Abstract
Introduction
Wire localization (WL) of non-palpable breast cancers on the day of surgery is uncomfortable for patients and impacts OR efficiency. Radioactive seed localization (RSL) before the day of surgery avoids these disadvantages. In this study we compare outcomes of our initial 6-month experience with RSL to those with WL in the preceding 6 months.
Methods
Lumpectomies for invasive or intraductal cancers localized with a single 125iodine seed (January-June 2012) were compared to those using 1 wire (July-December 2011). Surgeons and radiologists did not change. Positive and close margins were defined as tumor on ink and tumor ≤1mm from ink, respectively. Demographic and clinical characteristics and outcomes were compared between RSL and WL patients.
Results
There were 431 RSL and 256 WL lumpectomies performed. Clinicopathologic characteristics did not differ between groups. Most seeds (90%) were placed before the day of surgery. Positive margins were present in 7.7% of RSL versus 5.5% of WL patients, and 16.9% of RSL versus 19.9% of WL had close margins (p=0.38). The median operative time was longer for lumpectomy and sentinel lymph node biopsy (SLNB) in the RSL group (55 versus 48 minutes, p<0.0001). There was no significant difference in the volume of tissue excised between groups.
Conclusions
In the first 6 months of RSL, operative scheduling was simplified, while rates of positive and close margins were similar to those seen after many years of experience with WL. Operative time was slightly longer for RSL lumpectomy and SLNB; we anticipate this will decrease with experience.
Keywords: breast-conserving surgery, radioactive seed localization, wire localization
INTRODUCTION
The widespread uptake of breast cancer screening, combined with improvements in imaging technology, has resulted in the frequent diagnosis of small non-palpable breast cancers. Wire localization (WL) of non-palpable cancers on the day of surgery is the most common localizing technique employed when breast conservation is pursued, yet this procedure has several limitations. It is uncomfortable for patients[1, 2], carries a risk of wire displacement or fracture during patient transportation from the radiology department to the operating table, and can result in delays or scheduling conflicts in radiology that impact upon operating room efficiency.
Techniques have been developed to replace wire localization. Ultrasound-guided lumpectomy directed at the hematoma (HUG)[3] or a breast marker[4] avoids preoperative localization, but requires surgeon training and a clearly visible hematoma or marker. Radio-guided occult lesion localization (ROLL) involves injecting 99technetium into the lesion the day before, or morning of, surgery.[5] This can be combined with a technetium injection for sentinel lymph node (SLN) identification (SNOLL).[5, 6] Radioactive seed localization (RSL), positions a 4.5 mm 125iodine seed in the breast tissue to be excised. Seeds have a half-life of 60 days, theoretically allowing insertion months prior to surgery if required (e.g., before neoadjuvant chemotherapy).[7, 8] The feasibility of RSL has been reported in institutional series.[9, 10] A small multicenter randomized control trial of WL versus RSL demonstrated no difference in the rates of positive or close margins (p = 0.61), but surgery was shorter following seed localization (mean, 19.4 minutes versus 22.2 minutes, p < 0.001).[1]
In January 2012, the standard method of localizing non-palpable breast lesions at Memorial Sloan-Kettering Cancer Center (MSKCC) changed from WL to RSL. The aim of this study was to compare our initial 6-month experience with RSL to our established practice of WL. The primary outcomes were the rates of positive and close margins, and the resulting rate of re-operation to improve margins between the 2 groups. Secondary outcomes were the lumpectomy volume and operative time.
PATIENTS AND METHODS
Following institutional review board approval, data were obtained from a prospectively maintained, registered database and patient electronic medical records. Patients with non-palpable invasive breast cancer or ductal carcinoma in situ (DCIS) were eligible for inclusion if they were treated with breast-conserving surgery (BCS) at MSKCC and had localization performed with a single radioactive seed (January-June 2012) or a single wire (July-December 2011) (FIG 1). Patients were excluded if they had more than one seed or wire inserted to localize a lesion, received neoadjuvant chemotherapy, had previous ipsilateral breast cancer, had multicentric cancer, or stage IV breast cancer.
