Abstract
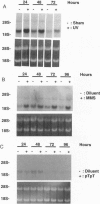
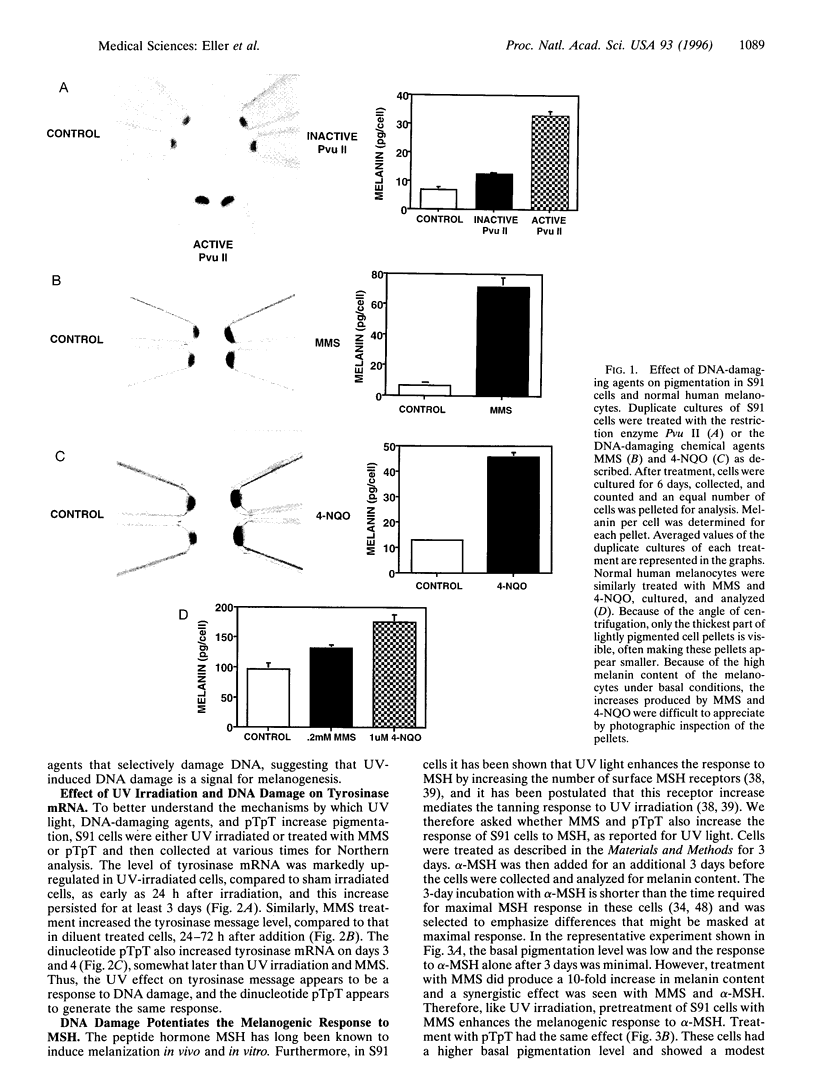
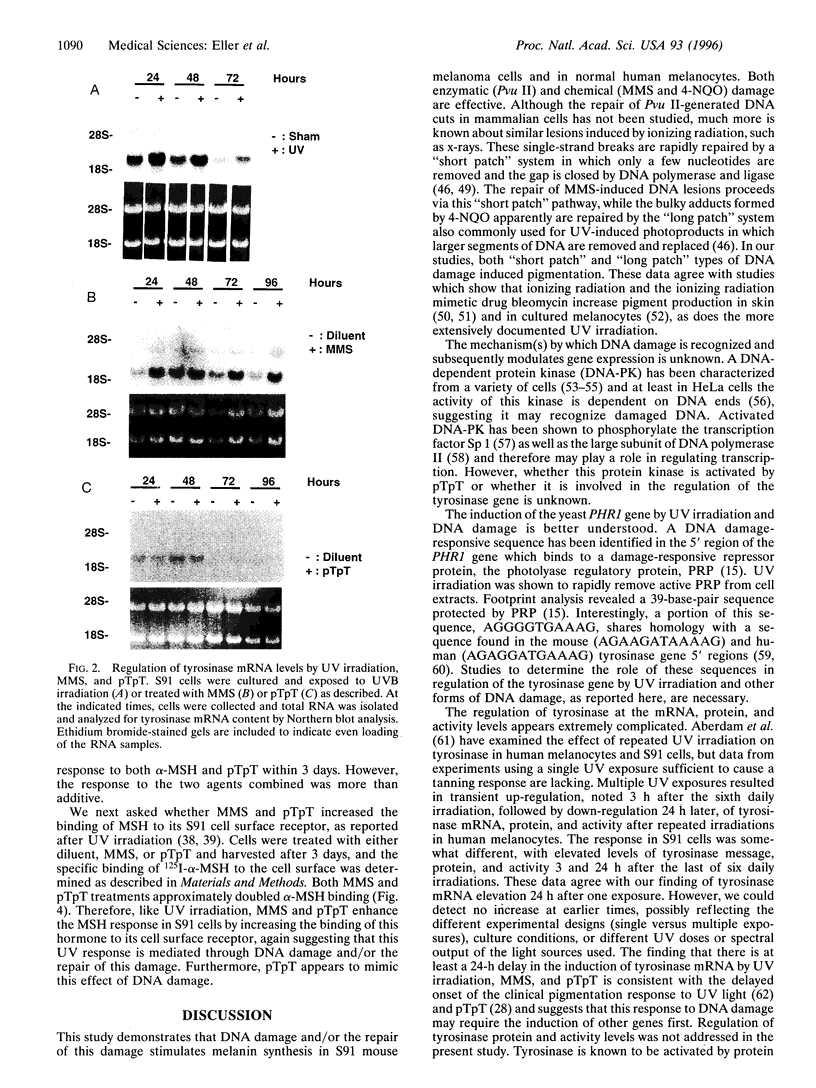
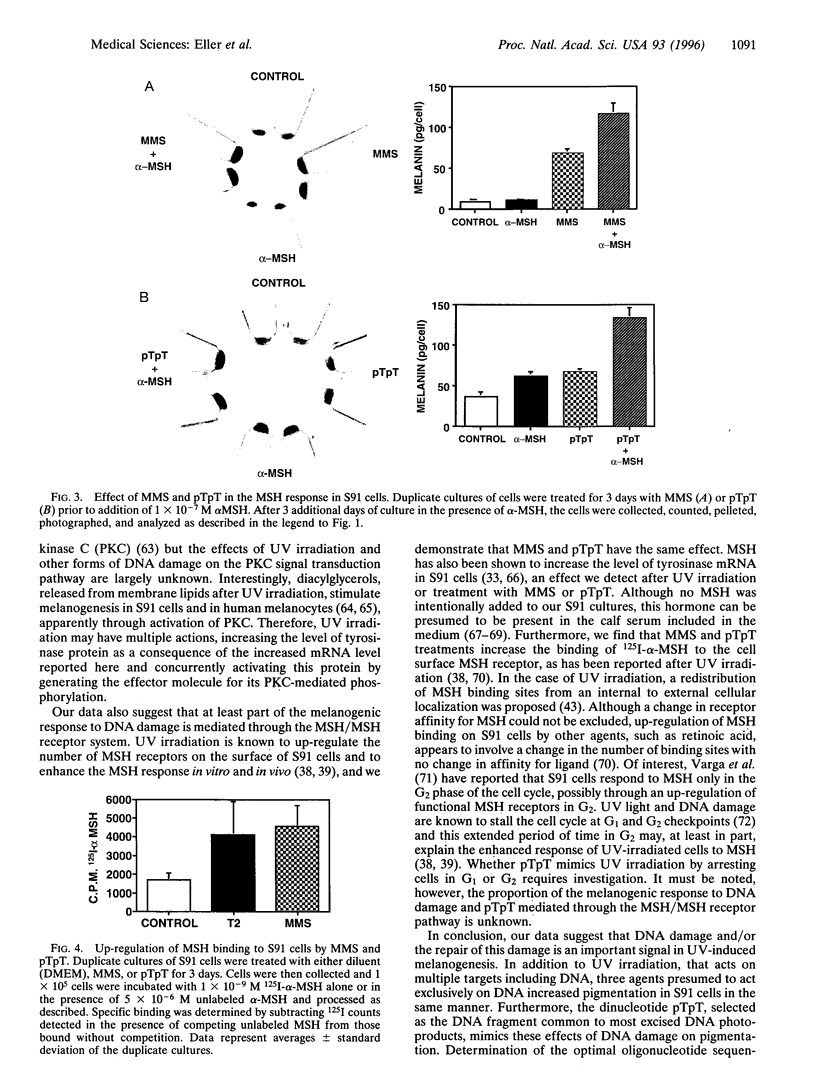
Although the ability of UV irradiation to induce pigmentation in vivo and in vitro is well documented, the intracellular signals that trigger this response are poorly understood. We have recently shown that increasing DNA repair after irradiation enhances UV-induced melanization. Moreover, addition of small DNA fragments, particularly thymine dinucleotides (pTpT), selected to mimic sequences excised during the repair of UV-induced DNA photoproducts, to unirradiated pigment cells in vitro or to guinea pig skin in vivo induces a pigment response indistinguishable from UV-induced tanning. Here we present further evidence that DNA damage and/or the repair of this damage increases melanization. (i) Treatment with the restriction enzyme Pvu II or the DNA-damaging chemical agents methyl methanesulfonate (MMS) or 4-nitroquinoline 1-oxide (4-NQO) produces a 4- to 10-fold increase in melanin content in Cloudman S91 murine melanoma cells and an up to 70% increase in normal human melanocytes, (ii) UV irradiation, MMS, and pTpT all upregulate the mRNA level for tyrosinase, the rate-limiting enzyme in melanin biosynthesis. (iii) Treatment with pTpT or MMS increases the response of S91 cells to melanocyte-stimulating hormone (MSH) and increases the binding of MSH to its cell surface receptor, as has been reported for UV irradiation. Together, these data suggest that UV-induced DNA damage and/or the repair of this damage is an important signal in the pigmentation response to UV irradiation. Because Pvu II acts exclusively on DNA and because MMS and 4-NQO, at the concentrations used, primarily interact with DNA, such a stimulus alone appears sufficient to induce melanogenesis. Of possible practical importance, the dinucleotide pTpT mimics most, if not all, of the effects of UV irradiation on pigmentation, tyrosinase mRNA regulation, and response to MSH without the requirement for antecedent DNA damage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberdam E., Roméro C., Ortonne J. P. Repeated UVB irradiations do not have the same potential to promote stimulation of melanogenesis in cultured normal human melanocytes. J Cell Sci. 1993 Dec;106(Pt 4):1015–1022. doi: 10.1242/jcs.106.4.1015. [DOI] [PubMed] [Google Scholar]
