Abstract
Over 90% of the U.S. population has detectable bisphenol-A (BPA) in their urine according to recent biomonitoring data. BPA is best known for its estrogenic properties, and most rodent research on the nervous system effects of BPA has focused on determining if chronic exposures during pre- and perinatal development have organizational effects on brain development and behavior. Estrogens also have important impacts on brain and behavior during adulthood, particularly in females during aging, but the impact of BPA on the adult brain is less studied. We have published a series of studies documenting that chronic exposure to various estrogens including 17β-estradiol, ERβ selective SERMs and soy phytoestrogens impairs performance of middle-aged female rats on an operant working memory task. The purpose of this study was to determine if chronic oral exposure to BPA would alter working memory on this same task. Ovariectomized (OVX) middle-aged Long Evans rats were tested on an operant delayed spatial alternation (DSA) task. Rats were treated for 8–10 weeks with either a 0 (vehicle control), 5 or 50 μg/kg bw/day oral bolus of BPA. A subset of the vehicle control rats were implanted with a Silastic implant containing 17β-estradiol (low physiological range) to serve as a positive control. All rats were tested for 25 sessions on the DSA task. BPA treatment did not influence performance accuracy on the DSA task, whereas 17β-estradiol significantly impaired performance, as previously reported. The results of this study suggest that chronic oral exposure to BPA does not alter working memory processes of middle-aged OVX rats assessed by this operant DSA task.
Keywords: bisphenol-A, working memory, middle-aged, OVX, DSA
1. Introduction
Bisphenol-A (BPA) is a high production volume chemical used in the manufacture of a variety of plastics and plastic-containing products (see Richter et al., 2007), and is found in an assortment of food and beverage containers (see also Erler and Novak, 2010). Human exposure to this chemical is ubiquitous (see Vandenberg et al., 2007), with the majority of exposures occurring orally from the diet (Rudel et al., 2011; von Goetz et al., 2010). Urinary concentrations of BPA in humans are quite variable (Ye et al., 2011), with estimates of daily exposure to adults in the general population ranging from 0.008 to 1.5 μg/kg bw/day (NTP, 2008; World Health Organization, 2010).
BPA is a synthetic estrogen that can bind the nuclear estrogen receptors (ERs), having a higher affinity for ERβ than for ERα (Matthews et al., 2001; Routledge et al., 2000; Takemura et al., 2005). It is considered a weak estrogen agonist as its binding affinity is approximately 10,000 fold lower than that of 17β-estradiol (Kupier et al., 1998). BPA also possesses both anti-estrogenic and anti-androgenic properties at some doses in particular tissues (Wolstenholme et al., 2011). The current U.S. tolerable daily intake (TDI) set by the EPA is 50μg/kg/day, but the safety of this oral reference dose has come under scrutiny as recent research suggests the possibility that BPA may have a non-monotonic dose response curve, with some effects in animal models occurring at doses well below the TDI (Vandenberg et al., 2009, 2012).
A large number of animal and human studies have been undertaken to assess the effects of exposure to BPA on a variety of health endpoints. Developmental exposures have been the major focus of research assessing the impact of BPA on learning and memory (see Golub et al., 2010; Palanza et al., 2008), but there is little clarity or consistency in the findings. In rodents, exposures at or below the TDI (≤50 μg/kg/day; see also Sekizawa, 2008) have resulted in a range of effects on memory processes, ranging from deficits, to no effect, to enhancements (Goncalvez et al., 2010; Jones and Watson, 2012; Poimenova et al., 2010; Ryan and Vandenbergh, 2006; Viberg et al., 2011; Xu et al., 2011). Studies also suggest that exposure to BPA during development can permanently alter the structure and organization of brain regions important to learning and memory (Matsuda et al., 2010; Xu et al., 2011).
In contrast, fewer studies have examined the effects of BPA exposure during adulthood on learning and memory of rodents, and all of the studies to date have been conducted in young OVX rats or gonadally-intact rats. A set of studies found performance on both object placement and recognition memory tasks - thought to be mediated by the hippocampus (Ennaceur et al., 1997) - were impaired by treatment with BPA. In these tasks, rats were given 3 min to explore 2 objects, and following a delay memory was tested by moving (placement) or replacing (recognition) one of the objects. A single injection of BPA blocked a 17β-estradiol induced improvement in both object placement and recognition memory in 3-month old ovariectomized (OVX) female rats (lowest effective dose 4 and 40 μg/kg respectively: Inagaki et al., 2012). Similar effects on both object recognition and placement tasks were seen following a single injection of a 40 μg/kg dose of BPA to gonadally-intact male rats (Eilam-Stock et al., 2012). Further, short term daily oral treatment (14–28 days) with either relatively high (20 mg/kg) or relatively low (2 or 20 μg/kg) doses of BPA impaired memory performance of young adult male rats or mice in the Morris water maze (Jain et al., 2011; Kim et al., 2011).
To date, no studies have assessed the potential of BPA to alter memory processes in aging rodents. This is an important period for estrogen action in the female brain as middle-age represents the time when the transition from cyclical estrogen production to low/null circulating estrogen levels occurs. The question of whether or not estrogen replacement during this period aides or impairs cognitive aging remains unresolved. Furthermore, there are very few studies addressing how exposures to estrogen-active toxicants such as BPA may alter brain and behavior during this time period. Importantly, 17β-estradiol exposures in aging OVX rodents have revealed both brain region and memory system dependent changes in behavior (see Frick, 2009), with estrogens typically enhancing performance on hippocampally-sensitive tasks (Daniel et al., 1997; Daniel and Dohanich, 2001; Davis et al., 2005; Korol and Kolo, 2002; Zurkovsky et al., 2006), but impairing performance on tasks mediated by the prefrontal and striatal systems (Davis et al., 2005; Korol and Kolo, 2002; Neese et al., 2010a; Wang et al., 2008, 2009, 2011; Zurkovsky et al., 2007).