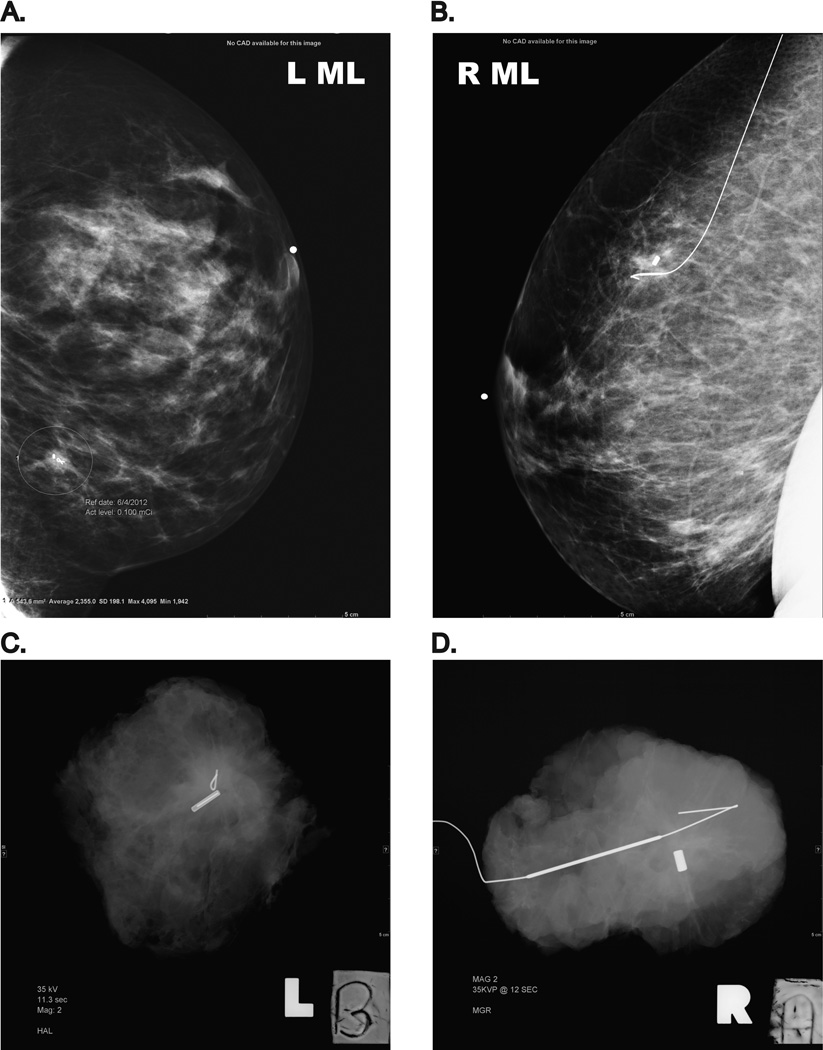
FIG 1.
Two 12 mm invasive ductal carcinomas excised following RSL or WL. (A and B): Mediolateral mammogram views performed following localization, demonstrating the radioactive seed and wire in position. (C and D): Specimen x-ray performed following the RSL and WL lumpectomies
RSL, radioactive seed localization; WL, wire localization
The surgeons and radiologists at our institution did not change between study time periods. Surgery was performed by 10 specialized breast surgeons. In December 2011, to facilitate the transition from WL to RSL, 2 to 4 initial cases of RSL were performed by each surgeon with a wire in place. The Node Seeker system and Gamma Probe (IntraMedical Imaging LLC, Los Angeles, CA), which detects both 99technetium and 125iodine, was used to perform the lumpectomy and SLN biopsy in RSL operations. During both study periods, lumpectomies were performed using the cavity shave approach[11], in which the index cancer was excised with a narrow margin of grossly normal breast tissue, and separate “shaved” margins were taken from the surgical cavity, oriented, and inked for evaluation.