- Altmeyer P., Stöhr L., Holzmann H. Seasonal rhythm of the plasma level of alpha-melanocyte stimulating hormone. J Invest Dermatol. 1986 Apr;86(4):454–456. doi: 10.1111/1523-1747.ep12285798. [DOI] [PubMed] [Google Scholar]
- Applegate L. A., Luscher P., Tyrrell R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991 Feb 1;51(3):974–978. [PubMed] [Google Scholar]
- BECKER S. W., Jr, FITZPATRICK T. B., MONTGOMERY H. Human melanogenesis; cytology and histology of pigment cells (melanodendrocytes). AMA Arch Derm Syphilol. 1952 May;65(5):511–523. doi: 10.1001/archderm.1952.01530240003001. [DOI] [PubMed] [Google Scholar]
- Bolognia J., Murray M., Pawelek J. UVB-induced melanogenesis may be mediated through the MSH-receptor system. J Invest Dermatol. 1989 May;92(5):651–656. doi: 10.1111/1523-1747.ep12696836. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T., Vancurová I., Sun I., Lou W., DeLeon S. A DNA-activated protein kinase from HeLa cell nuclei. Mol Cell Biol. 1990 Dec;10(12):6460–6471. doi: 10.1128/mcb.10.12.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A. K., Orlow S. J., Bolognia J. L., Pawelek J. M. Structural/functional relationships between internal and external MSH receptors: modulation of expression in Cloudman melanoma cells by UVB radiation. J Cell Physiol. 1991 Apr;147(1):1–6. doi: 10.1002/jcp.1041470102. [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Orlow S. J., Pawelek J. M. Stimulation of the receptor for melanocyte-stimulating hormone by retinoic acid. FEBS Lett. 1990 Dec 10;276(1-2):205–208. doi: 10.1016/0014-5793(90)80543-r. [DOI] [PubMed] [Google Scholar]
- De Luca M., Siegrist W., Bondanza S., Mathor M., Cancedda R., Eberle A. N. Alpha melanocyte stimulating hormone (alpha MSH) stimulates normal human melanocyte growth by binding to high-affinity receptors. J Cell Sci. 1993 Aug;105(Pt 4):1079–1084. doi: 10.1242/jcs.105.4.1079. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Devary Y., Rosette C., DiDonato J. A., Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993 Sep 10;261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- Donatien P. D., Hunt G., Pieron C., Lunec J., Taïeb A., Thody A. J. The expression of functional MSH receptors on cultured human melanocytes. Arch Dermatol Res. 1992;284(7):424–426. doi: 10.1007/BF00372074. [DOI] [PubMed] [Google Scholar]