Our research group has established a mnemonic impairing effect of 17β-estradiol on a working memory task in middle-aged (12-month) OVX Long-Evans rats, a rodent model of the perimenopausal woman (Neese et al., 2010a; Wang et al., 2009). Specifically, performance on the operant delayed spatial alternation (DSA) task, which requires a rat to alternate its responses between two retractable levels to receive a food reward, was impaired following chronic treatment (8–10 weeks) with a Silastic implant that delivered a physiological dose (20–30 pg/ml) of 17β-estradiol. The deficits were measured following short intertrial delays (3-, 6- and 9-sec) that have been shown to be sensitive to prefrontal cortical disruption (Chudasama and Muir, 1997; Harrison and Mair, 1996; Mair et al., 1998; Sloan et al., 2006; Van Haaren et al., 1985, 1988; Young et al., 1996). This deficit was largely paralleled by treatment with the ERβ agonist diarylpropionitrile (DPN), while treatment with the ERα agonist propyl pyrazole triol (PPT) had little effect (Neese et al., 2010a). In addition, chronic treatment with the soy phytoestrogen genistein, which has a higher binding affinity for ERβ than for ERα, also impaired DSA performance in aging OVX female rats (Neese et al., 2010b, 2012).
The purpose of this study was to determine the potential for BPA to impair performance on this DSA task in middle-aged OVX rats. BPA has been shown to disrupt the performance of young adult OVX rats treated with 17β-estradiol and gonadally-intact adult male rats on hippocampus-sensitive memory tasks, including the Morris water maze, object placement and object recognition tasks (Eilam-Stock et al., 2012; Inagaki et al., 2012; Jain et al., 2011; Kim et al., 2011), but no studies have been conducted in OVX aging female rodents treated with BPA. Given that our prior research has shown that chronic treatment with17β-estradiol or ERβ agonists impairs performance on the DSA task in aging OVX rats, we hypothesized that exposure to BPA would also impair performance in this model. Treatment doses (5 and 50 μg/kg) were selected to address lose-dose effects relevant to human exposures (see Vandenberg et al., 2012).
2. Methods
2.1. Animals and Exposure
One hundred fifty-seven 10–12 month old female Long-Evans retired breeder rats were obtained from Harlan (Indianapolis, IN) in three separate cohorts and maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were housed in a temperature and humidity controlled room (22°C, 40–55% humidity) on a 12-hour reverse light-dark cycle (lights off at 8:30 am). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health, 2002) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Rats were pair-housed by treatment group in polysulfone cages (45 × 24 × 20 cm) with woodchip bedding. All rats were subject to isoflurane gas anesthesia prior to OVX. A subset of rats were implanted with a Silastic capsule that contained 17β-estradiol at the time of OVX, and served as the positive control group (see Neese et al., 2010a; Wang et al., 2009). The Silastic capsule was 1 cm in length (1.5 mm i.d., 1.96 mm o.d.) and was plugged with silicone and dried overnight before packing with a 10% 17β-estradiol/cholesterol mixture (Sigma, St. Louis, MO), after which the other end was plugged with silicone. Capsules were soaked in sterile saline at 37°C overnight before insertion during surgery. Previous research in this lab has shown that these 17β-estradiol implants produce stable serum estradiol concentrations of about 20–30 pg/ml for at least 10 weeks (Neese et al., 2010a).
Standard rodent diets contain soy phytoestrogens which can influence performance of the DSA task (see Neese et al., 2010b, 2012). There is also a wide variability of soy content seen across lots of standard rodent chow (Brown and Setchell, 2001; Thigpen et al., 2004, 2007). To avoid this confound, the rats in this study were maintained on a low-soy diet (Harlan diet 2016, Madison, WI). BPA-free water (Ultra-Reverse Osmosis System, freedrinkingwater.com; confirmed BPA free via HPLC with CoulArray detection, personal communication, Fred Vom Saal) was available ad libitum in glass water bottles. Beginning one week after OVX surgery, rats were weighed daily and food was restricted to maintain them at 85% of their free-feeding body weights. Operant training began two weeks following OVX and occurred once daily, six days/week during the dark phase of the light cycle. Rats were fed one hour after the daily test session was completed.
2.2. BPA Treatment
BPA was purchased from Sigma-Aldrich (#239658, St. Louis, MO) and dissolved in a 2% ethanol solution prior to mixing in tocopherol stripped corn oil (MP Biomedicals, #901415, Solon, OH). BPA at 50, 5, or 0 (vehicle control) μg/kg/bw was delivered via an oral bolus once per day with a polysulfone pipette tip (#9400263, Thermo Scientific). All rats received a single treatment each day prior to training/testing, and a single treatment on Sunday (non-operant testing day). BPA is quickly metabolized following oral bolus exposure and this glucuronidated form is not estrogenic (Matthews et al., 2001). In rodents, peak serum concentrations of unconjugated BPA occur within 30–60 mins (see Doerge et al., 2010, 2011; Taylor et al., 2011). The operant DSA training and testing tasks typically take 45–60 minutes to complete, therefore rats were treated with BPA approximately 10–30 minutes prior to operant training or testing each day. Rats treated with 17β-estradiol (positive control) were also given a single oral dose of the 0 μg/kg treatment (vehicle control) prior to training or testing each day. Daily BPA treatments lasted for 8–10 weeks depending upon the number of sessions needed for individual rats to achieve criterion for response shaping, lever press training, and cued alternation training (Fig. 1 and see below). Few rats needed more than 9 weeks to complete training and testing, and the length of treatment did not differ across BPA exposure groups or cohorts.
