role for judgment of epileptic seizure or paroxysmal event for each clinical spell, but its interpretation is not always straightforward. If the clinical events without EEG correlate are strongly suggestive of seizures, we usually regard these spells as epileptic seizures. However, the electric/magnetic physiological profile of EEG negative epileptic seizures remains unknown. We present a 19-year-old male known to have epileptic seizures, in which both magnetoencephalography (MEG) unique and EEG/MEG spikes were seen.. Both types of spikes originated from the same source but the EEG/MEG spikes had significantly higher magnitude than the MEG-unique spikes. Therefore some epileptic seizures, even though generated identically, to the MEG-positive seizures could be EEG-negative due to the smaller magnitude.
Keywords: EEG-negative seizures, Magnetoencephalography, EEG/MEG spikes, MEG-unique spikes
INTRODUCTION
Video EEG, the essential tool for diagnosis of epilepsy, plays an important role for judgment of epileptic seizure or paroxysmal event for each clinical spell. This interpretation is not always straightforward, because certain types of seizures, such as simple partial seizures or epilepsy associated with brief tonic seizures (i.e. frontal lobe epilepsy), often do not show ictal EEG changes1. If the clinical events that show no EEG correlate are nevertheless strongly suggestive of seizures, we usually regard these spells as epileptic seizures. However, the electric/magnetic physiological profile of EEG negative epileptic seizures remains unknown.
Generally speaking the detection capability of EEG is limited spatially, requiring a synchronously discharging cortical area of 6 to 10 cm2 in order to manifest changes on the scalp EEG.2,3 Magnetoencephalography’s (MEG) spatial detection sensitivity is higher than that for EEG; involvement of 3 to 4 cm2 of cortex can be detected with MEG.3 Therefore, some spikes missed on EEG may be detected on MEG. Here we report a patient in whom the discrepant EEG and MEG ictal findings provided critical clues for our understanding of the physiological profile of EEG negative epileptic seizures. The detail offered by MEG is a new addition to our knowledge base.
CASE REPORT
The patient is a 19-year-old male whose seizure history started at 19 months of age. At age 12 years, he developed episodes of humming and hand automatism associated with reduced responsiveness --- the same type of seizures he had when he presented to us. In spite of aggressive antiepileptic treatment, seizures occurred daily. His birth and developmental history were normal and he performed above average in regular classes throughout school. No emotional, psychosocial, or family problems were known to exist. His other past history and family history were not remarkable.
Historical and recent EEGs had shown generalized spikes and intermittent slowing with maximum negativity in the right frontal area. Previous video-EEG monitoring captured not only seizures associated with right hemispheric EEG discharges, but also documented many spells with similar semiology that were associated with no abnormal EEG findings during the behavioral changes. Repeated conventional 1.5 T MRI (three at our institution, others elsewhere) detected no MRI lesions. Positron emission tomography at our institution indicated hypometabolism involving the right temporal and right frontal lobes. A new MRI study, this time at 3T, detected a subtle blurring at the gray-white matter junction in the right posterior orbitofrontal gyrus, suggesting malformation of cortical development (Fig. 1).
Fig. 1.