The tumor size, histologic type, grade, occurrence of lymphovascular invasion (LVI), and the presence of DCIS or an extensive intraductal component (EIC) were acquired from the pathology report. Estrogen receptor (ER), progesterone receptor (PR), and HER2/neu status were also obtained. Microinvasion was defined as a focus of invasive carcinoma measuring < 1 mm in size. An EIC was defined as the presence of intraductal carcinoma that was greater than 25 % of the invasive tumor mass and that was also present outside of the invasive tumor. The size of DCIS was approximated from the radiological size on preoperative imaging. Positive and close margins were defined as tumor on ink, and tumor ≤ 1 mm from ink, respectively.
The volume or weight of the lumpectomy specimen is not measured as part of routine pathology evaluation; however, the dimensions of the excised specimen are recorded. Hence, the volume of the initial excision was estimated using the formula for the volume of an ellipsoid: 4/3×π×½ length×½ width×½ height. The operative time was obtained from the electronic operative record which detailed the start and finish times; cases where bilateral or additional procedures were performed were excluded from the operative time analysis.
Demographics, clinical factors, and outcomes were compared between the RSL and WL groups using chi-square tests for categorical variables and Wilcoxon tests for continuous variables. Two-tailed statistical analysis was performed using SAS version 9.1 (SAS Institute, Cary, NC), and p-values less than 0.05 were considered statistically significant.
RESULTS
A total of 687 patients were included in the study; 431 had RSL from January-June 2012, and 256 had WL from July-December 2011. A considerable number of patients who had surgery from July-December 2011 were excluded because multiple wires were used for localization, or because during the transition period, both a seed and wire were used for localization (Table 1). Localization prior to the day of surgery was performed for 90% of the RSL group (median, 1 day preoperatively; range, 0–47 days); all wires were inserted on the morning of surgery.
Table 1.
The patient population examined in the RSL and WL groups.
| January-June 2012 | July-December 2011 | |
|---|---|---|
| Number of cancers localized | 467 | 379 |
| Excluded: | ||
| Transition: seed + wire | NA | 34 |
| Multiple wires/seeds | 10 | 78 |
| Previous ipsilateral cancer | 13 | 3 |
| Neoadjuvant therapy | 13 | 4 |
| Wire for re-excision | NA | 4 |
| Number included in study | 431 (Seed Group) | 256 (Wire Group) |
RSL, radioactive seed localization; WL, wire localization; NA, not applicable
There were no significant differences in the baseline characteristics of the patients in the 2 groups (Table 2). The median age at surgery did not differ and was approximately 60 years in both groups. Three-quarters of patients had surgery for an invasive carcinoma, and > 80% of invasive carcinomas were infiltrating ductal carcinomas. The median tumor size was 11 mm in each group. Approximately half of the invasive cancers were high grade, LVI was present in 20%, and DCIS was present in > 75% of cases. For patients with DCIS +/− microinvasion, the median radiological size of DCIS was 10 mm in each group, and the nuclear grade was low or intermediate in nearly 70% of cases.
Table 2.
Clinical and pathologic characteristics of the patients in RSL and WL groups.
| Characteristic | Seed Group (n = 431) | Wire Group (n = 256) | p-value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Age | years: median (range) | 59.7 (26.8–92.3) | 60.8 (30.6–86.1) | 0.31 | ||
| Tumor type | Infiltrating ductal | 261 | 60.6 | 169 | 66.0 | 0.28 |
| Infiltrating lobular (ILC) | 43 | 10.0 | 18 | 7.0 | ||
| Infiltrating other | 24 | 5.6 | 11 | 4.3 | ||
| DCIS + microinvasion | 11 | 2.6 | 11 | 4.3 | ||
| DCIS | 92 | 21.3 | 47 | 18.4 | ||
| Infiltrating carcinoma | (n = 328) | 76.1 | (n = 198) | 77.3 | ||
| Tumor size | mm: median (range) | 11.0 (1.0–41.0) | 11.0 (1.5–30.0) | 0.44 | ||