Figure 1.
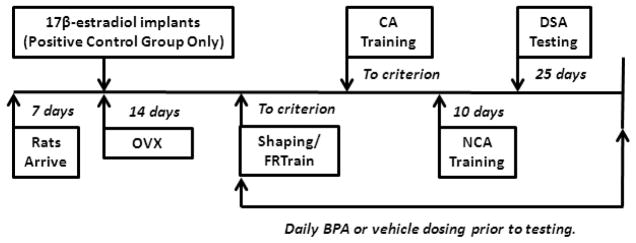
Treatment and testing schedule for BPA and vehicle dosing.
This study was conducted in three separate cohorts of rats; therefore cohort was included as a factor in all statistical analyses (see statistical analyses below). The final sample sizes for each treatment dose were as follows: 50 μg/kg/day BPA (BPA50), cohort 1, n=12; cohort 2, n=14; cohort 3, n=16 (total N=42): 5 μg/kg/day BPA (BPA5), cohort 1, n=12; cohort 2, n=13; cohort 3, n=15 (total N=40): 0 μg/kg/day BPA (oil), cohort 1, n=11; cohort 2, n=12; cohort 3, n=16 (total N=39): 10% 17β-estradiol (estradiol), cohort 1, n=9; cohort 2, n=11; cohort 3, n=16 (total N=36).
2.3. Operant Training and Testing
Behavioral training and testing was conducted in standard automated operant chambers (Med Associates Inc., St. Albans, VT) housed in sound-attenuated wooden boxes. All of the test chambers had the same features and dimensions: 21.6 cm high with a 29.2 cm × 24.8 cm stainless-steel grid floor that rested just above a tray filled with woodchip bedding. Soy-free 45-mg food pellets (5TUL, Test Diet, Richmond, IN) were dispensed through a pellet dispenser centered 2.5 cm above the floor on the operant panel. A pair of retractable response levers and a pair of stimulus cue lamps, one above each lever, were positioned symmetrically on both sides of the pellet dispenser. The levers were 5.7 cm from the midline and 7.0 cm above the floor. The cue lights were located 5.7 cm above the levers. Each chamber also contained a Sonalert tone generator, a white noise generator, and a house light located on the back wall. Experimental contingencies were programmed using the Med-State behavioral programming language (Med-Associates, Vermont).
2.4. Response shaping and lever press training
Rats were trained to press the response levers by using an autoshaping program (Neese et al., 2010a, 2010b; Wang et al., 2008, 2009; Widholm et al., 2001, 2004). Autoshaping test sessions were terminated after 60 min elapsed or 100 reinforcers were delivered. Criterion for this condition was set at 100 lever presses in two consecutive sessions. Following autoshaping, the rats were exposed to a continuous reinforcement schedule in which the lever associated with reinforcement alternated following delivery of every 5th reinforcer to prevent the rats from developing a lever or side preference. This cycle of alternating levers terminated after 100 reinforcers were received or 60 min had elapsed. A performance criterion of 100 reinforcers earned for three consecutive sessions was established for this condition.
2.5. Training Phases Prior to DSA
After lever press training, the rats were trained to alternate their responses prior to beginning testing on the DSA task (Neese et al., 2010a, 2010b; Wang et al., 2008, 2009; Widholm et al., 2004). The initial training schedule began with cued alternation (CA) training in which a cue light was illuminated to indicate the correct lever on each trial. Each correct cued lever press was reinforced. The lever associated with reinforcement was not altered following an incorrect response, i.e. the ‘correct’ lever continued to be the lever opposite of the lever associated with reinforcement in the most recent ‘correct’ trial. A 0-sec delay (<0.15 sec) was imposed between trials in this initial cued training phase, with the levers retracted and extended between trials. Rats were trained to a criterion of one session above chance defined as >60% correct presses. Next, a noncued alternation (NCA) task was presented where the cue light no longer indicated the correct lever, and both cue lights were illuminated when the levers were extended. Correct responses still consisted of alternating right and left lever presses. As for CA, the correct lever was again always the lever opposite of the one on the trial in which most recent ‘correct’ lever press occurred. Again, a 0-sec delay was imposed between opportunities to press with the levers retracting and extending between presses. Each rat was trained for 10 consecutive sessions on the NCA phase.
2.6. Delayed spatial alternation (DSA) testing
The final alternation phase was the variable DSA task. Following each lever press, variable intertrial delays of 0, 3, 6, 9, or 18 sec were randomly imposed between opportunities to press (Neese et al., 2010a, 2010b; Wang et al., 2008, 2009; Widholm et al, 2004). As in NCA training, both of the cue lights were illuminated when the levers were extended. There were 40 trials at each delay and a total of 200 trials per session. Intertrial delays were randomly balanced within each session and any specific delay was not presented on more than three consecutive trials. The correct lever was again always the lever opposite of the one on the trial in which most recent ‘correct’ lever press occurred. Each animal was tested for 25 consecutive sessions.
2.7. Statistical Analyses
The behavioral data were analyzed using SPSS for Windows, Version 19.0. Treatment group and cohort were included in the analyses as between subject factors and significance was set at p<0.05. When appropriate, Tukey post hoc tests were run for pair-wise comparisons.