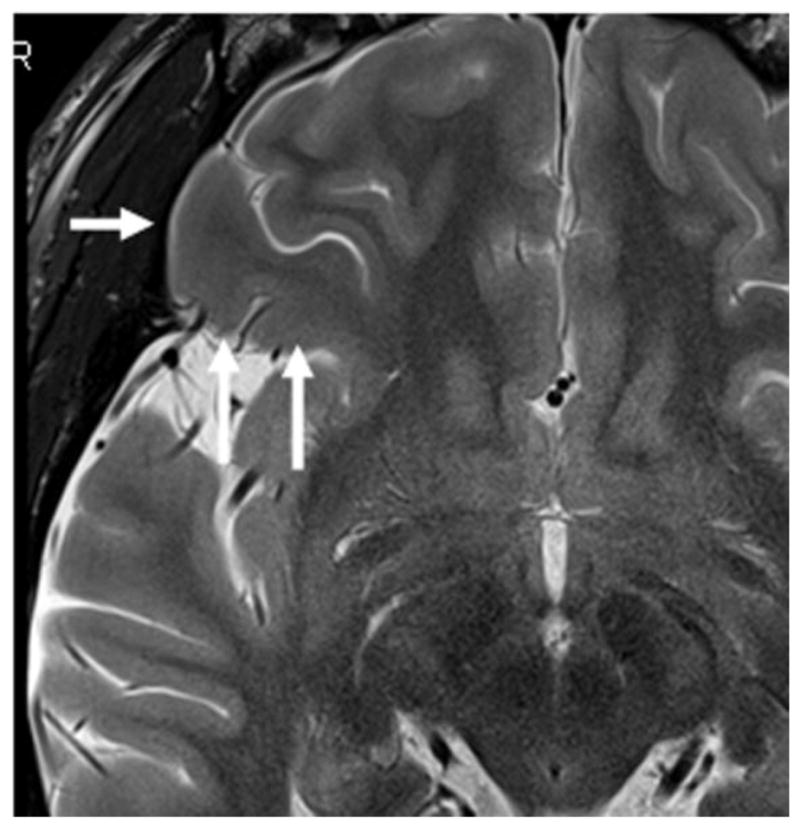
Axial T2- weighted image (3 Tesla) indicates a subtle blurring of the gray-white matter junction suggesting malformation of cortical development in the right orbitofrontal gyrus (white arrow).
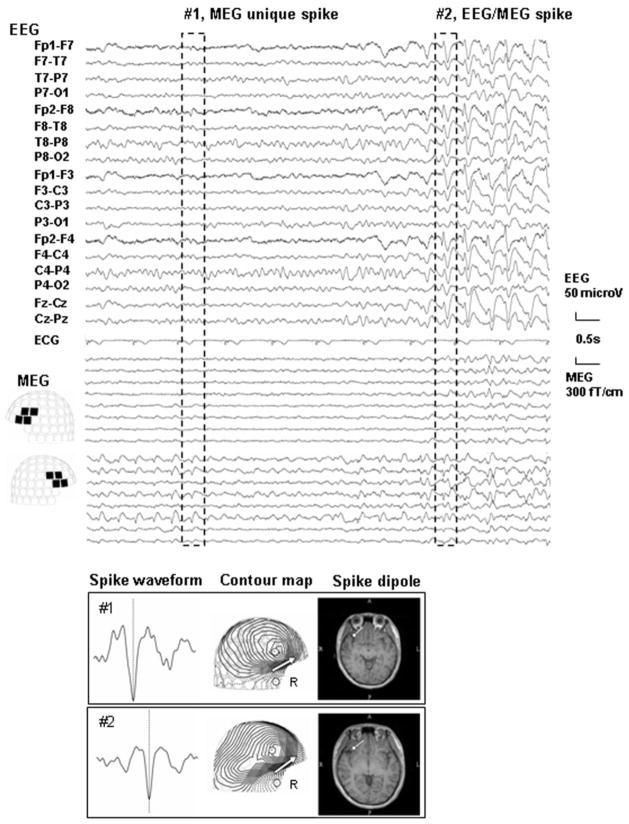
Subsequently, we performed simultaneous EEG and MEG in a magnetically shielded room; method details are described elsewhere.4 The patient had frequent electromagnetic seizures characterized by repetitive spike discharges without clinical symptom. Notably, spikes unique to MEG appeared antecedent to the appearance of spikes visible on both EEG and MEG. A representative plot is shown in Fig. 2 (upper column). Curiously, dipole localization (employing a single equivalent current dipole model) estimated both MEG unique spike (#1) and EEG/MEG spike (#2) in the same area, the right orbitofrontal gyrus (Fig. 2, lower column), conicident with the area indcated as abnormal on the 3T MRI.
Fig. 2.