- Eller M. S., Oleksiak M. F., McQuaid T. J., McAfee S. G., Gilchrest B. A. The molecular cloning and expression of two CRABP cDNAs from human skin. Exp Cell Res. 1992 Feb;198(2):328–336. doi: 10.1016/0014-4827(92)90387-n. [DOI] [PubMed] [Google Scholar]
- Eller M. S., Yaar M., Gilchrest B. A. DNA damage and melanogenesis. Nature. 1994 Dec 1;372(6505):413–414. doi: 10.1038/372413a0. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann P. S., Gilchrest B. A. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987 Oct;133(1):88–94. doi: 10.1002/jcp.1041330111. [DOI] [PubMed] [Google Scholar]
- Friedmann P. S., Wren F. E., Matthews J. N. Ultraviolet stimulated melanogenesis by human melanocytes is augmented by di-acyl glycerol but not TPA. J Cell Physiol. 1990 Feb;142(2):334–341. doi: 10.1002/jcp.1041420216. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y. Postreplication repair of alkylation damage to DNA of mammalian cells in culture. Cancer Res. 1975 Oct;35(10):2780–2789. [PubMed] [Google Scholar]
- Fuller B. B., Lunsford J. B., Iman D. S. Alpha-melanocyte-stimulating hormone regulation of tyrosinase in Cloudman S-91 mouse melanoma cell cultures. J Biol Chem. 1987 Mar 25;262(9):4024–4033. [PubMed] [Google Scholar]
- Garmyn M., Yaar M., Holbrook N., Gilchrest B. A. Immediate and delayed molecular response of human keratinocytes to solar-simulated irradiation. Lab Invest. 1991 Oct;65(4):471–478. [PubMed] [Google Scholar]
- Gilchrest B. A., Zhai S., Eller M. S., Yarosh D. B., Yaar M. Treatment of human melanocytes and S91 melanoma cells with the DNA repair enzyme T4 endonuclease V enhances melanogenesis after ultraviolet irradiation. J Invest Dermatol. 1993 Nov;101(5):666–672. doi: 10.1111/1523-1747.ep12371673. [DOI] [PubMed] [Google Scholar]
- Gordon L., Peacocke M., Gilchrest B. A. Induction of c-fos but not c-myc in S-91 cells by melanization signals. J Dermatol Sci. 1992 Jan;3(1):35–41. doi: 10.1016/0923-1811(92)90006-w. [DOI] [PubMed] [Google Scholar]
- Gordon P. R., Gilchrest B. A. Human melanogenesis is stimulated by diacylglycerol. J Invest Dermatol. 1989 Nov;93(5):700–702. doi: 10.1111/1523-1747.ep12319900. [DOI] [PubMed] [Google Scholar]