For CA, cumulative errors across all sessions served as the main measure of learning, and was analyzed using ANOVA for treatment group and cohort. For NCA, the overall proportion correct across the ten sessions served as the primary measure of learning, and was analyzed using a 4 (treatment group) × 3 (cohort) × 10 (session) mixed ANOVA where session was a repeated measures factor.
For DSA, the proportion correct across the 25 test sessions was first averaged across blocks of 5 test sessions to produce five 5-session test blocks. Proportion correct at each intertrial delay across the 25 test sessions was then analyzed using a mixed 4 (treatment group) × 3 (cohort) × 5 (block) × 5 (delay) repeated measures ANOVA with block (1 – 5) and delay (0, 3, 6, 9, 18 sec) serving as repeated measures factors.
Error pattern analyses were also conducted to determine if rats were more likely to respond incorrectly following a correct or an incorrect lever press. A ‘win-stay’ error was defined as an incorrect response on the same lever that had been correct on the previous trial, whereby the rat responded correctly on the n-1 trial, but incorrectly on the nth trial. A ‘lose-stay’ error was defined as an incorrect lever press on the same lever that had been incorrect on the previous trial, whereby the rat responded incorrectly on the n-1 trial as well as the nth trial. Win-stay and lose-stay errors were analyzed separately using a mixed 4 (treatment group) × 3 (cohort) × 5 (block) repeated measures ANOVA with block (1 – 5) serving as a repeated measures factor. The latency to lever press following a correct and an incorrect response were also analyzed separately using a mixed 4 (treatment group) × 3 (cohort) × 5 (block) repeated measures ANOVA with block (1 – 5) serving as a repeated measures factor.
3. Results
3.1. CA and NCA Training
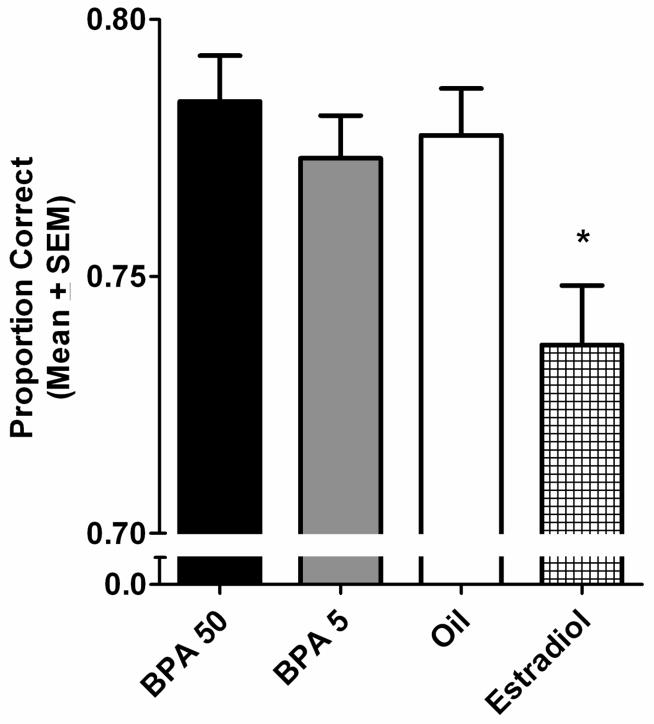
Treatment groups did not differ on errors committed during CA training. This was revealed by a non-significant ANOVA for treatment group, F(3,145)=0.142, p>0.05. Performance accuracy during NCA training also was not influenced by BPA treatment. However, a significant main effect of treatment group was uncovered for proportion correct during NCA training, F(3,145)=5.136, p=0.002. Post hoc analyses found the estradiol treated group to perform worse overall than did the BPA50, BPA5, and oil treated groups, p<0.05 (Fig. 2). Lastly, a main effect of session was uncovered, F(9,1305)=329.779, p<0.001. The performance of all groups improved across subsequent training sessions.
Figure 2.
Multiple comparison results of proportion correct during NCA training (Mean ± SEM). The estradiol treated group performed worse than did the BPA50, BPA5 and oil treated groups,* p<0.05.
3.2. DSA Testing
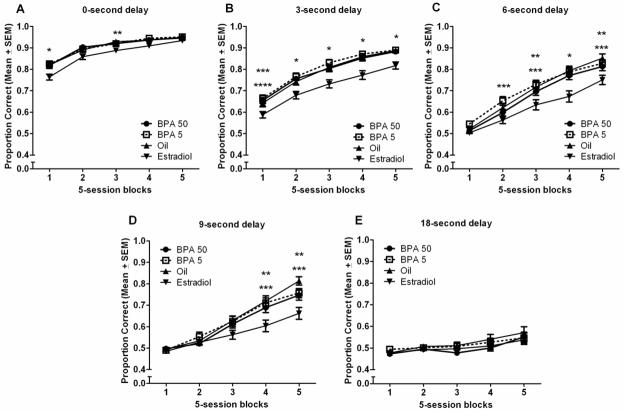
As with NCA training, BPA treatment did not alter performance during DSA testing. Again, a significant main effect of treatment group was uncovered, F(3,145)=6.097, p=0.001, with post hoc tests revealing that the estradiol treated group performed worse overall than did the BPA50, BPA5, and oil treated groups, p<0.05. A significant treatment group x block x delay effect was also uncovered for proportion correct, F(16,2320)=2.182, p=0.003. Again, the performance of the BPA treated rats did not differ significantly from that of the oil treated groups during any of the delays (Fig. 3). As previously published, we found a significant deficit in the performance of the 17β-estradiol treated rats, an effect most clearly seen following the 3- and 6-sec delays, p<0.05 (Fig. 3B and 3C). Lastly, a significant block x delay effect was uncovered, F(16,2320)=2.182, p<0.001. All groups tended to perform better across subsequent sessions of testing, except at the longest delay.