Upper Column EEG and MEG waveforms and dipole analysis of the spikes. The set of spikes exemplified by the discharge enclosed by the broken line and labeled as #1 were detected with MEG only (MEG unique) whereas the set labeled as #2 are typical of those that were detected with both EEG and MEG (EEG/MEG spike)
Lower column: A magnified spike waveform, MEG contour map, and spike dipole source locations on the patient’s individual MRI for spikes #1 and 2 are shown. MEG contour maps: Arrows on the contour maps reflect the estimated dipoles projected to the sensor surface. The solid line indicates the efflux of the magnetic field from the head surface, and the broken line indicates influx of the magnetic field into the head. MRI: The circle and bar on the MRI scans indicate the dipole location and orientation, respectively. Note that the patient has two types of spikes: MEG unique spikes (#1) and EEG/MEG spikes (#2). Spike dipoles of both are estimated on the same area, the right orbito-frontal area, where an axial T2- weighted image (3 Tesla) indicates as abnormal.
In an effort to understand the difference between the MEG unique spikes and the spikes seen on MEG and EEG, we compared their magnitudes. We selected a one-minute segment from the recording, in which both MEG unique spikes and EEG/MEG spikes were included. The two types of spikes were identified based on its morphology (spike outstanding from background, and following slow wave).5 The magnetic amplitude of MEG spike was measured (baseline to peak, see Fig. 3) 6 at the four sensors showing the highest responses over the right fronto-temporal area, and we calculated the average of the four responses (“average”). We found 28 MEG unique spikes and 14 EEG/MEG spikes. The “average” of the two groups was compared using the Mann-Whitney test; p values less than 0.05 were taken as significant. Mean and standard deviation of the “averaged” value of the MEG unique spikes (n=28) and the EEG/MEG spikes (n=14) are 232.6 ± 66.2 and 436.2 ± 143.0, respectively (p<0.01) (Table 1).
Fig. 3.
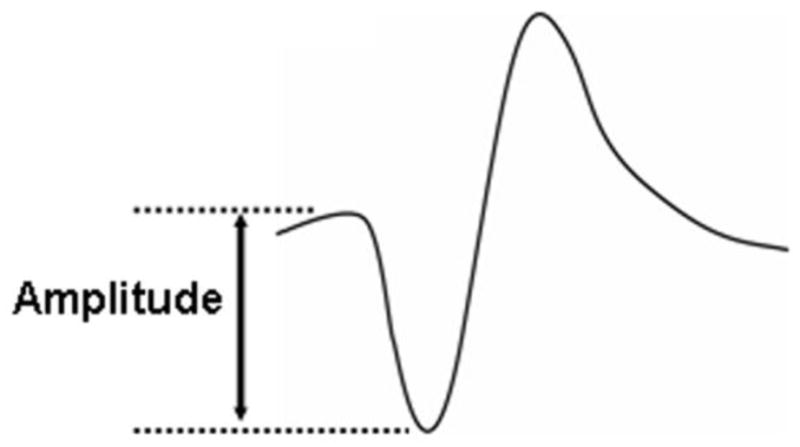
Spike amplitude of a MEG sensor is defined from baseline to peak.
Table 1.