| T1 | 300 | 91.5 | 176 | 88.9 | 0.33 | |
| T2 | 28 | 8.5 | 22 | 11.1 | ||
| Tumor grade | Not assessed/ILC | 36 | 11.0 | 19 | 9.6 | 0.57 |
| I | 50 | 15.2 | 29 | 14.6 | ||
| II | 88 | 26.8 | 47 | 23.7 | ||
| III | 154 | 47.0 | 103 | 52.0 | ||
| LVI present | 69 | 21.0 | 37 | 18.7 | 0.52 | |
| DCIS | No DCIS | 75 | 22.9 | 35 | 17.7 | 0.32 |
| DCIS present | 232 | 70.7 | 147 | 74.2 | ||
| EIC | 21 | 6.4 | 16 | 8.1 | ||
| Receptor subtype |
ER/PR+, HER2− | 287 (323*) | 88.9 | 173 (197*) | 87.8 | 0.90 |
| ER/PR+, HER2+ | 12 (323*) | 3.7 | 8 (197*) | 4.1 | ||
| ER/PR−, HER2+ | 5 (323*) | 1.5 | 2 (197*) | 1.0 | ||
| Triple negative | 19 (323*) | 5.9 | 14 (197*) | 7.1 | ||
| LN metastases | Not assessed | 6 | 1.8 | 2 | 1.0 | 0.45 |
| 0 | 262 | 79.9 | 166 | 83.8 | ||
| 1 or 2 | 52 | 15.9 | 24 | 12.1 | ||
| ≥ 3 | 8 | 2.4 | 6 | 3.0 | ||
| DCIS (+/− microinvasion) | (n = 103) | 23.9 | (n = 58) | 22.7 | ||
| Radiological size |
mm: median (range) | 10.0 (2.3–55.0) | 10.0 (1.2–50.0) | 0.61 | ||
| Nuclear grade | Low | 24 | 23.3 | 10 | 17.2 | 0.51 |
| Intermediate | 46 | 44.7 | 31 | 53.4 | ||
| High | 33 | 32.0 | 17 | 29.3 | ||
RSL, radioactive seed localization; WL, wire localization; T, tumor; ILC, infiltrating lobular carcinoma; LVI, lymphovascular invasion; DCIS, ductal carcinoma in situ; EIC, extensive intraductal component; ER+, estrogen receptor positive; PR+, progesterone receptor positive; HER2+, HER2/neu amplified; LN, lymph node
Some patients were missing receptor status variables. Denominators reflect number of patients with data available.
The outcomes of the RSL and WL operations are shown in Table 3. The rate of positive margins (7.7% versus 5.5%) and close margins (16.9% versus 19.9%) were not different between the RSL and WL groups (p = 0.38). Re-operation to improve the margin of excision was performed in 23.0% of patients in the RSL group and in 22.3% of patients in the WL group (p = 0.83). Similarly, there was no significant difference in the initial lumpectomy volume between the RSL group and the WL group (median, 21.2 cm3; range, 0.2–311.0 cm3 versus median, 19.0 cm3; range, 0.9–197.9 cm3; p = 0.074). Of note, this calculated volume did not include the separate cavity-shaved margins which were excised after removal of the seed or wire, and should not have been influenced by the localization method used.
Table 3.
Outcomes of patients in the RSL and WL groups.
| Outcome | Seed Group (n = 431) | Wire Group (n = 256) | p-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Margin Status: | |||||
| Clear | 325 | 75.4 | 191 | 74.6 | 0.38 |
| Tumor on ink | 33 | 7.7 | 14 | 5.5 | |
| Close (≤ 1mm from ink) | 73 | 16.9 | 51 | 19.9 | |
| Reoperation to Improve margins | 99 | 23.0 | 57 | 22.3 | 0.83 |
| No. | Median (range) | No. | Median (range) | ||
| Initial lumpectomy volume (cm3) | 429 | 21.2 (0.2–311.0) | 256 | 19.0 (0.9–197.9) | 0.074 |
| Operative time (minutes): | |||||
| All procedures | 403 | 50 (16–140) | 247 | 45 (12–150) | |
| Lumpectomy alone | 96 | 31 (16–72) | 46 | 33 (21–66) | 0.18 |
| Lumpectomy + SLNB | 301 | 55 (29–140) | 196 | 48 (12–110) | < 0.0001 |
| Lumpectomy + ALND | 6 | 80 (62–105) | 5 | 68 (50–150) | 0.86 |
RSL, radioactive seed localization; WL, wire localization; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection
The operative time could be assessed accurately for 403 RSL operations and for 247 WL operations. For lumpectomy alone (n= 142), there was no difference in the operative time between the RSL and WL groups (median, 31 minutes; range, 16–72 minutes versus median, 33 minutes; range, 21–66 minutes; p = 0.18). The same was true for lumpectomy and axillary lymph node dissection (ALND) (n= 11), (median, 80 minutes; range, 62–105 minutes versus median, 68 minutes; range, 50–150 minutes; p = 0.86). For lumpectomy and SLNB (n = 497), operative time was significantly longer for the RSL group than the WL group (median, 55 minutes; range, 29–140 minutes versus median, 48 minutes; range, 12–110 minutes; p < 0.0001).