- Gordon P. R., Gilchrest B. A. Human melanogenesis is stimulated by diacylglycerol. J Invest Dermatol. 1989 Nov;93(5):700–702. doi: 10.1111/1523-1747.ep12319900. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Hill H. Z., Setlow R. B. Comparative action spectra for pyrimidine dimer formation in cloudman S91 mouse melanoma and EMT6 mouse mammary carcinoma cells. Photochem Photobiol. 1982 May;35(5):681–684. doi: 10.1111/j.1751-1097.1982.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Hoganson G. E., Ledwitz-Rigby F., Davidson R. L., Fuller B. B. Regulation of tyrosinase mRNA levels in mouse melanoma cell clones by melanocyte-stimulating hormone and cyclic AMP. Somat Cell Mol Genet. 1989 May;15(3):255–263. doi: 10.1007/BF01534876. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., MacDonald J. J., Lees-Miller S., Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990 Oct 5;63(1):155–165. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- Kartasova T., Cornelissen B. J., Belt P., van de Putte P. Effects of UV, 4-NQO and TPA on gene expression in cultured human epidermal keratinocytes. Nucleic Acids Res. 1987 Aug 11;15(15):5945–5962. doi: 10.1093/nar/15.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Muramatsu T., Yamashina Y., Shirai T., Ohnishi T., Mori T. Melanin reduces ultraviolet-induced DNA damage formation and killing rate in cultured human melanoma cells. J Invest Dermatol. 1993 Nov;101(5):685–689. doi: 10.1111/1523-1747.ep12371676. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Wakulchik M., Kestler D., Barton D. E., Francke U., Lamoreux M. L., Whitney J. B., 3rd, Halaban R. Isolation, chromosomal mapping, and expression of the mouse tyrosinase gene. J Invest Dermatol. 1989 Nov;93(5):589–594. doi: 10.1111/1523-1747.ep12319693. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Wakulchik M., Haq A. K., Halaban R., Kestler D. Sequence analysis of mouse tyrosinase cDNA and the effect of melanotropin on its gene expression. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1301–1309. doi: 10.1016/s0006-291x(88)81370-6. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., MCGUIRE J. S. MELANOCYTE-STIMULATING HORMONE AND ADRENOCORTICOTROPHIC HORMONE. THEIR RELATION TO PIGMENTATION. N Engl J Med. 1964 Mar 12;270:539–546. doi: 10.1056/NEJM196403122701101. [DOI] [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Matsui M. S., DeLeo V. A. Induction of protein kinase C activity by ultraviolet radiation. Carcinogenesis. 1990 Feb;11(2):229–234. doi: 10.1093/carcin/11.2.229. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Creative blocks: cell-cycle checkpoints and feedback controls. Nature. 1992 Oct 15;359(6396):599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Ogawa T. Regulation in repressor inactivation by RecA protein. Adv Biophys. 1990;26:33–49. doi: 10.1016/0065-227x(90)90006-f. [DOI] [PubMed] [Google Scholar]