Figure 3.
(A–E). Multiple comparison results of proportion correct across five 5-session blocks of testing, sorted by intertrial delay (Mean ± SEM). * The estradiol treated group performed worse than did all treatment groups (p<0.05). ** The estradiol treated group performed worse than did the oil treated group (p<0.05). *** The estradiol treated group performed worse than did the BPA5 treated group (p<0.05). **** The estradiol treated group performed worse than did the BPA 50 treated group (p<0.05).
3.3. Error pattern analyses
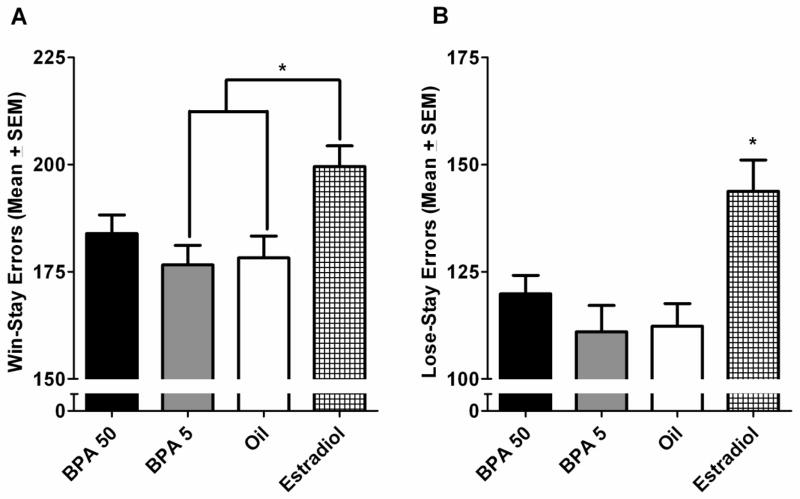
A significant main effect of treatment group was uncovered for both win-stay, F(3,145)=4.173, p=0.007, and lose-stay errors, F(3,145)=6.286, p<0.001. Error rates in the BPA treated rats did not differ significantly from the oil treated group, and, similar to our previously published data, the estradiol treated group made more win-stay and lose-stay errors than did the oil treated group, p<0.05 (Fig. 4A and 4B). A main effect of block was also uncovered for both win-stay, F(4,580)=208.533, p<0.001, and lose-stay errors, F(4,580)=363.233, p<0.001. All treatment groups committed fewer errors across subsequent sessions of testing.
Figure 4.
Multiple comparison results of error patterns (Mean ± SEM). (A) Win-stay errors committed across five 5-session blocks of DSA testing. The estradiol treated group committed more errors than did the BPA5 and oil treated groups, *p<0.05. (B) Total lose-stay errors committed on the DSA task across 25 sessions of testing. The estradiol treated group committed more errors overall than did the other three treatment groups, *p<0.05.
3.4. Latencies to lever press
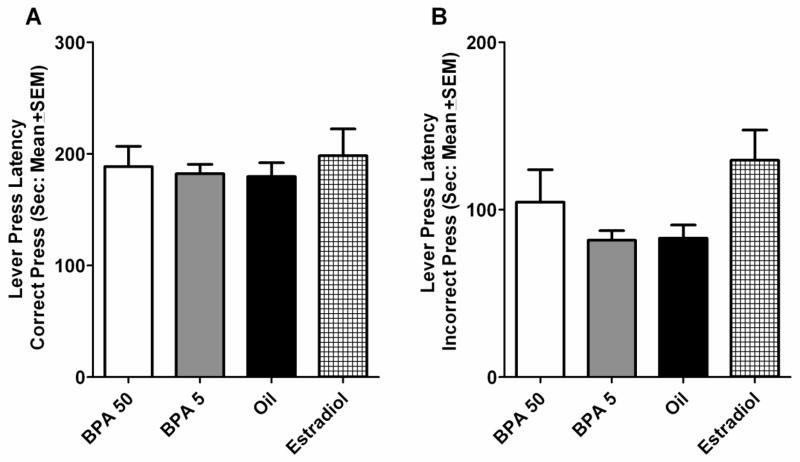
A main effect of block was also uncovered for latency to respond after a correct press, F(4,580)=5.460, p=0.006. Latencies tended to decrease across sessions of testing. A main effect of treatment group was uncovered for latency to respond after an incorrect press, F(3,145)=3.341, p=0.021. Post hoc analyses failed to uncover any significant differences between treatment groups, but the BPA5 and oil treated groups tended to have shorter latencies to respond (Fig. 4., p<0.10). A main effect of block was also uncovered for latency to respond after an incorrect press, F(4,580)=70.323, p<0.001. Latencies tended to decrease across sessions of testing.
3.5. Cohort Effects
Few effects of testing cohort were uncovered. Importantly, BPA treatment did not interact with cohort on any measure, rather the 1st cohort of rats appeared to perform slightly worse than the other two cohorts overall. Main effects of cohort were uncovered during NCA training, F(2,145)=9.761, p<0.001, and DSA testing, F(8,580)=2.454, p=0.028. The 1st cohort performed significantly worse than both the 2nd and 3rd cohorts during NCA training, p<0.05, and worse than the 2nd cohort at the 18 sec intertrial delay during DSA testing, p<0.05. A significant block x cohort effect was also uncovered for lose-stay errors, F(8,580)=3.093, p=0.010. Follow-up analyses found that, in the 2nd block of testing, the 2nd cohort of rats committed more errors than did the 1st and 3rd cohorts.