Mean value of top 4 high amplitude of sensors of each spike
|
|
|||
|---|---|---|---|
| EEG−/MEG+ | EEG+/MEG+ | ||
|
|
|||
| 176 | 274 | (fT/cm) | |
| 290 | 652 | ||
| 249 | 366 | ||
| 270 | 611 | ||
| 211 | 464 | ||
| 205 | 348 | ||
| 306 | 599 | ||
| 189 | 245 | ||
| 137 | 341 | ||
| 97 | 568 | ||
| 243 | 269 | ||
| 342 | 585 | ||
| 186 | 445 | ||
| 169 | 340 | ||
| 264 | |||
| 180 | |||
| 356 | |||
| 286 | |||
| 306 | |||
| 311 | |||
| 131 | |||
| 166 | |||
| 197 | |||
| 208 | |||
| 236 | |||
| 241 | |||
| 302 | |||
| 260 | |||
|
|
|||
| Number | 28 | 14 | |
| Range | (97–342) | (245–652) | |
| mean | 232.6 | 436.2 | |
| SD | 66.2 | 143.0 | |
|
|
|||
| p<0.01 | |||
DISCUSSION
This patient represented an unusual opportunity to compare MEG-unique spikes and EEG/MEG spikes, in the same subject. We believe that these results; 1) MEG unique spikes appearing antecedent to the EEG/MEG spikes, and 2) statistically higher mean magnetic amplitude in EEG/MEG spike than that of MEG unique spike, demonstrates, from a single focus in the same patient, MEG’s higher spike sensitivity. Based on the similar spike distribution, magnetic field contour map, and dipole location of the the two types of spikes (Fig. 2), we also conclude that despite an identical mechanism of seizure generation epileptic seizures may often be EEG-negative (but seen only with MEG) due to their small magnitude
Based on previous reports,1 we speculate that our findings are related to the extent of the cortical areas that is involved in epileptic activity and its directional profile; i.e. we expect the tangential component to be more prominent than radial one.1 In addition to its increased detection of epileptic activity, the excellent spatial resolution of MEG localized the spike sources to the MRI lesion, in contrast to EEG which showed a much more diffuse focus. These findings are consistent with MEG’s demonstrated increased sensitivity to epileptic discharges7 and better spatial resolution.3
MEG’s higher sensitivity to spikes may provide us with a new and complementary view of seizure evaluation. In this particular case, MEG demontrated seizure onset earlier than observed on EEG and explained the clinical episodes that had been seen previously without electrophysiological change. This view may also provide insight when the findings of other medical examinations combined with simultaneous EEG are difficult to interpret, such as positron emission tomography or single photon emission computed tomography.
As a single case, our findings illustrate the potential for MEG to improve our understanding of the physiological profile of EEG-negative epileptic seizures. Further investigation and accumulation of similar cases will reveal its consistency and significance.
Acknowledgments
This work was supported in part by grants from the National Institute of Biomedical Imaging and BioEngineering R01-EB009048 and R01-EB002010, and from the National Institute of General Medical Sciences DP2 OD006469-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benbadis SR, LaFrance WC., Jr . Clinical features and the role of video-EEG monitoring. In: Schachter SC, LaFrance WC Jr, editors. Gates and Rowan’s Nonepileptic Seizures. 3. Cambridge, UK: Cambridge University Press; 2010. pp. 38–50. [Google Scholar]
- 2.Cooper R, Winter AL, Crow HJ, Walter WG. Comparison of subcortical and scalp activity using chronically indwelling electrodes in man. Electroencephalogr Clin Neurophysiol. 1965;18:217–228. doi: 10.1016/0013-4694(65)90088-x. [DOI] [PubMed] [Google Scholar]
- 3.Barkley GL, Baumgartner C. MEG and EEG in epilepsy. J Clin Neurophysiol. 2003;20:163–178. doi: 10.1097/00004691-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Salayev KA, Nakasato N, Ishitobi M, Shamoto H, Kanno A, Iinuma K. Spike orientation may predict epileptogenic side across cerebral sulci containing the estimated equivalent dipole. Clin Neurophysiol. 2006;117:1836–1843. doi: 10.1016/j.clinph.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- 6.Santiuste M, Nowak R, Russi A, Tarancon T, Oliver B, Ayats E, et al. Simultaneous magnetoencephalography and intracranial EEG registration: technical and clinical aspects. J Clin Neurophysiol. 2008;25:331–339. doi: 10.1097/WNP.0b013e31818e7913. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki M, Pestana E, Burgess RC, Lüders HO, Shamoto H, Nakasato N. Detection of epileptiform activity by human interpreters: blinded comparison between electroencephalography and magnetoencephalography. Epilepsia. 2005;46:59–68. doi: 10.1111/j.0013-9580.2005.21104.x. [DOI] [PubMed] [Google Scholar]