DISCUSSION
The ability to accurately localize non-palpable lesions with a “wire” revolutionized breast cancer surgery after its first description in 1965.[12] The procedure however, requires patients to first report to the radiology department for localization and await transportation to the operating room with a wire protruding from the breast, which is often uncomfortable and carries a small risk of dislodgement or fracturing. The need for same day localization also typically precludes placing such an operation as the first case on the operating room schedule, and additional delays related to difficult wire placements or syncope during wire localization often interfere with operating room efficiency, particularly at institutions with a high daily volume of localization procedures.
Following the introduction of RSL at our institution, 90% of localizations were performed prior to the day of surgery, simplifying operating room scheduling and minimizing patient inconvenience on the morning of surgery. In addition, as it is our practice to perform the radioisotope injection (99technetium) for SLNB on the day prior to surgery, seed placement was scheduled on the same day as the isotope injection to minimize patient travel whenever possible. Same-day RSL was reserved for patients who were unable to travel to the hospital prior to surgery. The 10 participating surgeons felt that RSL simplified operative scheduling and patient flow in the operating room. Although not directly measured in our study, others have reported that the pain of the localization is similar between RSL and WL[13, 14]; yet patients reported that RSL was more convenient.[13] In a small Canadian randomized controlled trial, using a Likert scale, patients rated RSL less painful than WL; however, a similar level of anxiety was experienced by both patient groups.[1]
The primary outcome of this study was to compare our rates of complete excision of non-palpable breast cancers localized with RSL versus WL. Characteristics known to influence the likelihood of positive margins, including tumor size, presence of an EIC, or pure DCIS, did not differ significantly between groups. No significant differences in completeness of excision related to localization technique were observed, in spite of the fact that surgeons had many years of experience with WL and were in the initial 6 months of using RSL. Negative margins, defined as tumor not touching ink, were obtained at initial excision in 92.3% of RSL cases and 94.5% of WL cases. Close margins were present in 16.9% and 19.9%, respectively. In their multicenter randomized controlled trial, Lovrics et al. compared the outcome of 152 patients allocated to RSL and 153 patients allocated to WL for breast cancer.[1] An intention-to-treat analysis was performed; however, 21 patients (14%) randomized to the RSL group had WL performed, 3 of whom had both RSL and WL. In contrast to the eligibility criteria for our study, more than one seed or wire was used to perform localization in 9.9% and 13.7% of cases, respectively. The rate of positive and close margins in this study also did not differ significantly by localization method. In the Netherlands, a retrospective comparison of RSL in 71 patients and ROLL in 83 patients following neoadjuvant chemotherapy has recently been performed. Positive pathological margins were found in 13% of both the RSL and ROLL groups, and the rate of re-operation was similar, at 8% and 7%, respectively.[15]
We also found no significant difference in lumpectomy specimen volume based on localization technique, similar to the Canadian randomized trial of RSL versus WL in which both the specimen volume and weight did not differ by localization technique.[1]
The median operative time for patients in the RSL group was 5 minutes longer than for those in the WL group. However, operative times for lumpectomy alone or lumpectomy with ALND did not differ between groups. The median time to perform lumpectomy with SLNB was 7 minutes longer in RSL patients than in WL patients (median, 55 minutes versus median, 48 minutes, p < 0.0001), suggesting that this difference was related to the logistics of the probe used for both RSL and sentinel node detection. Prior to seed localization, the C-Trak system (Care Wise Medical, Morgan Hill, CA), with a simple on/off switch, was used for SLNB. The Node Seeker system, which detects both 125iodine and 99technetium (IntraMedical Imaging LLC, Los Angeles, CA), has a complex operating system which requires a longer start-up time, and the settings must be changed between the lumpectomy and SLNB procedures. In contrast to our experience, Lovrics et al. found that the mean operative time was shorter for RSL than WL (19.4 minutes versus 22.2 minutes, p < 0.001).[1] However, it is unclear how these operative times were obtained; following initial lumpectomy, the specimen radiograph was used to direct cavity re-excision in 47% of operations, and almost all (98%) patients had a SLNB or ALND performed.