4. Discussion
This study failed to find any influence of a daily oral bolus of 5 or 50 μg/kg/day BPA on performance of this operant DSA task in middle-aged OVX rats. In contrast, the positive control, 17β-estradiol, impaired DSA performance in middle-aged rats to the same degree as previously reported (Neese et al., 2010a; Wang et al., 2009). Three separate replicates (cohorts) were tested and, summing across the three cohorts, included a total of 36–42 rats per exposure group. The large number of rats tested in this experiment were needed in order to determine if BPA produced any subtle effects on memory that may have been missed in a smaller study. In addition, confirming these effects across a series of cohorts ensures that any treatment effect, or lack thereof, was confirmed in each replicate. Given these large group sizes, it is very unlikely that the lack of significant findings reported here is due to a lack of sufficient statistical power to detect subtle treatment effects.
4.1. Relationship to previous research
The finding that chronic BPA treatment failed to alter prefrontally-mediated working memory in OVX rats is supported by a recent study which suggests that acute BPA does not influence memory in an animal model that uses a low or null estrogen background. In that study, treatment of 3-month old OVX rats with a single dose of BPA (range of 1–400 μg/kg) failed to alter performance on hippocampally-mediated object placement or recognition memory tasks (Inagaki et al., 2012). Interestingly, BPA did block the ability of 17β-estradiol (and 17α-estradiol) to enhance the performance of OVX female rats on these tasks. This suggests an anti-estrogenic effect of BPA on memory consolidation in this paradigm. Estrogens have differential effects on memory processes thought to be mediated by different brain systems (see Frick, 2009), but the lack of effect on this prefrontally-mediated memory task and on a hippocampally-mediated memory task (see Inagaki et al., 2012) suggests little effect of low doses of BPA on general mnemonic function in OVX female rats. Conversely, BPA treatment of gonadally-intact adult male and female rats at doses below 50 μg/kg impaired hippocampally-mediated memory processes in other studies (Eilam-Stock et al., 2012; Inagaki et al., 2012; Jain et al., 2011), evidence that exposure to this chemical at doses below the TDI may impair some aspects of mnemonic function in rats with significant levels of circulating hormones.
A 17β-estradiol treatment group was included as a positive control group in this study (see also Cory-Slechta et al., 2001; Li et al., 2008) in order to control for Type II error, and to ensure the study had adequate power to detect expected differences (Cory-Slechta et al., 2001). The effects of BPA on behavior in the published literature have been inconsistent (see Hengstler et al., 2011 and introduction), making the inclusion of a positive control group within each testing cohort even more important. Importantly, we found a treatment effect of 17β-estradiol that was entirely consistent with our previously published findings. Specifically, 17β-estradiol treatment produced a delay-dependent decrement in the performance of middle-aged OVX female rats (Neese et al., 2010a; Wang et al., 2009), suggesting that these deficits are related to memory, and not such cognitive domains as attention and/or motivation (Pontecorvo et al., 1996; Van Hest and Stecker, 1996; see also the more detailed discussion of this topic in Wang et al., 2009). As discussed, we have reported this task to be sensitive to other ERβ selective compounds, including the ERβ agonist DPN (Neese et al., 2010a), and the soy phytoestrogen genistein (Neese et al., 2010b, 2012). The relative binding affinity of genistein to ERβ is much higher than that of BPA (Kuiper et al., 1998), and relatively high doses of genistein treatment (3.4 mg/kg/day) produced only modest effects on the performance of this DSA task (Neese et al., 2012). Therefore, if the decrements in DSA performance are driven by ERβ agonism, it is not that surprising that these treatment doses of BPA, which were chosen to reflect the range of human exposure (NTP, 2008; World Health Organization, 2010), failed to alter performance. It is important to note that BPA also binds the membrane estrogen receptor GPR30 (Alonso-Magdalena et al., 2012; Thomas and Dong, 2006), but the role of this receptor in DSA performance is currently not known.
4.2. Treatment dose and timing
Some controversy exists about the exact half-life of unconjugated BPA in rodent blood (see Doerge et al., 2010, 2011, 2012; Sieli et al., 2011; Taylor et al., 2011). The glucuronidated form of BPA is not estrogenic (Matthews et al., 2001), but it appears that, in rodents, maximum blood levels of unconjugated BPA occur within 30–60 mins following an oral bolus exposure (Doerge et al., 2010, 2011; Taylor et al., 2011). Therefore, the dosing paradigm used in this study should have resulted in peak unconjugated BPA concentrations during the daily testing period, and it is unlikely that the lack of an effect is related to the timing of daily treatment.
4.3. Effects of Testing Cohort
We conducted this study in 3 replicates, or cohorts, spaced approximately 4 months and testing cohort had little effect on performance. Cohort effects, when present, did not interact with treatment group on the main measures of learning during the training phases (CA and NCA). In addition, cohort did not interact with treatment group on performance accuracy or errors committed during DSA testing. Overall, subtle differences were measured between testing cohorts, but these differences were not systematic and appear to be unrelated to BPA exposure.
4.3. Strengths of Study
A major strength of this study was that we used a long-term daily oral dosing paradigm rather than an injection/implant source of exposure (see also Li et al., 2008). This more accurately models the route of exposure in humans. Many precautions were also taken to limit BPA exposure from outside sources, including housing rats in BPA-free caging and giving ad libitum access to water that was both confirmed to be BPA free and delivered in BPA free glass bottles. In addition to limiting sources of exogenous BPA exposure, we were also careful to reduce exposure to other exogenous estrogens via the diet. Another strength of this study is that we used age-appropriate rats to model the perimenopausal woman (Cory-Slechta et al., 2001; Crofton et al., 2004; Li et al., 2008). In contrast, many studies use OVX young rats as a rodent model of human menopause. In addition, we used a behavioral paradigm that we have shown to be sensitive to a variety of estrogens (Neese et al., 2010a, 2010b, 2012; Wang et al., 2008, 2009), and we tested effects across three cohorts of animals to confirm these findings were consistent across replicates. This resulted in a total N of 36–42 rats per treatment group at the conclusion the experiment. Lastly, we included a positive control group (discussed above) in each cohort (Cory-Slechta et al., 2001; Crofton et al., 2004; Li et al., 2008).