- Okoro A. N. Albinism in Nigeria. A clinical and social study. Br J Dermatol. 1975 May;92(5):485–492. [PubMed] [Google Scholar]
- Orlow S. J., Hotchkiss S., Pawelek J. M. Internal binding sites for MSH: analyses in wild-type and variant Cloudman melanoma cells. J Cell Physiol. 1990 Jan;142(1):129–136. doi: 10.1002/jcp.1041420116. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Repair replication in mammalian cells after x-irradiation. Mutat Res. 1972 Feb;14(2):225–235. doi: 10.1016/0027-5107(72)90049-8. [DOI] [PubMed] [Google Scholar]
- Park H. Y., Russakovsky V., Ohno S., Gilchrest B. A. The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. J Biol Chem. 1993 Jun 5;268(16):11742–11749. [PubMed] [Google Scholar]
- Parrish J. A., Jaenicke K. F., Anderson R. R. Erythema and melanogenesis action spectra of normal human skin. Photochem Photobiol. 1982 Aug;36(2):187–191. doi: 10.1111/j.1751-1097.1982.tb04362.x. [DOI] [PubMed] [Google Scholar]
- Peak J. G., Woloschak G. E., Peak M. J. Enhanced expression of protein kinase C gene caused by solar radiation. Photochem Photobiol. 1991 Mar;53(3):395–397. doi: 10.1111/j.1751-1097.1991.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Peterson K. R., Ossanna N., Thliveris A. T., Ennis D. G., Mount D. W. Derepression of specific genes promotes DNA repair and mutagenesis in Escherichia coli. J Bacteriol. 1988 Jan;170(1):1–4. doi: 10.1128/jb.170.1.1-4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S. R., Dvir A., Anderson C. W., Dynan W. S. DNA binding provides a signal for phosphorylation of the RNA polymerase II heptapeptide repeats. Genes Dev. 1992 Mar;6(3):426–438. doi: 10.1101/gad.6.3.426. [DOI] [PubMed] [Google Scholar]
- Ponnazhagan S., Hou L., Kwon B. S. Structural organization of the human tyrosinase gene and sequence analysis and characterization of its promoter region. J Invest Dermatol. 1994 May;102(5):744–748. doi: 10.1111/1523-1747.ep12376924. [DOI] [PubMed] [Google Scholar]
- Protić M., Roilides E., Levine A. S., Dixon K. Enhancement of DNA repair capacity of mammalian cells by carcinogen treatment. Somat Cell Mol Genet. 1988 Jul;14(4):351–357. doi: 10.1007/BF01534643. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Weinstein I. B. Inducible cellular responses to ultraviolet light irradiation and other mediators of DNA damage in mammalian cells. Cell Biol Toxicol. 1990 Jan;6(1):105–126. doi: 10.1007/BF00135030. [DOI] [PubMed] [Google Scholar]