Radiation safety issues related to the process of RSL in our institution are overseen by the Radiation Safety Service, Department of Medical Physics, and these have recently been described.[16, 17]. The RSL program licensing required routine and emergency training of all persons involved in handling of seeds. An inventory log (database) tracked the receipt, storage, radiological implantation, surgical excision, explantation in pathology, and decay in storage of all seeds [17]. The median seed radioactivity at the time of implantation was 83 μCi, and the median dose rate from patients with a single seed was 9.5 μSv per hour at contact and 0.5 μSv per hour at 1 meter. The low dose of radioactivity associated with RSL has been previously shown: with a mean excision of 4cm of breast tissue, the maximum dose to residual breast tissue approximates that of a 2-view mammogram [18]. In general, seed placement occurred within 1 week of surgery. In 2 patients, surgery was performed 30 and 47 days after placement due to acute medical conditions necessitating postponement of surgery. Both seeds were retrieved without incident. Adverse events related to RSL were not encountered during this study, but have occurred in our institution. Technical difficulties during radiological seed placement required additional wire localization in one case [16], 1 seed used to localize an axillary lymph node could not safely be retrieved and was left in-situ [17], and 1 seed was dropped and temporarily lost during pathological explantation. During surgical excision, care must be taken when using suctioning or surgical sponges close to the specimen to avoid unintentionally displacing the seed.
In summary, during the first 6-months of RSL in our institution, 90% of localizations were performed prior to the day of surgery, the rates of positive and close margins did not differ from those seen following many years of experience with WL, and specimen volume was not significantly increased. A minimal increase in operating time was seen in RSL patients undergoing both lumpectomy and SLNB, which we anticipate will decrease as surgeons and nursing staff become familiar with the new equipment. The 10 participating surgeons felt that RSL simplified operative scheduling and patient flow in the operating room.
ACKNOWLEDGEMENTS
This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
This study was presented in part as a poster at the 2013 Society of Surgical Oncology Annual Cancer Symposium, National Harbor, MD. March 7–9, 2013.
CONFLICT OF INTEREST None.
REFERENCES
- 1.Lovrics PJ, Goldsmith CH, Hodgson N, et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol. 2011;18:3407–3414. doi: 10.1245/s10434-011-1699-y. [DOI] [PubMed] [Google Scholar]
- 2.Rampaul RS, Bagnall M, Burrell H, et al. Randomized clinical trial comparing radioisotope occult lesion localization and wire-guided excision for biopsy of occult breast lesions. Br J Surg. 2004;91:1575–1577. doi: 10.1002/bjs.4801. [DOI] [PubMed] [Google Scholar]
- 3.Arentz C, Baxter K, Boneti C, et al. Ten-year experience with hematoma-directed ultrasound-guided (HUG) breast lumpectomy. Ann Surg Oncol. 2010;17(Suppl 3):378–383. doi: 10.1245/s10434-010-1230-x. [DOI] [PubMed] [Google Scholar]
- 4.Klein RL, Mook JA, Euhus DM, et al. Evaluation of a hydrogel based breast biopsy marker (HydroMARK(R)) as an alternative to wire and radioactive seed localization for non-palpable breast lesions. J Surg Oncol. 2012;105:591–594. doi: 10.1002/jso.22146. [DOI] [PubMed] [Google Scholar]