4.6 Future Studies
These results do not discount the potential for BPA to alter other aspects of behavior in aging OVX rats. Developmental exposure to BPA can alter nonmnemonic behavioral domains, including anxiety and exploratory behavior (see Golub et al., 2010; Palanza et al., 2008). In humans, gestational BPA exposure has been linked to increased externalizing behaviors and altered emotional behavior in 3- to 5-year old children (Braun et al., 2009; 2011; Perera et al., 2012). Further, 17β-estradiol treatment can also reduce anxiety behaviors in rodents (Bowman et al., 2002). Therefore, an important area for future studies is BPA effects on social/emotional behaviors in animal models. Additionally, the focus of this study was modeling the perimenopausal woman, but other studies suggest acute BPA treatment can interfere with memory consolidation in young cycling rats (see Inagaki et al., 2012). Our previous research found that the performance of young adult cycling rats did not differ from that of OVX rats in this same DSA task (Wang et al., 2008), but this does not preclude the possibility that BPA exposure could alter DSA performance in cycling rats. Therefore future research should also assess the effects of chronic BPA exposure on DSA performance in young, gonadally-intact female rodents.
4.5. Summary and Conclusions
Overall, this study failed to show any effect of chronic BPA treatment in middle-aged OVX rats on an operant DSA task that is sensitive to the effects of a variety of estrogens (Neese et al., 2010a, 2010b, 2012; Wang et al., 2008, 2009). Little difference was measured between the BPA and oil treated groups, whereas the positive control group (17β-estradiol) showed a reliable deficit in the acquisition of this operant DSA task.
Figure 5.
ANOVA results of lever press latencies (Mean ± SEM). (A) Latency to lever press following a correct response across five 5-session blocks of DSA testing. No differences were measured between treatment groups, p>0.05. (B) Latency to lever press following an incorrect response across five 5-session blocks of DSA testing. No differences were measured between treatment groups, p>0.05.
Highlights.
Estrogens can impair memory processes in aging female rats.
Bisphenol-A (BPA) is a ubiquitous chemical with mixed estrogen agonist/antagonist properties.
We treated ovariectomized middle-aged rats with BPA.
BPA treatment did not alter working memory processes in middle-aged OVX rats.
Acknowledgments
The authors would like to thank Mindy Howe for her help with OVX surgeries and daily animal care. In addition, we would like to thank Fred Vom Saal for confirming that our reverse osmosis system produced BPA-free drinking water. This research was supported by the National Institute on Aging Grant P01 AG024387 (SLS) and the P50 AT006268 from ODS, NCAAM, and NCI (SLS). Steven Neese also received support from the National Institute of Environmental Health Sciences Grant T32 ES007326.
Footnotes
5. Conflict of Interest Statement
The authors have no potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Magdalena P, Ropero AB, Soriano S, Garcia-Arevealo M, Ripoll C, Fuentes E, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355:201–7. doi: 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–10. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–52. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Crofton KM, Foran JA, Ross JF, Sheets LP, et al. Methods to identify and characterize developmental neurotoxicity for human health risk assessment. I: behavioral effects. Environ Health Perspect. 2001;109 (Suppl 1):79–91. doi: 10.1289/ehp.01109s179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Makris SL, Sette WF, Medez E, Raffaele KC. A qualitative retrospective analysis of positive control data in developmental neurotoxicity studies. Neurotoxicol Teratol. 2004;26:345–52. doi: 10.1016/j.ntt.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediated the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbair S, Mizumori SJY. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fischer JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol. 2010;247:158–65. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fischer JW. Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice: inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol Lett. 2011;207:298–305. doi: 10.1016/j.toxlet.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fischer JW. Pharmacokinetics of bisphenol A in serum and adipose tissue following intravenous administration to adult female CD-1 mice. Toxicol Lett. 2012;211:114–19. doi: 10.1016/j.toxlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Eilam-Stock T, Serrano S, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci. 2012;126:175–85. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Erler C, Novak J. Bisphenol A exposure: human risk and health policy. J Pediatr Nurs. 2010;25:400–7. doi: 10.1016/j.pedn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Wu KL, Kaufman FL, Li LH, Morna-Messen F, Zeise L, et al. Bisphenol A: developmental toxicity from early prenatal exposure. Birth Defects Res B Dev Reprod Toxicol. 2010;89:441–66. doi: 10.1002/bdrb.20275. [DOI] [PubMed] [Google Scholar]
- Goncalves CR, Cunha RW, Barros DM, Martinez PE. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environ Toxicol Pharmacol. 2010;30:195–201. doi: 10.1016/j.etap.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Mair RG. A comparison of the effects of frontal cortical and thalamic lesions of measures of spatial learning and memory in the rat. Behav Brain Res. 1996;75:195–206. doi: 10.1016/0166-4328(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41:263–91. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–67. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Kumar M, Suranagi UD, Mediratta PK. Protective effect of N-acetylcysteine on bisphenol A-induced cognitive dysfunction and oxidative stress in rats. Food Chem Toxicol. 2011;49:1404–09. doi: 10.1016/j.fct.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm Beh. 2012;61:605–10. doi: 10.1016/j.yhbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Kim ME, Park Hr, Gong EJ, Choi SY, Kim HS, Lee J. Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem Toxicol. 2011;49:3383–89. doi: 10.1016/j.fct.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29:504–19. doi: 10.1016/j.neuro.