- Rosen C. F., Gajic D., Jia Q., Drucker D. J. Interaction of TPA and ultraviolet B radiation in regulation of ODC gene expression in rat keratinocytes. Am J Physiol. 1992 Nov;263(5 Pt 1):C1103–C1110. doi: 10.1152/ajpcell.1992.263.5.C1103. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Arimura A., Kastin A. J. Hypothalamic regulatory hormones. Science. 1973 Jan 26;179(4071):341–350. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- Schauer E., Trautinger F., Köck A., Schwarz A., Bhardwaj R., Simon M., Ansel J. C., Schwarz T., Luger T. A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994 May;93(5):2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian J., Kraus B., Sancar G. B. Expression of the yeast PHR1 gene is induced by DNA-damaging agents. Mol Cell Biol. 1990 Sep;10(9):4630–4637. doi: 10.1128/mcb.10.9.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian J., Sancar G. B. A damage-responsive DNA binding protein regulates transcription of the yeast DNA repair gene PHR1. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11251–11255. doi: 10.1073/pnas.88.24.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Smith A. I., Funder J. W. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988 Feb;9(1):159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., Bae I., Chen C. Y., Gilmer T. M., Kastan M. B., O'Connor P. M., Fornace A. J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994 Nov 25;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Dipasquale A., Pawelek J., McGuire J. S., Lerner A. B. Regulation of melanocyte stimulating hormone action at the receptor level: discontinuous binding of hormone to synchronized mouse melanoma cells during the cell cycle. Proc Natl Acad Sci U S A. 1974 May;71(5):1590–1593. doi: 10.1073/pnas.71.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. I., Hunt T., Jackson R. J., Anderson C. W. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985 Jan;4(1):139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh D. B., Alas L., Kibitel J., O'Connor A., Carrier F., Fornace A. J., Jr Cyclobutane pyrimidine dimers in UV-DNA induce release of soluble mediators that activate the human immunodeficiency virus promoter. J Invest Dermatol. 1993 Jun;100(6):790–794. doi: 10.1111/1523-1747.ep12476573. [DOI] [PubMed] [Google Scholar]