- 5.Monti S, Galimberti V, Trifiro G, et al. Occult breast lesion localization plus sentinel node biopsy (SNOLL): experience with 959 patients at the European Institute of Oncology. Ann Surg Oncol. 2007;14:2928–2931. doi: 10.1245/s10434-007-9452-2. [DOI] [PubMed] [Google Scholar]
- 6.Lavoue V, Nos C, Clough KB, et al. Simplified technique of radioguided occult lesion localization (ROLL) plus sentinel lymph node biopsy (SNOLL) in breast carcinoma. Ann Surg Oncol. 2008;15:2556–2561. doi: 10.1245/s10434-008-9994-y. [DOI] [PubMed] [Google Scholar]
- 7.Alderliesten T, Loo CE, Pengel KE, et al. Radioactive seed localization of breast lesions: an adequate localization method without seed migration. Breast J. 2011;17:594–601. doi: 10.1111/j.1524-4741.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- 8.van Riet YE, Maaskant AJ, Creemers GJ, et al. Identification of residual breast tumour localization after neo-adjuvant chemotherapy using a radioactive 125 Iodine seed. Eur J Surg Oncol. 2010;36:164–169. doi: 10.1016/j.ejso.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.McGhan LJ, McKeever SC, Pockaj BA, et al. Radioactive seed localization for nonpalpable breast lesions: review of 1,000 consecutive procedures at a single institution. Ann Surg Oncol. 2011;18:3096–3101. doi: 10.1245/s10434-011-1910-1. [DOI] [PubMed] [Google Scholar]
- 10.van Riet YE, Jansen FH, van Beek M, et al. Localization of non-palpable breast cancer using a radiolabelled titanium seed. Br J Surg. 2010;97:1240–1245. doi: 10.1002/bjs.7097. [DOI] [PubMed] [Google Scholar]
- 11.Corben AD, Morrow M. Margins in breast cancer surgery. In: El-Tamer MB, editor. Principles and techniques in oncoplastic breast cancer surgery. Singapore: World Scientific Publishing Co.; 2012. pp. 65–85. [Google Scholar]
- 12.Dodd GD, Fry K, Delany W. Preoperative localization of occult carcinoma of the breast. In: Nealton TP, editor. Management of the patient with cancer. Philadelphia: Saunders; 1965. pp. 88–113. [Google Scholar]
- 13.Hughes JH, Mason MC, Gray RJ, et al. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. Breast J. 2008;14:153–157. doi: 10.1111/j.1524-4741.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 14.Gray RJ, Pockaj BA, Karstaedt PJ, Roarke MC. Radioactive seed localization of nonpalpable breast lesions is better than wire localization. Am J Surg. 2004;188:377–380. doi: 10.1016/j.amjsurg.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Donker M, Drukker CA, Valdes Olmos RA, et al. Guiding Breast-Conserving Surgery in Patients After Neoadjuvant Systemic Therapy for Breast Cancer: A Comparison of Radioactive Seed Localization with the ROLL Technique. Ann Surg Oncol. 2013 doi: 10.1245/s10434-013-2921-x. [DOI] [PubMed] [Google Scholar]
- 16.Sung JS, King V, Thornton CM, et al. Safety and efficacy of radioactive seed localization with I-125 prior to lumpectomy and/or excisional biopsy. Eur J Radiol. 2013 doi: 10.1016/j.ejrad.2013.04.008. Article in press, http://dx.doi.org/10.1016/j.ejrad.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Dauer LT, Thornton C, Miodownik D, et al. Radioactive Seed Localization with Iodine-125 for Non-1 Palpable Lesions Prior to Breast Lumpectomy and/or Excisional Biopsy: Methodology, Safety and Experience of Initial Year. Health Phys. 2013 doi: 10.1097/HP.0b013e31829c03e1. Article in press. [DOI] [PubMed] [Google Scholar]
- 18.Pavlicek W, Walton HA, Karstaedt PJ, Gray RJ. Radiation safety with use of I-125 seeds for localization of nonpalpable breast lesions. Acad Radiol. 2006;13:909–915. doi: 10.1016/j.acra.2006.03.017. [DOI] [PubMed] [Google Scholar]