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MV. Lesions of the frontal cortex, hippocampus, and intralaminar nuclei have distinct effects on remembering in rats. Behav Neurosci. 1998;112:772–92. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Saika S, Amano K, Shimizu E, Sajiki J. Changes in the brain monoamine levels in neonatal rats exposed to bisphenol A at low doses. Chemosphere. 2010;78:894–906. doi: 10.1016/j.chemosphere.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol a glucuronide, with estrogen receptors α and β. Chem Res Toxicol. 2001;14:149–57. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- National Research Council Institute for Laboratory Animals Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- National Toxicology Program. NTP-CEHR monograph on the potential human reproductive and developmental effects of bisphenol A. 2008 NIH publication No. 08-5994. Available from: http://ntp.niehs.nih.gov/ [PubMed]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Horm Beh. 2010a;58:878–90. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Wang VC, Doerge DR, Woodling KA, Andrade JE, Helferich WG, Korol DL, Schantz SL. Impact of dietary genistein on aging and executive function in rats. Neurotoxicol Teratol. 2010b;32:200–11. doi: 10.1016/j.ntt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Bandara SB, Doerge DR, Helferich WG, Korol DL, Schantz SL. Effects of multiple daily genistein treatments on delayed alternation and a differential reinforcement of low rates of responding task in middle-aged rats. Neurotoxicol Teratol. 2012;34:187–95. doi: 10.1016/j.ntt.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 2008;108:150–7. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. Prenatal bisphenol A exposure and child behavior in an inner city cohort. Environ Health Perspect. 2012;120:1190–4. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167:741–49. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Sahgal A, Stecker T. Further developments in the measurement of working memory in rodents. Brain Res Cog Brain Res. 1996;3:205–13. doi: 10.1016/0926-6410(96)00007-9. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) α and ERβ. J Biol Chem. 2000;275:35986–93. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–20. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Beh. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sekizawa J. Low-dose effects of bisphenol A: a serious threat to human health? J Toxicol Sci. 2008;33:389–403. doi: 10.2131/jts.33.389. [DOI] [PubMed] [Google Scholar]
- Sieli PT, Jasarevic E, Warzak DA, Mao J, Ellersieck MR, Liao C, et al. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect. 2011;119:1260–65. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–26. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Takemura H, Ma J, Sayama K, Terao Y, Zhu BT, Shimoi K. In vitro and vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology. 2005;207:215–21. doi: 10.1016/j.tox.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, et al. Similarity if bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perpect. 2011;119:422–30. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR J. 2004;45:410–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, et al. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–1726. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–9. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, De Bruin JP, Heinsbroek RP, Van de Poll NE. Delayed spatial response alternation: effects of delay-interval duration and lesions of the medial prefrontal cortex on response accuracy of male and female Wistar rats. Behav Brain Res. 1985;18:41–49. doi: 10.1016/0166-4328(85)90167-6. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Van Zijderveld G, Van Hest A, De Bruin JP, Van Eden CG, Van de Poll NE. Acquisition of conditional associations and operant delayed spatial response alternation: effects of lesions in the medial prefrontal cortex. Behav Neurosci. 1988;102:481–88. doi: 10.1037//0735-7044.102.4.481. [DOI] [PubMed] [Google Scholar]
- Van Hest A, Steckler T. Effects of procedural parameters on response accuracy: lessons from delayed (non-)matching procedures in animals. Brain Res Cog Brain Res. 1996;3:193–203. doi: 10.1016/0926-6410(96)00006-7. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons W. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Buratovic S, Eriksson P. Dose-dependent behavioral disturbances after a single neonatal bisphenol A dose. Toxicology. 2011;290:187–94. doi: 10.1016/j.tox.2011.09.006. [DOI] [PubMed] [Google Scholar]
- von Goetz N, Wormuth M, Scheringer M, Hungerbuhler K. Bisphenol A: how the most relevant exposure sources contribute to total consumer exposure. Risk Anal. 2012;30:473–87. doi: 10.1111/j.1539-6924.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Wang VC, Sable HJK, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm Beh. 2009;56:382–90. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Scahntz SL. Estradiol impairs response inhibition in young and middle-aged, but not old rats. Neurotoxicol Teratol. 2011;33:405–14. doi: 10.1016/j.ntt.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: Sex-specific deficits in associative ability and inhibitory control. Toxicol Appl Pharmacol. 2001;174:188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–89. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Toxicological and health aspects of bisphenol A; report of joint FAO/WHO expert meeting. 2010 Available from: www.who.int/foodsafety/chem/chemicals/bisphenol/en/
- Xu X, Li T, Luo Q, Hong X, Xie L, Tian D. Bisphenol-A rapidly enhanced passive avoidance memory and phosphorylation of NMDA receptor subunits in hippocampus of young rats. Toxicol Appl Pharmacol. 2011;255:221–8. doi: 10.1016/j.taap.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–8. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HL, Stevens AA, Converse E, Mair RG. A comparison of temporal decay in place memory tasks in rats (Rattus norvegicus) with lesions affecting thalamus, frontal cortex, or the hippocampal system. Behav Neurosci. 1996;110:1244–1260. doi: 10.1037//0735-7044.110.6.1244. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Boyd S, Brown SL, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]